Biohybrid Actuators for Soft Robotics: Challenges in Scaling Up
Abstract
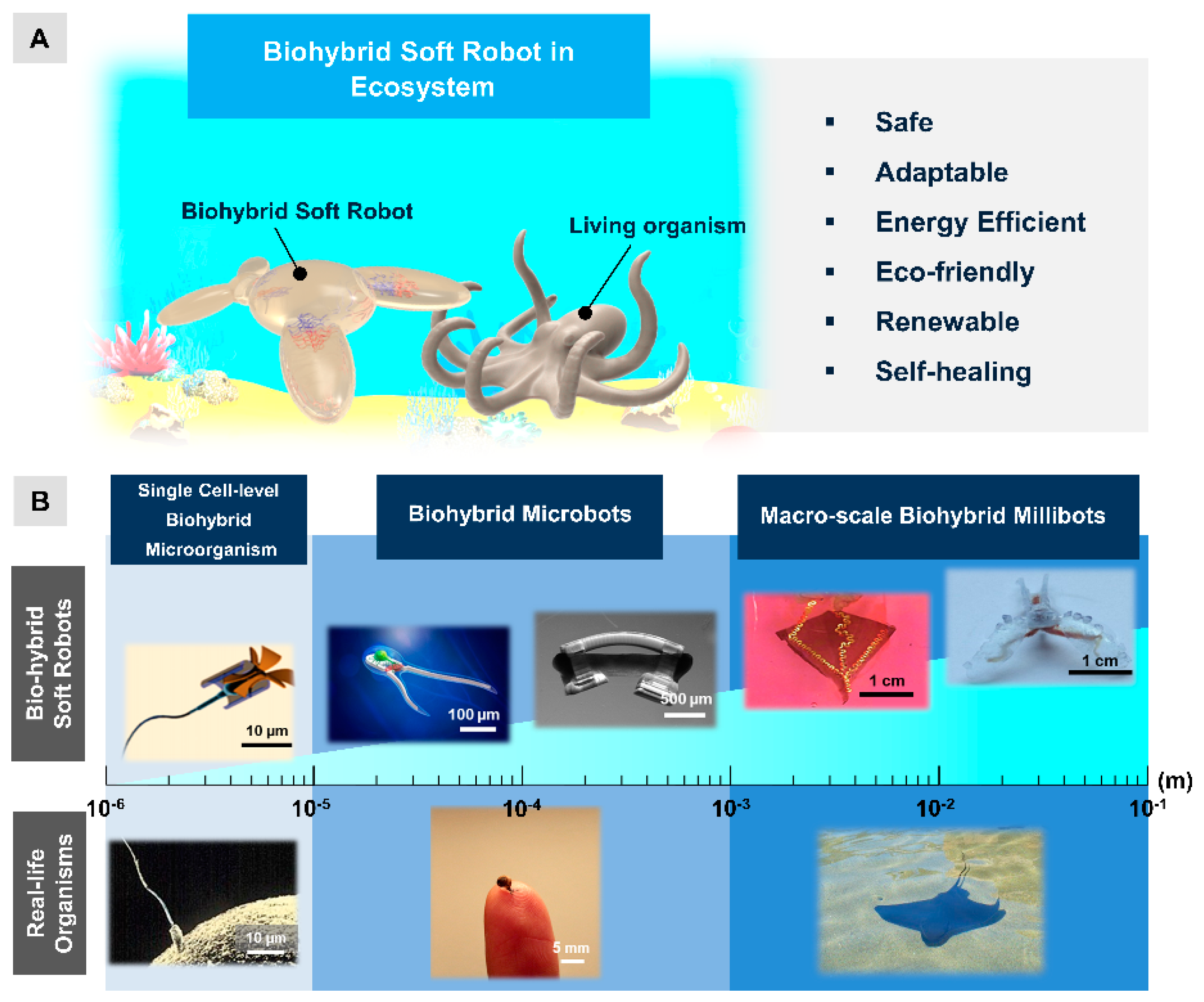
:1. Introduction
2. Challenges in Scaling Up Biohybrid Actuators
2.1. Vascularization
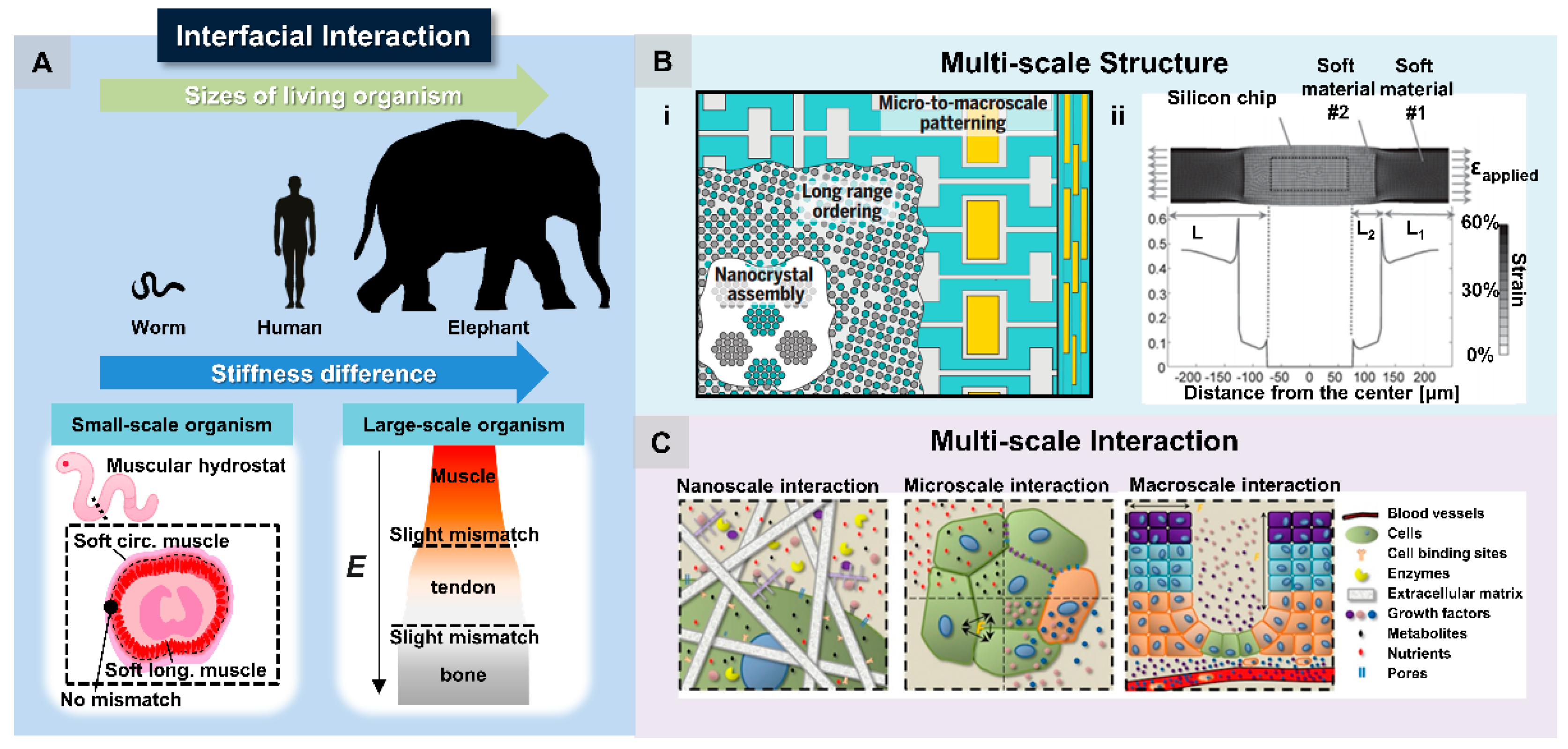
2.2. Interfacial Interaction
2.3. Innervation: Control Methods
2.3.1. Electrical Stimulation
2.3.2. Optical Stimulation
2.3.3. Neuromuscular Co-Culture
3. Perspectives and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ricotti, L.; Trimmer, B.; Feinberg, A.W.; Raman, R.; Parker, K.K.; Bashir, R.; Sitti, M.; Martel, S.; Dario, P.; Menciassi, A. Biohybrid actuators for robotics: A review of devices actuated by living cells. Sci. Robot. 2017, 2, eaaq0495. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yu, Y.; Chen, Z.; Bian, F.; Ye, F.; Sun, L.; Zhao, Y. Biohybrid robotics with living cell actuation. Chem. Soc. Rev. 2020, 49, 4043–4069. [Google Scholar] [CrossRef]
- Rich, S.I.; Wood, R.J.; Majidi, C. Untethered soft robotics. Nat. Electron. 2018, 1, 102–112. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Kim, S.; Laschi, C.; Trimmer, B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol. 2013, 31, 287–294. [Google Scholar] [CrossRef]
- Shepherd, R.F.; Ilievski, F.; Choi, W.; Morin, S.A.; Stokes, A.A.; Mazzeo, A.D.; Chen, X.; Wang, M.; Whitesides, G.M. Multigait soft robot. Proc. Natl. Acad. Sci. USA 2011, 108, 20400–20403. [Google Scholar] [CrossRef] [Green Version]
- Kheirikhah, M.M.; Rabiee, S.; Edalat, M.E. A review of shape memory alloy actuators in robotics. In Proceedings of Robot Soccer World Cup; Springer: Berlin/Heidelberg, Germany, 2010; pp. 206–217. [Google Scholar]
- Lendlein, A.; Gould, O.E. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Youn, J.-H.; Jeong, S.M.; Hwang, G.; Kim, H.; Hyeon, K.; Park, J.; Kyung, K.-U. Dielectric elastomer actuator for soft robotics applications and challenges. Appl. Sci. 2020, 10, 640. [Google Scholar] [CrossRef] [Green Version]
- Polygerinos, P.; Correll, N.; Morin, S.A.; Mosadegh, B.; Onal, C.D.; Petersen, K.; Cianchetti, M.; Tolley, M.T.; Shepherd, R.F. Soft robotics: Review of fluid-driven intrinsically soft devices; manufacturing, sensing, control, and applications in human-robot interaction. Adv. Eng. Mater. 2017, 19, 1700016. [Google Scholar] [CrossRef]
