Abstract
Although infections with Cyclospora cayetanensis are prevalent worldwide, many aspects of this parasite’s life cycle and transmission remain unknown. Humans are the only known hosts of this parasite. Existing information on its endogenous development has been derived from histological examination of only a few biopsy specimens. Its asexual and sexual stages occur in biliary-intestinal epithelium. In histological sections, its stages are less than 10 μm, making definitive identification difficult. Asexual (schizonts) and sexual (gamonts) are located in epithelial cells. Male microgamonts have two flagella; female macrogametes contain wall-forming bodies. Oocysts are excreted in feces unsporulated. Sporulation occurs in the environment, but there are many unanswered questions concerning dissemination and survival of C. cayetanensis oocysts. Biologically and phylogenetically, C. cayetanensis closely resembles Eimeria spp. that parastize chickens; among them, E. acervulina most closely resembles C. cayetanensis in size. Here, we review known and unknown aspects of its life cycle and transmission and discuss the appropriateness of surrogates best capable of hastening progress in understanding its biology and developing mitigating strategies.
1. Introduction
Cyclosporiasis is a problem worldwide. Several recent papers review prevalence, clinical symptoms, diagnosis, epidemiology, and treatment of cyclosporiasis [1,2,3,4,5,6]. The objectives of this paper are to review known and unknown aspects of its life cycle and transmission, and to review the appropriateness of surrogates best capable of hastening progress in understanding its biology and developing mitigating strategies.
2. Etiology
Among at least 20 species in the genus Cyclospora, Cyclospora cayetanensis is the only species known to infect humans [7]. Other congeners infect animals ranging from arthropods to non-human primates [8]. There are no in vitro or in vivo methods known for propagating C. cayetanensis; this severely limits development of new control strategies. Attempts to infect several species of laboratory animals, including non-human primates with C. cayetanensis were unsuccessful [9]. Information on its endogenous developmental stages came from histological examination of limited biopsy material of digestive tissues [10,11]. Considering these limitations, we discuss here taxonomic, phylogenetic, molecular relationships of C. cayetanensis with other closely related organisms suitable as surrogates to explore its biology and control.
3. Taxonomy
Cyclospora cayetanensis is classified as a coccidian parasite, in the phylum Apicomplexa, family Eimeriidae. All members of Eimeriidae have a one-host, fecal-oral cycle. Eimeria species are among the most prevalent parasites of livestock and poultry; infections are so prevalent that it is very difficult to raise livestock free of Eimeria [12]. Eimeria are host-specific, and infections are generally confined to gastro-intestinal system. Indeed, Eimeria species causing poultry coccidiosis impose high costs to poultry production, worldwide. As with Cyclospora spp., oocysts of Eimeria are excreted in feces unsporulated (non-infectious), and sporulation occurs outside in the environment. Although Eimeria oocysts (encasing two sporocysts, each with four sporozoites) morphologically differ from those of Cyclospora (encasing two sporocysts, each with two sporozoites), they are phylogenetically related [13]. Cyclospora comprise a monophyletic group which share common ancestor with all species of Eimeria, to the exclusion of other extant taxa [13].
4. Life Cycle of C. cayetanensis
The life cycle of C. cayetanensis remains incompletely defined. The best-characterized stage is the oocyst. Humans are thought to be infected by ingesting food or water contaminated with oocysts (Figure 1).
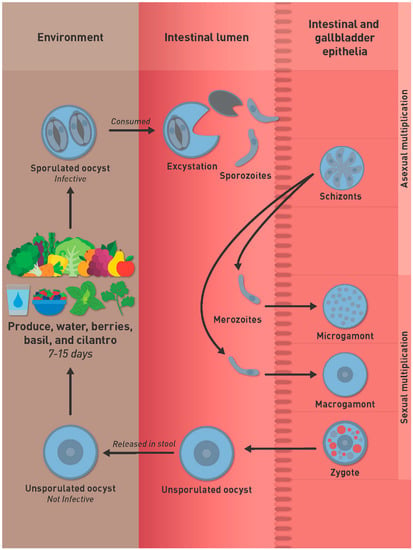
Figure 1.
Proposed life cycle of Cyclospora cayetanensis.
If a susceptible human ingests sporulated oocysts in contaminated food or water, the sporozoites inside the sporocysts excyst in the gut lumen and invade intestinal-biliary epithelial cells where the sporozoites transform into schizonts. Sexual multiplication occurs at the same site as asexual cycle. Microgamonts (male) contain flagellated microgemetes that fertilize macrogamonts to form the zygote. Oocysts then are excreted unsporulated in the feces. The prepatent period is thought to be around one week. Unsporulated oocysts are not infectious, they need to sporulate to became infective for a host. Under laboratory conditions, at 22 °C and 30 °C, sporulation will take around 7–14 days to occur outside the host [6].
