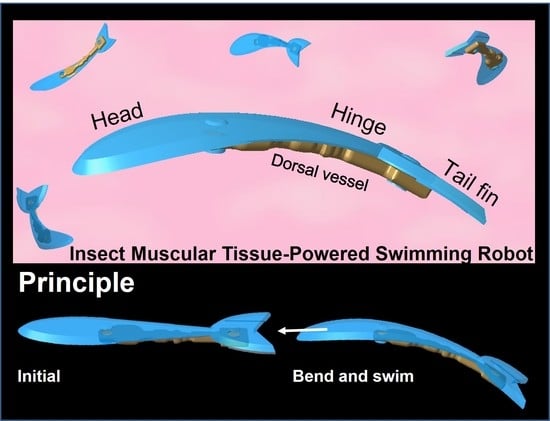
Insect Muscular Tissue-Powered Swimming Robot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect and its DV Preparation
2.2. Measurement of DV Contractile Force
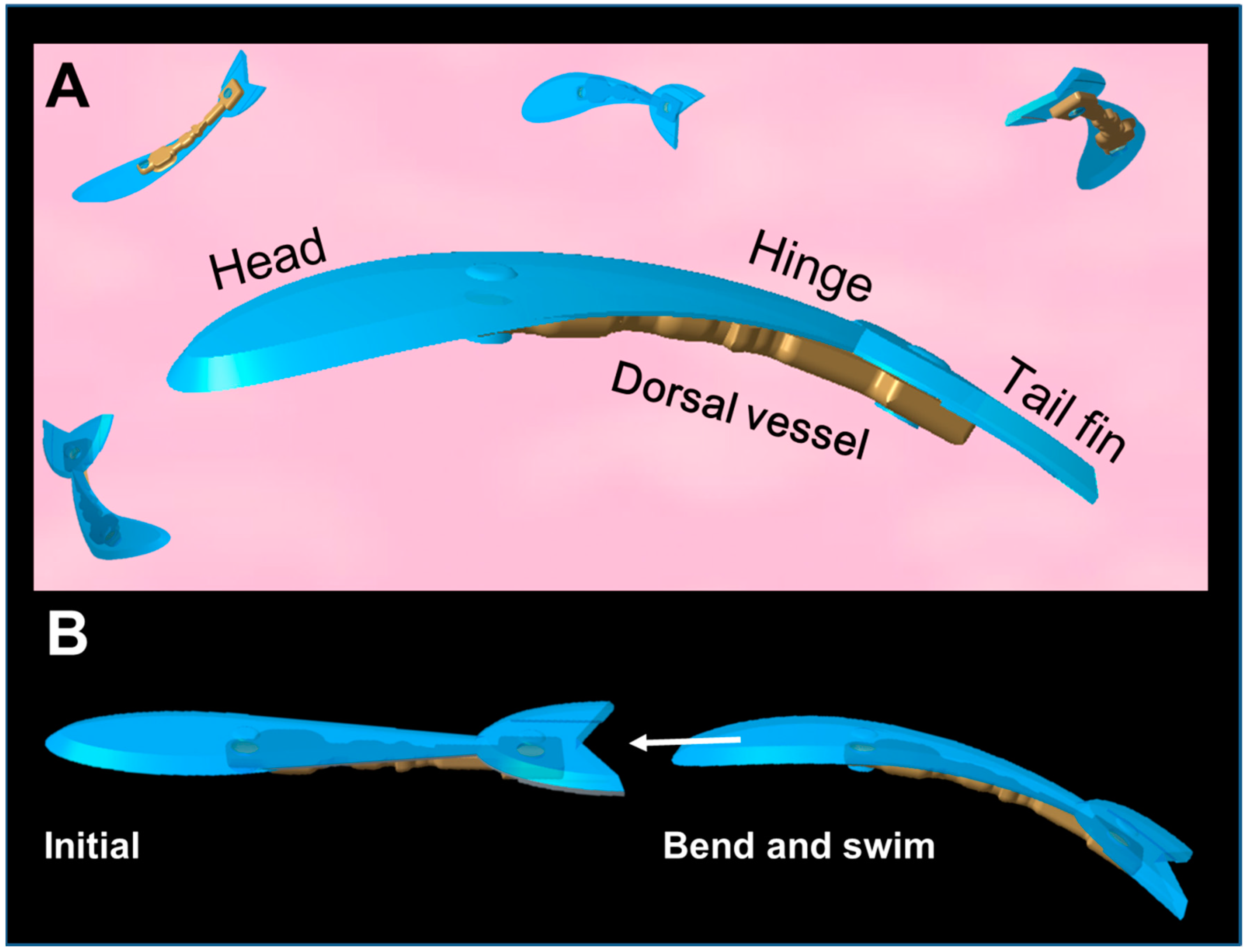
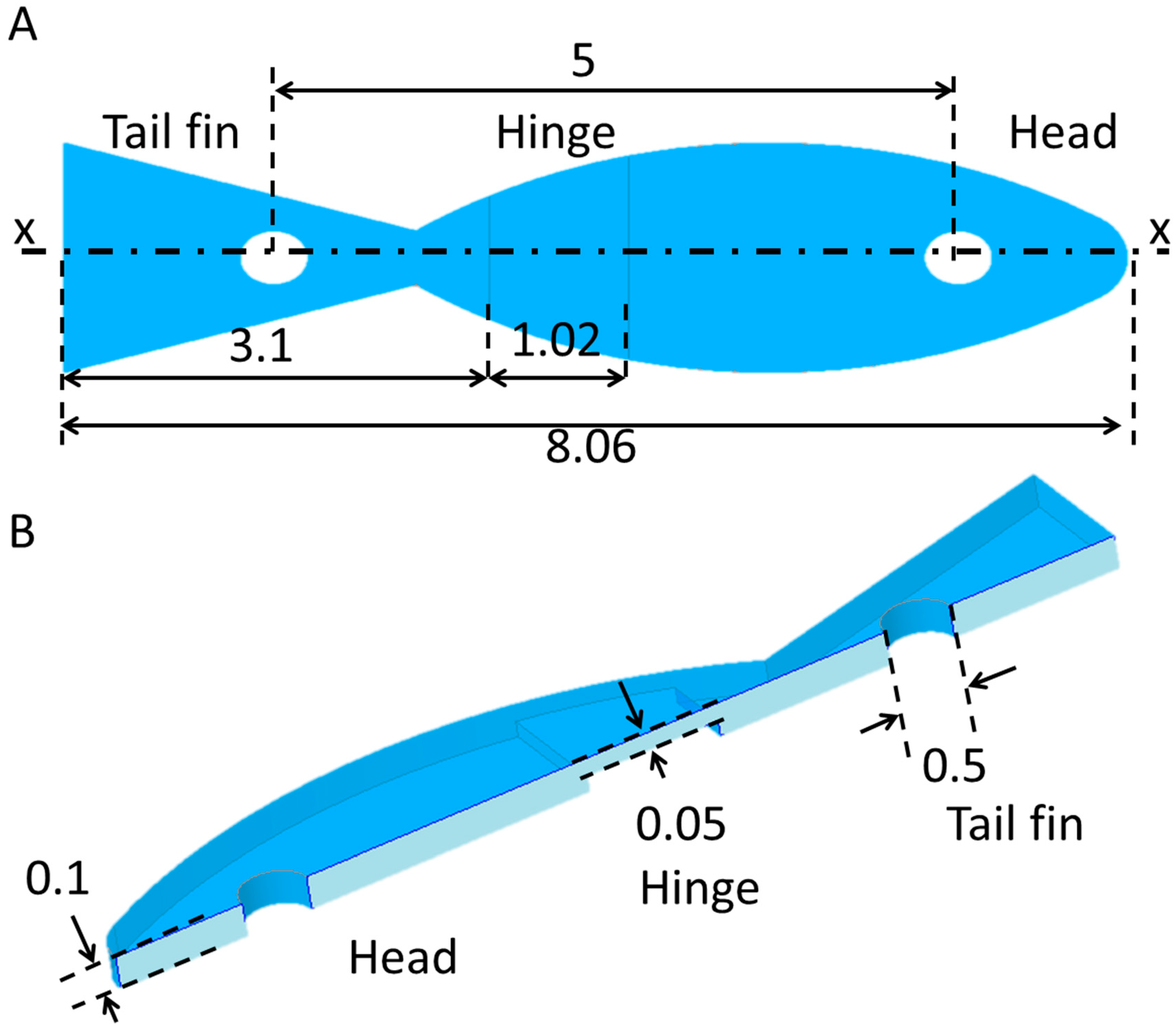
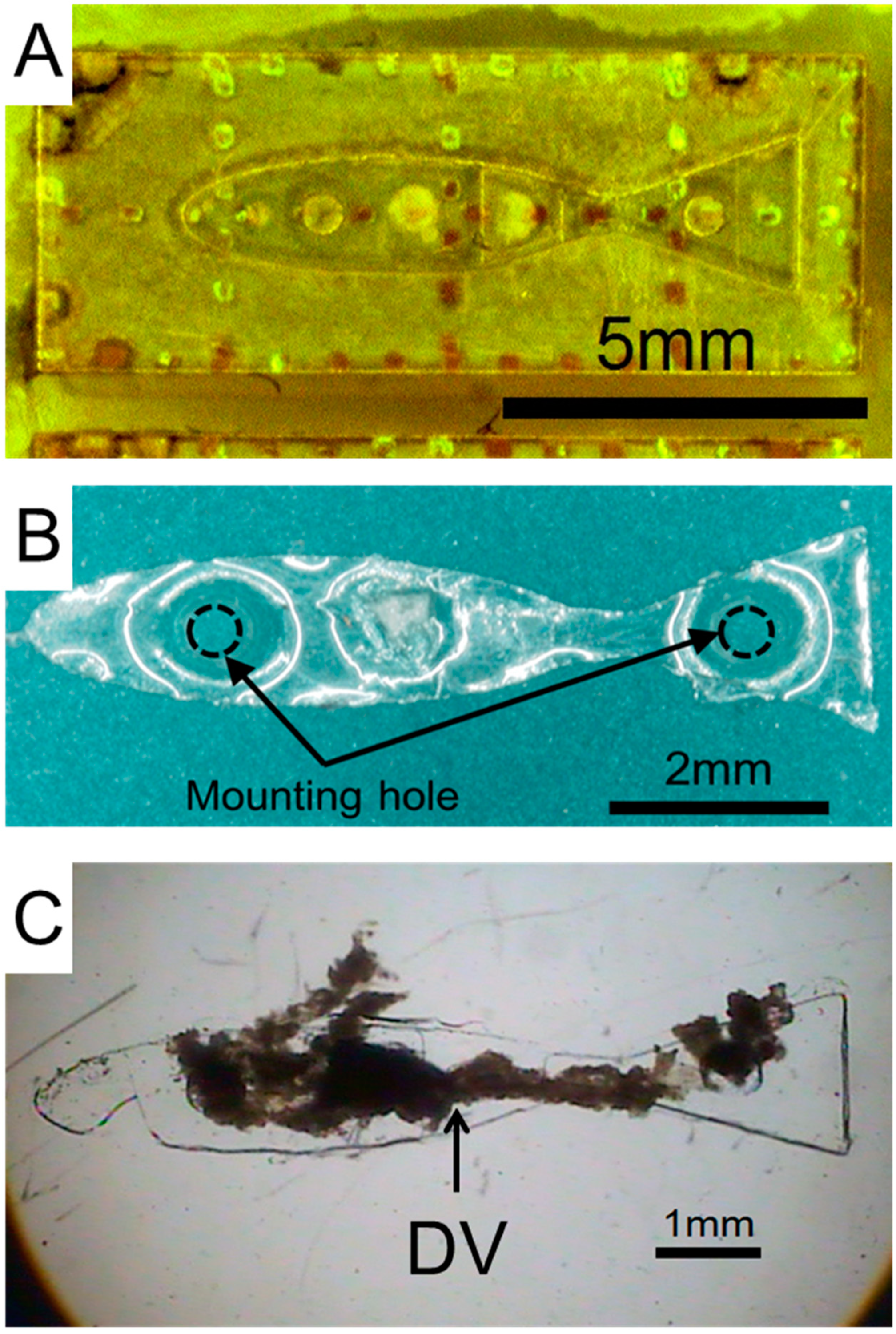
2.3. Design and Fabrication of the Prototype Swimming Robot
2.4. Fabrication of Prototype Robot Body
2.5. Assembly of DV Tissue onto the Prototype Robot
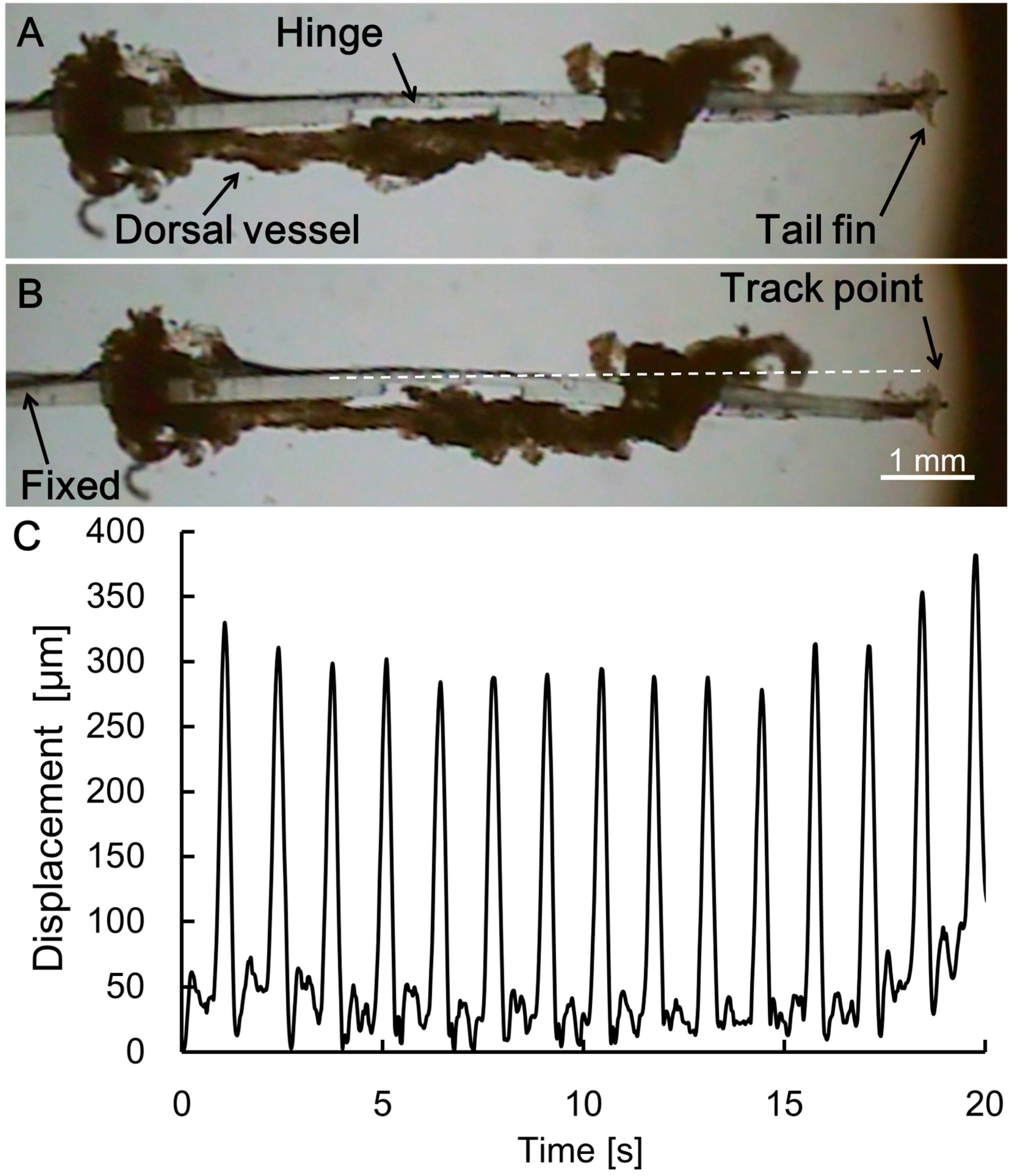
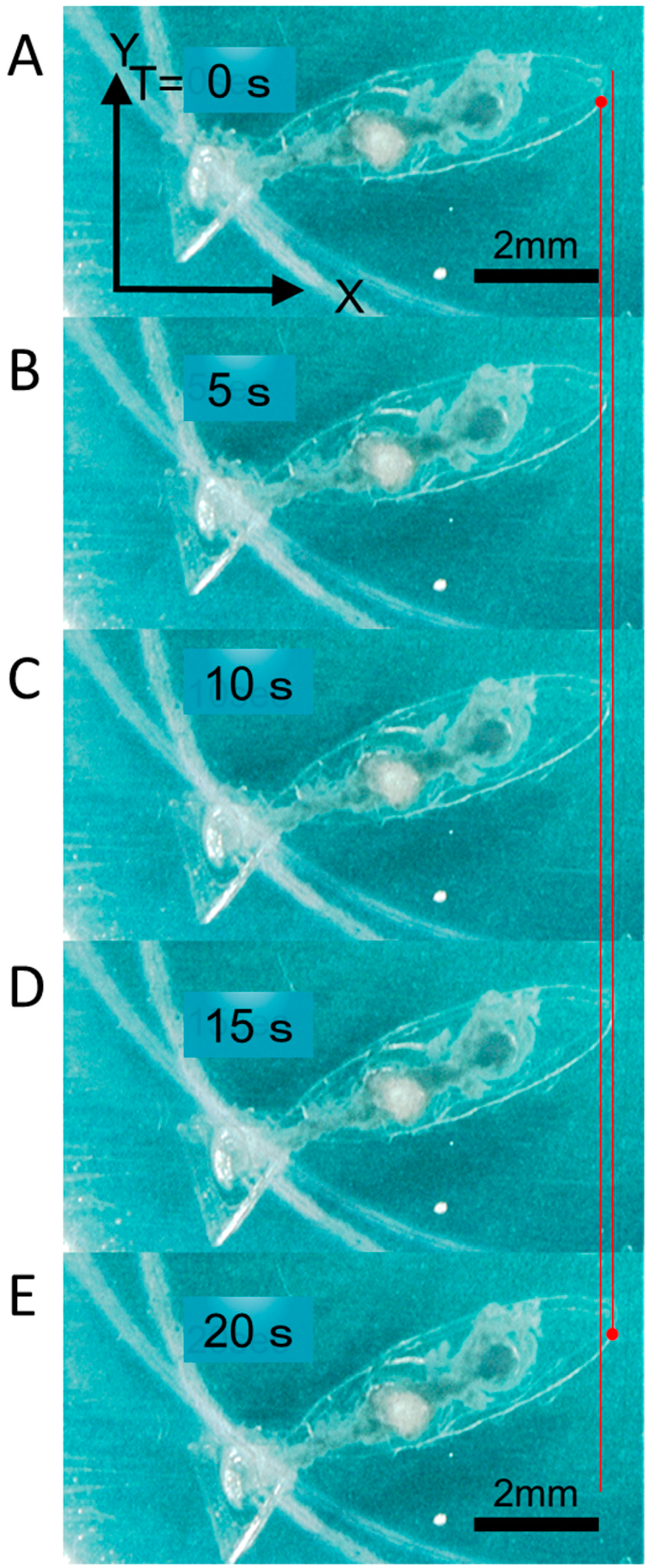
2.6. Image Analysis for Evaluation of the Swimming Robot
3. Results and Discussion
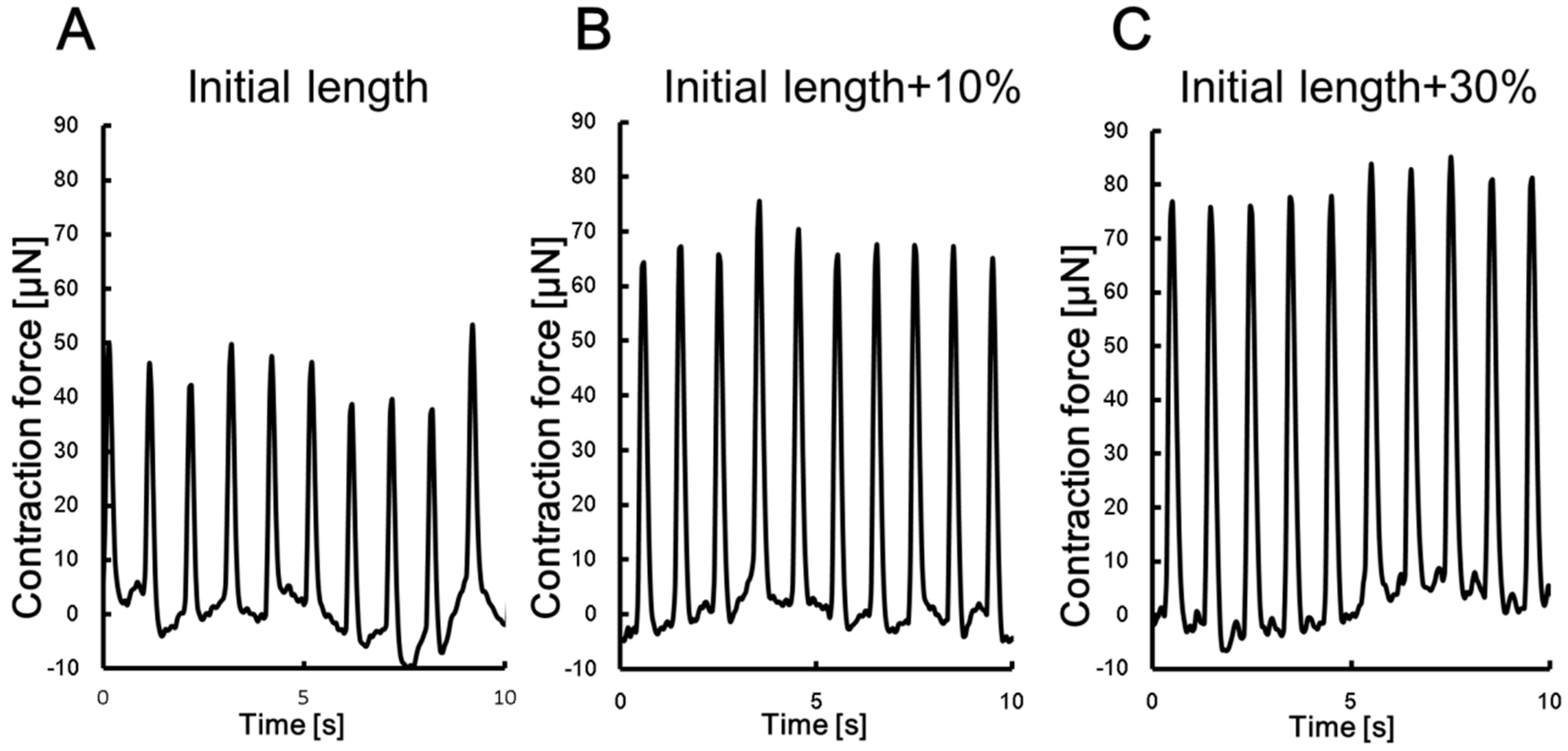
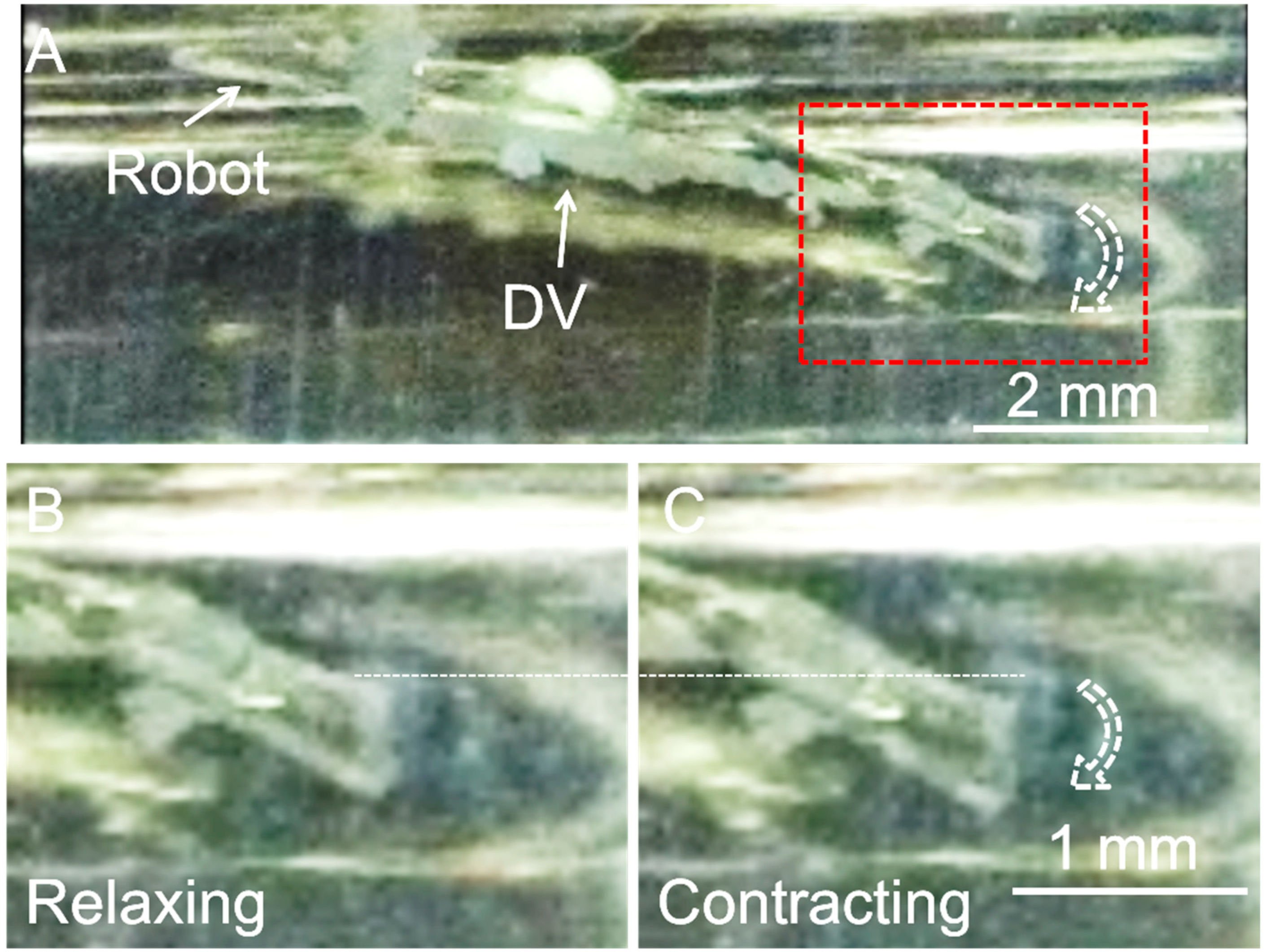
3.1. Measurement of DV Contractile Force
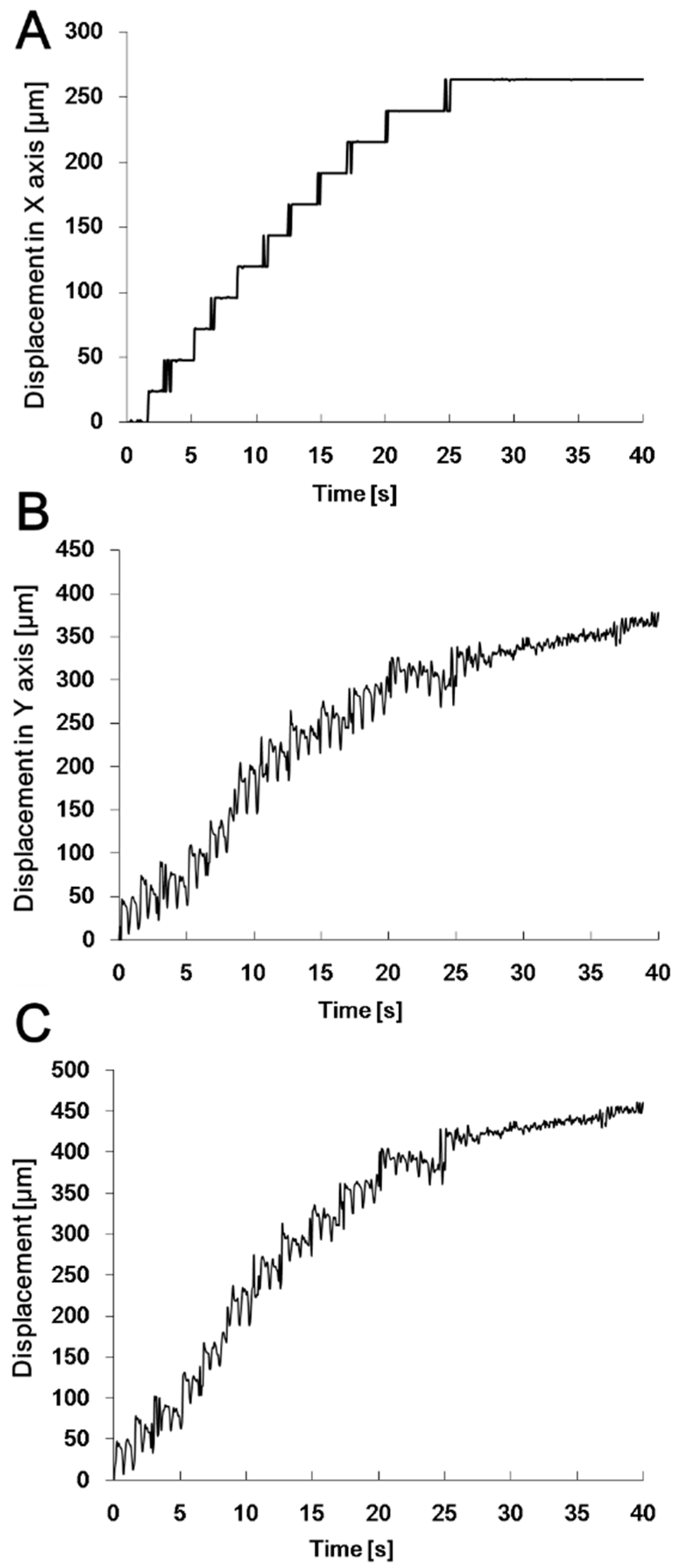
3.2. Motion Analysis of the Swimming Robot Tail Fin