- Hughes, J.; Culha, U.; Giardina, F.; Guenther, F.; Rosendo, A.; Iida, F. Soft manipulators and grippers: A review. Front. Robot. AI 2016, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Madden, J.D.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial muscle technology: Physical principles and naval prospects. IEEE J. Ocean. Eng. 2004, 29, 706–728. [Google Scholar] [CrossRef]
- Shin, S.R.; Migliori, B.; Miccoli, B.; Li, Y.C.; Mostafalu, P.; Seo, J.; Mandla, S.; Enrico, A.; Antona, S.; Sabarish, R. Electrically driven microengineered bioinspired soft robots. Adv. Mater. 2018, 30, 1704189. [Google Scholar]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-hybrid micromotor for targeted drug delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-J.; Gazzola, M.; Park, K.S.; Park, S.; Di Santo, V.; Blevins, E.L.; Lind, J.U.; Campbell, P.H.; Dauth, S.; Capulli, A.K. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, O.; Zhang, X.; Nuethong, S.; Pagan-Diaz, G.J.; Bashir, R.; Gazzola, M.; Saif, M.T.A. Neuromuscular actuation of biohybrid motile bots. Proc. Natl. Acad. Sci. USA 2019, 116, 19841–19847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, R.; Cvetkovic, C.; Uzel, S.G.; Platt, R.J.; Sengupta, P.; Kamm, R.D.; Bashir, R. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 2016, 113, 3497–3502. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, A.W.; Feigel, A.; Shevkoplyas, S.S.; Sheehy, S.; Whitesides, G.M.; Parker, K.K. Muscular thin films for building actuators and powering devices. Science 2007, 317, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Appiah, C.; Arndt, C.; Siemsen, K.; Heitmann, A.; Staubitz, A.; Selhuber-Unkel, C. Living Materials Herald a New Era in Soft Robotics. Adv. Mater. 2019, 31, 1807747. [Google Scholar] [CrossRef]
- Webster, V.A.; Chapin, K.J.; Hawley, E.L.; Patel, J.M.; Akkus, O.; Chiel, H.J.; Quinn, R.D. Aplysia californica as a novel source of material for biohybrid robots and organic machines. In Proceedings of the Conference on Biomimetic and Biohybrid Systems, Living Machines 2016, Edinburgh, Scotland, 18–22 July 2016; pp. 365–374. [Google Scholar]