4.1. Oocysts
The original description of C. cayetanensis elucidated most of the features presently understood about its oocysts [7]. Unsporulated oocysts in fresh fecal smears are spheroidal, 8–10 µm in diameter, with little or no size variation. The oocyst wall is colorless, thin (<1 µm), and bilayered. The inner layer encloses a central, undivided mass called the sporont; the sporont almost fills the oocyst and has many globules [7]. Sporulation occurs outside the host. Polar body and oocyst residuum are present. In fully-sporulated oocysts, there are two sporocysts, each with two sporozoites. Sporocysts are ovoidal, ~4 × 6 µm, and contain Stieda and substieda bodies, and a large residuum. The Stieda bodies are plug-like structures involved in sporozoite excystation [12]. The sporozoites are elongated, ~1 × 9 µm [7]. Whether C. cayetanensis sporozoites have crystalloid body or refractile bodies is not known. In Eimeria species, crystalloid bodies are considered virus-like particles; refractile bodies are proteinaceous in nature and are linked to host cell invasion [12].
4.2. Asexual Multiplication
After people ingest sporulated oocysts, sporozoites are believed (based on by analogy to more detailed data from various species of Eimeria) to excyst in the gut lumen and multiply, asexually, in intestinal epithelial cells. A few sporozoites may spill over and invade bile ducts and the gallbladder. Based on histological examination of endoscopic biopsies of duodenum or upper jejunum of Peruvian patients, asexual stages were observed in intestinal epithelial cells (presumably enterocytes) [10]. Only a few parasites were observed, and thus results were pioneering but preliminary. These stages were termed Types I and II meronts. Type I meronts each contained 8–12 small (3–4 μm long) merozoites; Type II meronts each contained four (12–15 μm) merozoites. However, the size of these merozoites was not confirmed by others [11]. The mode of division was not described.
Recently, an opportunity arose to study endogenous development of C. cayetanensis in an immunosuppressed cyclosporiasis patient with severe abdominal symptoms [11]. The entire gallbladder from this case had been surgically removed and fixed for histological and transmission electron microscopic (TEM) examination. In this gallbladder, profuse parasitization was observed; the diagnosis was confirmed by the presence of C. cayetanensis oocysts in feces and PCR, using DNA extracted from the gallbladder [11]. Cyclospora cayetanensis stages were found only in epithelial cells, located in a parasitophorous vacuole of host cytoplasm (Figure 2). Mature schizonts were 7.6 × 5.1 μm and contained up to 10 merozoites. The size of merozoites could not be determined but appeared to be less than 6 μm long. By TEM examination, schizonts divided by schizogony (where the parasite nucleus divided into five or more nuclei before segregating into distinct merozoites). A residual body was present in some, but not in others. The number of generations of schizonts could not be determined because of overcrowding. By light microscopy, merozoites and schizonts in different phases of development appeared within the same host cell or in adjacent host cells. Whether the course of schizont development in this case was influenced by the immunosuppressive status of the patient could not be ascertained. Thus, caution is warranted before extrapolating; future studies should determine whether such asynchronous development occurs in immunocompetent patients. We are aware of no archived biopsy material available to support such studies.
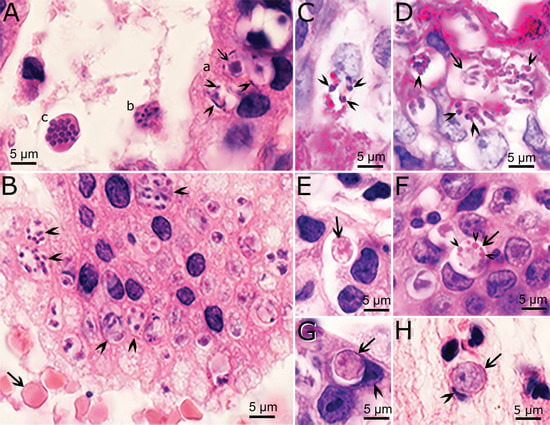
Figure 2.