3.3. Motion Analysis of the Swimming Robot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Umedachi, T.; Vikas, V.; Trimmer, B.A. Softworms: The design and control of non-pneumatic, 3D-printed, deformable robots. Bioinspir. Biomim. 2016, 11, 025001. [Google Scholar] [CrossRef]
- Slesarenko, V.; Engelkemier, S. Strategies to Control Performance of 3D-Printed, Cable-Driven Soft Polymer Actuators: From Simple Architectures to Gripper Prototype. Polymers 2018, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Yalikun, Y.; Noguchi, Y.; Kamamichi, N.; Tanaka, Y. Atmospheric-Operable 3D Printed Walking Bio-Robot Powered by Muscle-Tissue of Earthworm. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 7595–7600. [Google Scholar]
- Stanton, M.M.; Trichet-Paredes, C.; Sanchez, S. Applications of three-dimensional (3D) printing for microswimmers and bio-hybrid robotics. Lab Chip 2015, 15, 1634–1637. [Google Scholar] [CrossRef]
- Chan, V.; Asada, H.H.; Bashir, R. Utilization and control of bioactuators across multiple length scales. Lab Chip 2014, 14, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W. Biological Soft Robotics. Annu. Rev. Biomed. Eng. 2015, 17, 243–265. [Google Scholar] [CrossRef]
- Morishima, K.; Tanaka, Y.; Ebara, M.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. Demonstration of a bio-microactuator powered by cultured cardiomyocytes coupled to hydrogel micropillars. Sens. Actuators B Chem. 2006, 119, 345–350. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morishima, K.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. An actuated pump on-chip powered by cultured cardiomyocytes. Lab Chip 2006, 6, 362–368. [Google Scholar]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip 2007, 7, 207–212. [Google Scholar] [CrossRef]