- Grebenyuk, S.; Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Leijten, J.; Khademhosseini, A. From nano to macro: Multiscale materials for improved stem cell culturing and analysis. Cell Stem Cell 2016, 18, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Naghieh, S.; Sarker, M.; Karamooz-Ravari, M.R.; McInnes, A.D.; Chen, X. Modeling of the mechanical behavior of 3D bioplotted scaffolds considering the penetration in interlocked strands. Appl. Sci. 2018, 8, 1422. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Kalmykov, A.; Huang, C.; Bliley, J.; Shiwarski, D.; Tashman, J.; Abdullah, A.; Rastogi, S.K.; Shukla, S.; Mataev, E.; Feinberg, A.W. Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci. Adv. 2019, 5, eaax0729. [Google Scholar] [CrossRef] [Green Version]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Okano, K.; Hsu, H.-Y.; Li, Y.-K.; Masuhara, H. In situ patterning and controlling living cells by utilizing femtosecond laser. J. Photochem. Photobiol. C Photochem. Rev. 2016, 28, 1–28. [Google Scholar] [CrossRef]
- Cerchiari, A.; Garbe, J.C.; Todhunter, M.E.; Jee, N.Y.; Pinney, J.R.; LaBarge, M.A.; Desai, T.A.; Gartner, Z.J. Formation of spatially and geometrically controlled three-dimensional tissues in soft gels by sacrificial micromolding. Tissue Eng. Part C Methods 2015, 21, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Onoe, H.; Takeuchi, S. Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci. Robot. 2018, 3, eaat4440. [Google Scholar] [CrossRef] [Green Version]
- Pollet, A.M.; den Toonder, J.M. Recapitulating the Vasculature using organ-on-chip technology. Bioengineering 2020, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Kier, W.M.; Smith, K.K. Tongues, tentacles and trunks: The biomechanics of movement in muscular-hydrostats. Zool. J. Linn. Soc. 1985, 83, 307–324. [Google Scholar] [CrossRef]
- Begley, M.R.; Gianola, D.S.; Ray, T.R. Bridging functional nanocomposites to robust macroscale devices. Science 2019, 364, eaav4299. [Google Scholar] [CrossRef] [PubMed]
- Naserifar, N.; LeDuc, P.R.; Fedder, G.K. Material gradients in stretchable substrates toward integrated electronic functionality. Adv. Mater. 2016, 28, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.; Anuradha, E.; Solomon, F.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Bono, N.; Pezzoli, D.; Levesque, L.; Loy, C.; Candiani, G.; Fiore, G.B.; Mantovani, D. Unraveling the role of mechanical stimulation on smooth muscle cells: A comparative study between 2D and 3D models. Biotechnol. Bioeng. 2016, 113, 2254–2263. [Google Scholar] [CrossRef]