Stages of Cyclospora cayetanensis in the gallbladder of an immunosuppressed patient. (A,B,E–H) Hematoxylin and eosin stain. (C,D) Periodic acid Schiff (PAS) reaction, counter-stained with hematoxylin. Bar applies to all figures. (A) The glandular epithelium is on the right and lumen on the left. (a) Three microgamonts in epithelial cells. Note peripherally located microgametes (arrowheads) around a large residual body (arrow). (b, c) Schizonts free in the lumen of a gland. Note cross section (c) of merozoites in a schizont. (B) Desquamated epithelium in lumen of a gland. Note numerous schizonts (arrowheads) and red blood cells (arrow) for size comparison. (C) A schizont with PAS-positive (arrowheads) merozoites. (D) Several schizonts with variable PAS-positive granules (arrowheads). Arrow points to a PAS-negative schizont that has merozoites attached to a residual body (arrow). (E) An early macrogamont (arrow). (F) A late macrogamont (arrow) with eosinophilic wall-forming bodies (arrowheads). The host cell nucleus is indented. (G) An intracellular oocyst (arrow) in the epithelium. Note indented host cell nucleus (arrowhead). (H) An oocyst (arrow) free in lumen of gallbladder. Arrowhead points to indented host cell nucleus [11].
Asexual Multiplication in Related Coccidia
Details of the asexual division process can aid diagnosis and illustrate the close affinity of C. cayetanensis with certain other coccidia. Considerable confusion surrounds the terminology used to describe asexual stages of coccidia. For the benefit of researchers not familiar with developmental cycles of coccidia, a brief account is provided here. Meront and schizont each describe stages undergoing asexual division. “Meront” has been used to denote various asexual stages, irrespective of division status [12]. Thus, it lacks perfect specificity. Some coccidia (for example, Toxoplasma gondii) replicate by “endodyogeny”, wherein each mother cell produces, and is consumed by, exactly two daughter cells. Others (for example, species of Sarcocystis) divide by endopolygeny, where the mother cell’s nucleus becomes a mass of connected lobes before subdividing into many daughter cells [12].
In species of Eimeria, schizonts divide by schizogony. After their sporozoites invade the host cell, the parasite nucleus divides into four or more nuclei before any merozoites are formed. After a defined number of multiplication cycles (termed generations), characteristic of each species of Eimeria, parasites from the final merozoite generation initiate the sexual cycle. The number of generations is fixed for each Eimeria species, and is not altered by immunosuppression [12]. Merozoites from each generation of schizonts are morphologically distinct.
Schizogony in C. cayetanensis resembles that in Eimeria. The lack of sequential specimens for study has, to date, precluded determination of the number of generations it undergoes prior to initiating the sexual cycle. Nonetheless, the occurrence of merozoites of different sizes suggests the existence of multiple generations of schizonts [12]. The development of merozoites by schizogony was confirmed by TEM examination. The presence of Type II merozoites could not be confirmed.
Cystoisospora belli is another coccidian that also parasitizes the small intestine and biliary system in humans [14,15]. In Cystoisospora, different generations of schizonts/Types can be found in the same host cell, because schizonts can rupture inside the host cell and merozoites can multiply within the same host cell without exiting the host cell (Figure 3). Additionally, immature schizonts can retain the shape of merozoites (Figure 3). Whether this type of multiplication also occurs in C. cayetanensis remains to be determined. Because C. belli stages (including merozoites, gamonts and oocysts) are more than twice the size of their counterparts in C. cayetanensis, differential diagnosis is possible to the trained eye using light microscopy [15]. Genetic assays also support differential diagnosis [16,17,18].
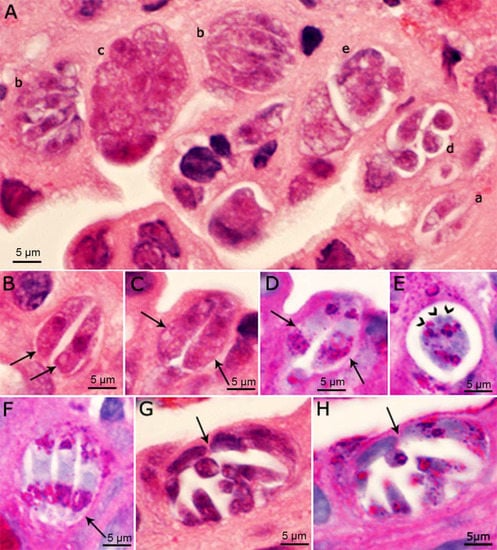
Figure 3.