- Park, J.; Kim, I.C.; Baek, J.; Cha, M.; Kim, J.; Park, S.; Lee, J.; Kim, B. Micro pumping with cardiomyocyte-polymer hybrid. Lab Chip 2007, 7, 1367–1370. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujita, H. Fluid driving system for a micropump by differentiating iPS cells into cardiomyocytes on a tent-like structure. Sens. Actuators B Chem. 2015, 210, 267–272. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Yang, S.; Baek, J.; Kim, B.; Lee, S.H.; Yoon, E.-S.; Chun, K.; Park, S. Establishment of a fabrication method for a long-term actuated hybrid cell robot. Lab Chip 2007, 7, 1504–1508. [Google Scholar] [CrossRef]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W.; Feigel, A.; Shevkoplyas, S.S.; Sheehy, S.; Whitesides, G.M.; Parker, K.K. Muscular thin films for building actuators and powering devices. Science 2007, 317, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, J.C.; Lee, H.; Feinberg, A.W.; Ripplinger, C.M.; Mccain, M.L.; Grosberg, A.; Dabiri, J.O.; Parker, K.K. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 2012, 30, 792–797. [Google Scholar] [CrossRef]
- Herr, H.; Dennis, R.G. A swimming robot actuated by living muscle tissue. J. Neuroeng. Rehabil. 2004, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of miniaturized walking biological machines. Sci. Rep. 2012, 2, 857. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Gazzola, M.; Park, K.S.; Park, S.; Di Santo, V.; Blevins, E.L.; Lind, J.U.; Campbell, P.H.; Dauth, S.; Capulli, A.K.; et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Schmidt, J.J.; Montemagno, C.D. Self-assembled microdevices driven by muscle. Nat. Mater. 2005, 4, 180–184. [Google Scholar] [CrossRef]