- Urciuolo, A.; Serena, E.; Ghua, R.; Zatti, S.; Giomo, M.; Mattei, N.; Vetralla, M.; Selmin, G.; Luni, C.; Vitulo, N. Engineering a 3D in vitro model of human skeletal muscle at the single fiber scale. PLoS ONE 2020, 15, e0232081. [Google Scholar] [CrossRef]
- Dixon, T.A.; Cohen, E.; Cairns, D.M.; Rodriguez, M.; Mathews, J.; Jose, R.R.; Kaplan, D.L. Bioinspired three-dimensional human neuromuscular junction development in suspended hydrogel arrays. Tissue Eng. Part C Methods 2018, 24, 346–359. [Google Scholar] [CrossRef]
- Krieger, J.; Park, B.-W.; Lambert, C.R.; Malcuit, C. 3D skeletal muscle fascicle engineering is improved with TGF-β1 treatment of myogenic cells and their co-culture with myofibroblasts. PeerJ 2018, 6, e4939. [Google Scholar] [CrossRef]
- Webster, V.A.; Young, F.R.; Patel, J.M.; Scariano, G.N.; Akkus, O.; Gurkan, U.A.; Chiel, H.J.; Quinn, R.D. 3D-printed biohybrid robots powered by neuromuscular tissue circuits from Aplysia californica. In Proceedings of the Conference on Biomimetic and Biohybrid Systems, Living Machines 2017, San Francisco, CA, USA, 25–28 July 2017; pp. 475–486. [Google Scholar]
- Wang, T.; Migliori, B.; Miccoli, B.; Shin, S.R. Bioinspired Soft Robot with Incorporated Microelectrodes. JoVE (J. Vis. Exp.) 2020, 156, e60717. [Google Scholar] [CrossRef]
- Pagan-Diaz, G.J.; Zhang, X.; Grant, L.; Kim, Y.; Aydin, O.; Cvetkovic, C.; Ko, E.; Solomon, E.; Hollis, J.; Kong, H. Simulation and fabrication of stronger, larger, and faster walking biohybrid machines. Adv. Funct. Mater. 2018, 28, 1801145. [Google Scholar] [CrossRef]
- Webster-Wood, V.A.; Akkus, O.; Gurkan, U.A.; Chiel, H.J.; Quinn, R.D. Organismal engineering: Toward a robotic taxonomic key for devices using organic materials. Sci. Robot. 2017, 2, eaap9281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Pagan-Diaz, G.; Gapinske, L.; Kim, Y.; Suh, J.; Solomon, E.; Harris, J.F.; Nam, S.; Bashir, R. Integration of Graphene Electrodes with 3D Skeletal Muscle Tissue Models. Adv. Healthc. Mater. 2020, 9, 1901137. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.; Levin, O.; Aviram, A.; Isakov, E.; Susak, Z. Muscle fatigue in interrupted stimulation: Effect of partial recovery on force and EMG dynamics. J. Electromyogr. Kinesiol. 1997, 7, 51–65. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Hasnan, N.; Abdul Wahab, A.K.; Davis, G.M. Strategies for rapid muscle fatigue reduction during FES exercise in individuals with spinal cord injury: A systematic review. PLoS ONE 2016, 11, e0149024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvetkovic, C.; Rich, M.H.; Raman, R.; Kong, H.; Bashir, R. A 3D-printed platform for modular neuromuscular motor units. Microsyst. Nanoeng. 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, A.; Sethumadhavan, A.; Krishnan, U.M. Toward building the neuromuscular junction: In vitro models to study synaptogenesis and neurodegeneration. ACS Omega 2019, 4, 12969–12977. [Google Scholar] [CrossRef] [Green Version]
- Bakooshli, M.A.; Lippmann, E.S.; Mulcahy, B.; Iyer, N.; Nguyen, C.T.; Tung, K.; Stewart, B.A.; van den Dorpel, H.; Fuehrmann, T.; Shoichet, M. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife 2019, 8, e44530. [Google Scholar] [CrossRef]
- Pimashkin, A.; Gladkov, A.; Mukhina, I.; Kazantsev, V. Adaptive enhancement of learning protocol in hippocampal cultured networks grown on multielectrode arrays. Front. Neural Circuits 2013, 7, 87. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, P.; Ko, S.H.; Majidi, C.; W. Feinberg, A.; A. Webster-Wood, V. Biohybrid Actuators for Soft Robotics: Challenges in Scaling Up. Actuators 2020, 9, 96. https://doi.org/10.3390/act9040096
Won P, Ko SH, Majidi C, W. Feinberg A, A. Webster-Wood V. Biohybrid Actuators for Soft Robotics: Challenges in Scaling Up. Actuators. 2020; 9(4):96. https://doi.org/10.3390/act9040096
Chicago/Turabian StyleWon, Phillip, Seung Hwan Ko, Carmel Majidi, Adam W. Feinberg, and Victoria A. Webster-Wood. 2020. "Biohybrid Actuators for Soft Robotics: Challenges in Scaling Up" Actuators 9, no. 4: 96. https://doi.org/10.3390/act9040096
APA StyleWon, P., Ko, S. H., Majidi, C., W. Feinberg, A., & A. Webster-Wood, V. (2020). Biohybrid Actuators for Soft Robotics: Challenges in Scaling Up. Actuators, 9(4), 96. https://doi.org/10.3390/act9040096