Asexual stages of Cystoisospora belli in histological sections of bile duct of a patient. Bar applies to all parts. (A–C,G) = Hematoxylin and eosin stain; (D–F,H) = PAS counter stained with hematoxylin. The luminal side of sections is on the top. (A) Several meronts in one microscopic field. (a) Paired merozoites in one pv. (b) Meront with four or more merozoites completely filling the pv. (c) Meront containing developing merozoites. The organisms are cut in cross-section; the nuclei appear larger than in merozoites. (d) Four merozoites in a pv with spaces between merozoites. (e) Immature meront. (B) Paired crescent-shaped merozoites. (C,D) The same paired organisms/meronts after staining with HE (C) and PAS (D). Note the polar PAS-positivity. The parasite nuclei are masked by PAS staining. (E) Meront with mero- zoites budding (arrowheads). Note PAS-positivity. (F) Meront (arrow) with three merozoites with polar PAS-positivity. (G,H) The same mature meront (arrow) after staining with HE (G) and PASH (H) [14].
4.3. Sexual Stages
Sexual stages of C. cayetanensis are smaller than 10 µm and occur in the same location as schizonts [11]. The male microgamonts measure 6.6 × 5.2 µm and contain fewer than 20 microgametes organized around a residual body. Each microgamete measures up to 2 µm long and is flagellated. Female macrogametes contain distinctive eosinophilic wall-forming bodies that vary in size and are less than 1 µm in HE-stained sections. Macrogamonts measure 5.8–6.5 × 5.3–6.5 µm. Oocysts in sections are unsporulated and have a diameter of 5.7–7.5 µm. The TEM examination confirmed the histologic findings [11].
5. Need for Surrogates for Studying C. cayetanensis
Only rarely have scientists been positioned to directly study C. cayatanensis infections in vivo. Even access to oocysts is limited, undermining efforts to understand their maturation and senescence. What we know of human pathology and the parasite’s development in human hosts has been derived from just a few case studies. Ethical constraints essentially preclude the ability to examine infections under defined or replicated conditions, and the absence of any in vitro or animal model for studying this organism further impede progress in identifying vulnerabilities that might be exploited to strengthen prevention or cure.
Fortunately, a wealth of data from natural and experimental Eimeria infections provide helpful context for interpreting what scant evidence we do have (see above). Furthermore, Eimeria can and should be exploited as surrogates for evaluating methods requiring large numbers of C. cayetanensis oocysts. For example, maturation of oocysts of Eimeria acervulina (a common poultry parasite) entails upregulation of a suite of genes most of which have homologues in C. cayetanensis [19]. These abundant parasites, which pose no risk to the health of human investigators, can speed efforts to evaluate methods to filter parasites from irrigation water, and treat food in ways that may render such contaminants harmless, or treat infections when prevention fails.
6. Molecular Tools and Comparative Genomics
Below, we briefly review insights from comparative genetics and genomics of Cyclospora illustrating the potential, and limits, of related organisms as surrogates to inform us of opportunities to better understand, and manage, this elusive but important human enteric pathogen [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
6.1. Small-Subunit rDNA Sequence
Molecular tools have revolutionized the exploration of biodiversity. Sequencing nuclear-encoded small-subunit rDNA gene (18S) has especially aided reconstruction of relationships among organisms for which morphological taxonomy is challenging [20]. Comparisons of SSU-rRNA sequences between human and primate isolates of Cyclospora spp. identified 98% similarity in that gene [21], validating their distinctness but testifying to their close evolutionary relationship. SSU-rDNA was also utilized to understand the relatedness among closely related species. Strikingly, examination of SSU-rRNA sequences revealed a 94 to 98% sequence similarity between Cyclospora and Eimeria species. The neighbor-joining tree constructed using 18S rDNA sequences suggests C. cayetanensis is most closely related to Eimeria species that infect birds (Figure 4A). Thus, molecular analyses of nuclear SSU rDNA sequences suggest that C. cayetanensis is closely related to other coccidia, especially members of the genus Eimeria in the family Eimeriidae.
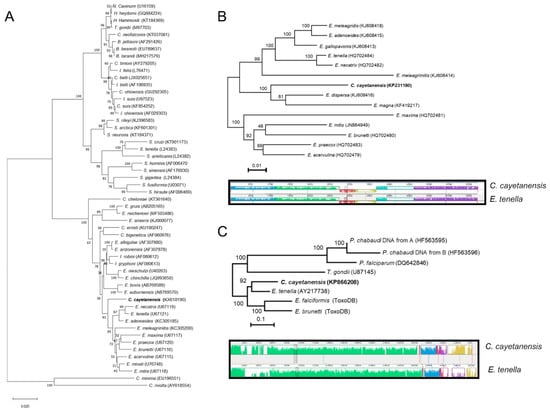
Figure 4.
Phylogenetic relationship between C. cayetanensis and Eimeria species. Maximum likelihood phylogenetic tresses were constructed using (A) 18S rDNA, (B) mitochondrial genome, and (C) apicoplast genome. Synteny in genome organizations between Cyclospora cayetanensis and E. tenella is shown in the mitochondrial (B) and apicoplast (C) genomes. (B,C) were modified with permission from Refs. [30,34].
This molecule serves as the target for FDA-validated assays for diagnosing contamination of certain types of fresh produce with C. cayetanensis from fresh produce and is also used to diagnose infections in clinical samples [22,23,24,25]. The sensitivity of assays targeting this gene benefits from the fact that each genome of the parasite encodes multiple copies of the SSU rRNA. Recently, a real-time qPCR assay targeting the C. cayetanensis 18S rDNA gene has been optimized to improve detection sensitivity, as validated in the FDA Bacteriological Analytical Manual (BAM) for C. cayetanensis testing [24].
6.2. Organellar Genomes
Organellar genomes, residing in the mitochondrion and apicoplast, illuminate relatedness among closely related apicomplexan species because they are inherited maternally without genetic recombination [26,27]. Recently, the mitochondrial and apicoplast genomes of C. cayetanensis have been sequenced and compared with closely related Eimeria species [28,29,30,31]. As with other coccidian parasites, the mitochondrial genome of C. cayetanensis is 6229 bp in size with 33% GC content [28,29,30,31]. The mitochondrial genome is organized in a linear molecule with approximately 513 copies; these are arranged in concatemeric structures with a head-tail configuration, as in other Eimeria mitochondrial genomes. As with other coccidian parasites, the mitochondrial genome of C. cayetanensis encodes three protein-coding genes (cox1, cox3, and cytB), in addition to 14 large subunit (LSU) and nine small subunit (SSU) fragmented rRNA genes [30]. Comparing the amino acids comprising the mitochondrially-encoded proteins of C. cayatanensis to those of closely-related species of Eimeria documented sequence similarities ranging from 90–97% for cytbB, 93–97% for cox1, and 83–93% for cox3 [30]. Additionally, comparative phylogenomic analysis of mitochondrial genomes also revealed the monophyletic clustering of C. cayetanensis with the rabbit-infecting parasite E. magna (Figure 4B) [30]. Comparing the mitochondrial genomes of isolates of C. cayetanensis identified 8 single nucleotide polymorphisms and one 7-bp multiple-nucleotide in the junction area between genome copies [28,32].
The apicoplast of C. cayetanensis, a non-photosynthetic plastid derived from a secondary symbiosis, is 34,155 bp in length, with a GC content of 22%, and harbors 29 protein-coding genes, 33 tRNA, and four rRNA genes [28,33]. Genes are encoded bidirectionally. A recent comparative study of sequence variation of apicoplast genomes of C. cayetanensis identified 25 single nucleotide differences amongst 11 isolates from several geographical locations, in addition to a unique 30 bp-long repeat insertion sequence in a Nepalese sample [28,33].
Comparing the apicoplast genome of C. cayetanensis to that of Eimeria tenella (Table 1) revealed complete conservation of gene order (synteny) and strong (85.6%) sequence conservation (Figure 4C) [28]. Thus, the genetic similarity between Eimeria species and C. cayetanensis in each organellar genomes substantiate avian Eimeria as a sensible surrogate to study drug treatment, and therapeutics specifically targeting the organellar genomes.

Table 1.
Comparison of genomic features of apicomplexan parasites.
6.3. Nuclear Genome
Currently, 39 nuclear genomes of C. cayetanensis have been assembled, using either short paired-end reads from Illumina sequencing (Illumina, San Diego, CA, USA) or Roche GS-FLX 454 sequencing (454 Life Sciences, Branford, CT, USA, available online at https://www.ncbi.nlm.nih.gov/assembly/?term=cyclospora, accessed on 15 November 2021) [34,35]. Of these 39 genomes, CcayRef3 (GCF_002999335) and CHN_HEN01 (GCF_000769155) presently serve as the reference genomes of C. cayetanensis. The total nuclear genome size is estimated to be ~44 Mb (Table 1) [34,35]. These genomes have roughly similar GC composition (52%) and are predicted to encode ~7500 genes; they have been resolved to 738 contigs (Table 1). Not surprisingly, comparative genome analysis revealed that the genome organization, metabolic capabilities, and potential invasion mechanisms are very similar to those of E. tenella [34]. When compared with more distantly related relatives that form tissue-cysts (such as T. gondii), these monoxenous parasites are notably reduced in rhoptry protein kinases, phosphatases, and serine protease inhibitors. This seems significant because of the dominance of such protein domains in tissue-cyst forming coccidian parasites; this underscores the special suitability of Eimeria as surrogates to study the enigmatic Cyclospora spp. Note, however, that even these close relatives differ, reminding us of the uniqueness of each biological species: a family of TA4-type surface antigens (SAG), highly abundant in E. tenella, have not been detected in C. cayetanensis [34]. In addition, plant-like AP2 transcription factors are slightly more abundant in C. cayetanensis than in E. tenella, consistent with these being the major transcription factors in apicomplexans [34].
6.4. Developmentally-Regulated Genes
Genome assembly and annotation for Cyclospora and for its Eimeria relatives remain far from complete, impeding efforts to understand their comparative biology. Nonetheless, a recent study of sporulation in E. acervulina suggests that similar genes in each species may be important regulators or effectors of parasite maturation [19]. Maturing cohorts of E. acervulina undergo concerted changes in the expression of many genes; most of these increase their expression over the course of sporulation. Genes strongly expressed throughout sporulation included ATPases (like EAH 4100 and EAH 4110) and oocyst wall proteins (like EAH 33530). Members assigned to the SAG family of surface antigens (EAH 59200, 59950, and 11680) were expressed to a much greater extent in mature than in immature oocysts. Although the function of many other genes remains unknown, guided at best by predictions from gene ontology, it is notable that about two-thirds of these genes have strongly conserved genes in C. cayetanensis. Until it becomes possible to propagate and study C. cayetanensis (in animal models, in vitro, or in organoid cultures), Eimeria surrogates offer a biologically sensible means to experiment with parasites, to define their biology, tolerances, and vulnerabilities.
7. Conclusions
To counter the harms posed by C. cayetanensis, producers, public health authorities, clinicians and researchers require more empirical studies of this enigmatic parasite. However, the episodic nature of its transmission and the absence of in vitro or in vivo means to propagate the parasite severely limit such opportunities. Fortunately, a wealth of information exists for the veterinary parasites which Cyclospora most closely resembles. These similarities span transmission, development, genetics, pathogenesis, and molecular biology. The experimental opportunities afforded by these readily studied surrogates should hasten progress in understanding, and better managing, threats to food safety and public health.
Author Contributions
J.P.D., A.K. and B.M.R. participated in writing this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by USDA Project “Detection and Control of Foodborne Parasites for Food Safety”: 8042-32000-113-00D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest.
References
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and cyclosporiasis: An update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Cyclosporiasis-updates on clinical presentation, pathology, clinical diagnosis, and treatment. Microorganisms 2021, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Gasser, R.B. Human cyclosporiasis. Lancet Infect. Dis. 2019, 19, e226–e236. [Google Scholar] [CrossRef]
- Li, J.; Cui, Z.; Qi, M.; Zhang, L. Advances in cyclosporiasis diagnosis and therapeutic intervention. Front. Cell Infect. Microbiol. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Hadjilouka, A.; Tsaltas, D. Cyclospora cayetanensis-major outbreaks from ready to eat fresh fruits and vegetables. Foods 2020, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.C.; Cinar, H.N.; Dubey, J.P. Coccidiosis in humans. In Coccidiosis in Livestock, Poultry, Companion Animals, and Humans; Dubey, J.P., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 268–312. [Google Scholar]
- Ortega, Y.R.; Gilman, R.H.; Sterling, C.R. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J. Parasitol. 1994, 80, 625–629. [Google Scholar] [CrossRef]
- Solarczyk, P. Host range of Cyclospora species: Zoonotic implication. Acta Protozool. 2021, 60, 13–20. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Ortega, Y.R.; Hanes, D.E.; Nace, E.K.; Do, R.Q.; Robl, M.G.; Won, K.Y.; Gavidia, C.; Sass, N.L.; Mansfield, K.; et al. Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. J. Parasitol. 2000, 86, 577–582. [Google Scholar] [CrossRef]
- Ortega, Y.R.; Nagle, R.; Gilman, R.H.; Watanabe, J.; Miyagui, J.; Quispe, H.; Kanagusuku, P.; Roxas, C.; Sterling, C.R. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J. Infect. Dis. 1997, 176, 1584–1589. [Google Scholar] [CrossRef]
- Dubey, J.P.; Almeria, S.; Mowery, J.; Fortes, J. Endogenous developmental cycle of the human coccidian Cyclospora cayetanensis. J. Parasitol. 2020, 106, 295–307. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Jenkins, M.C.; Bauer, C. Biology of intestinal coccidia. In Coccidiosis in Livestock, Poultry, Companion Animals, and Humans; Dubey, J.P., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 1–36. [Google Scholar]
- Thompson, P.; Rosenthal, B.M. Phylogeny of coccidian parasites. In Coccidiosis in Livestock, Poultry, Companion Animals, and Humans; Dubey, J.P., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 37–41. [Google Scholar]
- Dubey, J.P.; Almeria, S. Cystoisospora belli infections in humans: The past 100 years. Parasitology 2019, 146, 1490–1527. [Google Scholar] [CrossRef]
- Dubey, J.P.; Evason, K.J.; Walther, Z. Endogenous development of Cystoisospora belli in intestinal and biliary epithelium of humans. Parasitology 2019, 146, 865–872. [Google Scholar] [CrossRef]
- Resende, D.V.; Pedrosa, A.L.; Correia, D.; Cabrine-Santos, M.; Lages-Silva, E.; Meira, W.S.; Oliveira-Silva, M.B. Polymorphisms in the 18S rDNA gene of Cystoisospora belli and clinical features of cystoisosporosis in HIV-infected patients. Parasitol. Res. 2011, 108, 679–685. [Google Scholar] [CrossRef]
- Taniuchi, M.; Verweij, J.J.; Sethabutr, O.; Bodhidatta, L.; Garcia, L.; Maro, A.; Kumburu, H.; Gratz, J.; Kibiki, G.; Houpt, E.R. Multiplex polymerase chain reaction method to detect Cyclospora, Cystoisospora, and Microsporidia in stool samples. Diagn. Microbiol. Infect. Dis 2011, 71, 386–390. [Google Scholar] [CrossRef]
- Woon, S.A.; Yang, R.; Ryan, U.; Boan, P.; Prentice, D. Chronic Cystoisospora belli infection in an immunocompetent Myanmar refugee—Microscopy is not sensitive enough. BMC Infect. Dis. 2016, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.S.; O’Brien, C.N.; Jenkins, M.C.; Rosenthal, B.M. Dynamically expressed genes provide candidate viability biomarkers in a model coccidian. PLoS ONE 2021, 16, e0258157. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Leasi, F.; Obertegger, U.; Kieneke, A.; Barraclough, T.G.; Fontaneto, D. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proc. Natl. Acad. Sci. USA 2012, 109, 16208–16212. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.L.; da Silva, A.J.; Lilley, B.G.; Pieniazek, N.J. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp. n., and C. papionis sp. n. Emerg. Infect. Dis. 1999, 5, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Ortega, Y.; Simpson, S.; Kerdahi, K. Genetic characterization of human-pathogenic Cyclospora cayetanensis parasites from three endemic regions at the 18S ribosomal RNA locus. Infect. Genet. Evol. 2014, 22, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.; Yildirim, I.H.; Tas Cengiz, Z.; Karaman, U. Single-strand conformation polymorphism-based genetic characterization of the Cyclospora cayetanensis strains collected from different provinces in Turkey. Ann. Agric. Environ. Med. 2021, 28, 267–270. [Google Scholar] [CrossRef]
- Murphy, H.R.; Cinar, H.N.; Gopinath, G.; Noe, K.E.; Chatman, L.D.; Miranda, N.E.; Wetherington, J.H.; Neal-McKinney, J.; Pires, G.S.; Sachs, E.; et al. Interlaboratory validation of an improved method for detection of Cyclospora cayetanensis in produce using a real-time PCR assay. Food Microbiol. 2018, 69, 170–178. [Google Scholar] [CrossRef]
- Li, N.; Ye, J.; Arrowood, M.J.; Ma, J.; Wang, L.; Xu, H.; Feng, Y.; Xiao, L. Identification and morphologic and molecular characterization of Cyclospora macacae n. sp. from rhesus monkeys in China. Parasitol. Res. 2015, 114, 1811–1816. [Google Scholar] [CrossRef][Green Version]
- Lim, L.; McFadden, G.I. The evolution, metabolism and functions of the apicoplast. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 749–763. [Google Scholar] [CrossRef]
- Greiner, S.; Sobanski, J.; Bock, R. Why are most organelle genomes transmitted maternally? Bioessays 2015, 37, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Guo, Y.; Zhang, L.; Rowe, L.A.; Roellig, D.M.; Frace, M.A.; Li, N.; Liu, S.; Feng, Y.; Xiao, L. Genetic similarities between Cyclospora cayetanensis and cecum-infecting avian Eimeria spp. in apicoplast and mitochondrial genomes. Parasit. Vectors 2015, 8, 358. [Google Scholar] [CrossRef]
- Ogedengbe, M.E.; Qvarnstrom, Y.; da Silva, A.J.; Arrowood, M.J.; Barta, J.R. A linear mitochondrial genome of Cyclospora cayetanensis (Eimeriidae, Eucoccidiorida, Coccidiasina, Apicomplexa) suggests the ancestral start position within mitochondrial genomes of eimeriid coccidia. Int. J. Parasitol. 2015, 45, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Cinar, H.N.; Gopinath, G.; Jarvis, K.; Murphy, H.R. The complete mitochondrial genome of the foodborne parasitic pathogen Cyclospora cayetanensis. PLoS ONE 2015, 10, e0128645. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.S.; Barta, J.R.; Whale, J.; Hofstetter, J.N.; Casillas, S.; Barratt, J.; Talundzic, E.; Arrowood, M.J.; Qvarnstrom, Y. Mitochondrial junction region as genotyping marker for Cyclospora cayetanensis. Emerg. Infect. Dis. 2019, 25, 1314–1319. [Google Scholar] [CrossRef]
- Gopinath, G.R.; Cinar, H.N.; Murphy, H.R.; Durigan, M.; Almeria, M.; Tall, B.D.; DaSilva, A.J. A hybrid reference-guided de novo assembly approach for generating Cyclospora mitochondrion genomes. Gut. Pathog. 2018, 10, 15. [Google Scholar] [CrossRef]
- Cinar, H.N.; Qvarnstrom, Y.; Wei-Pridgeon, Y.; Li, W.; Nascimento, F.S.; Arrowood, M.J.; Murphy, H.R.; Jang, A.; Kim, E.; Kim, R.; et al. Comparative sequence analysis of Cyclospora cayetanensis apicoplast genomes originating from diverse geographical regions. Parasit. Vectors 2016, 9, 611. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Frace, M.A.; Tang, K.; Arrowood, M.J.; Moss, D.M.; et al. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef] [PubMed]
- Qvarnstrom, Y.; Wei-Pridgeon, Y.; Li, W.; Nascimento, F.S.; Bishop, H.S.; Herwaldt, B.L.; Moss, D.M.; Nayak, V.; Srinivasamoorthy, G.; Sheth, M.; et al. Draft genome sequences from Cyclospora cayetanensis oocysts purified from a human stool sample. Genome Announc. 2015, 3, e01324-15. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.P.; Li, Y.; Sateriale, A.; Brooks, K.L.; Tracey, A.; Sanders, M.J.; Ansell, B.R.E.; Jex, A.R.; Cooper, G.W.; Smith, E.D.; et al. Long-read assembly and comparative evidence-based reanalysis of Cryptosporidium genome sequences reveals expanded transporter repertoire and duplication of entire chromosome ends including subtelomeric regions. Genome Res. 2021, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Brayton, K.A.; Lau, A.O.; Herndon, D.R.; Hannick, L.; Kappmeyer, L.S.; Berens, S.J.; Bidwell, S.L.; Brown, W.C.; Crabtree, J.; Fadrosh, D.; et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007, 3, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Khan, A.; Behnke, M.S.; Namasivayam, S.; Swapna, L.S.; Hadjithomas, M.; Karamycheva, S.; Pinney, D.; Brunk, B.P.; Ajioka, J.W.; et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun. 2016, 7, 10147. [Google Scholar] [CrossRef]
- Blazejewski, T.; Nursimulu, N.; Pszenny, V.; Dangoudoubiyam, S.; Namasivayam, S.; Chiasson, M.A.; Chessman, K.; Tonkin, M.; Swapna, L.S.; Hung, S.S.; et al. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. mBio 2015, 6, e02445-14. [Google Scholar] [CrossRef]
- Khan, A.; Fujita, A.W.; Randle, N.; Regidor-Cerrillo, J.; Shaik, J.S.; Shen, K.; Oler, A.J.; Quinones, M.; Latham, S.M.; Akanmori, B.D.; et al. Global selective sweep of a highly inbred genome of the cattle parasite Neospora caninum. Proc. Natl. Acad. Sci. USA 2019, 116, 22764–22773. [Google Scholar] [CrossRef]
- Berna, L.; Marquez, P.; Cabrera, A.; Greif, G.; Francia, M.E.; Robello, C. Reevaluation of the Toxoplasma gondii and Neospora caninum genomes reveals misassembly, karyotype differences, and chromosomal rearrangements. Genome Res. 2021, 31, 823–833. [Google Scholar] [CrossRef]
- Xia, J.; Venkat, A.; Bainbridge, R.E.; Reese, M.L.; Le Roch, K.G.; Ay, F.; Boyle, J.P. Third-generation sequencing revises the molecular karyotype for Toxoplasma gondii and identifies emerging copy number variants in sexual recombinants. Genome Res. 2021, 31, 834–851. [Google Scholar] [CrossRef]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D.; et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).