- Akiyama, Y.; Sakuma, T.; Funakoshi, K.; Hoshino, T.; Iwabuchi, K.; Morishima, K. Atmospheric-operable bioactuator powered by insect muscle packaged with medium. Lab Chip 2013, 13, 4870–4880. [Google Scholar] [CrossRef]
- Baryshyan, A.L.; Domigan, L.J.; Hunt, B.; Trimmer, B.A.; Kaplan, D.L. Self-assembled insect muscle bioactuators with long term function under a range of environmental conditions. RSC Adv. 2014, 4, 39962. [Google Scholar] [CrossRef]
- Akiyama, Y.; Odaira, K.; Iwabuchi, K.; Morishima, K. Long-term and room temperature operable bio-microrobot powered by insect heart tissue. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Cancun, Mexico, 23–27 January 2011; pp. 145–148. [Google Scholar]
- Akiyama, Y.; Odaira, K.; Iwabuchi, K.; Morishima, K. Long-term and room temperature operable bioactuator powered by insect dorsal vessel tissue. Lab Chip 2009, 9, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Odaira, K.; Sakiyama, K.; Hoshino, T.; Iwabuchi, K.; Morishima, K. Rapidly-moving insect muscle-powered microrobot and its chemical acceleration. Biomed. Microdevices 2012, 14, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Iwabuchi, K.; Furukawa, Y.; Morishima, K. Biological contractile regulation of micropillar actuator driven by insect dorsal vessel tissue. In Proceedings of the 2008 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 501–505. [Google Scholar]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measuring mechanical properties of cell sheets by a tensile test using a self-attachable fixture. J. Robot. Mechatron. 2013, 25, 603–610. [Google Scholar] [CrossRef]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measurement system for biomechanical properties of cell sheet. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 1010–1015. [Google Scholar]
- Akiyama, Y.; Iwabuchi, K.; Furukawa, Y.; Morishima, K. Fabrication and evaluation of temperature-tolerant bioactuator driven by insect heart cells. In Proceedings of the Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, CA, USA, 12–16 October 2008; pp. 1669–1671. [Google Scholar]
- Uesugi, K.; Shimizu, K.; Akiyama, Y.; Hoshino, T.; Iwabuchi, K.; Morishima, K. Contractile Performance and Controllability of Insect Muscle-Powered Bioactuator with Different Stimulation Strategies for Soft Robotics. Soft Robot. 2016, 3, 13–22. [Google Scholar] [CrossRef]
- Uesugi, K.; Akiyama, Y.; Yamato, M.; Okano, T.; Hoshino, T.; Morishima, K. Development of cell-sheet handling tool for measurement of cell sheet adhesion force. In Proceedings of the 2009 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 9–11 November 2009; pp. 614–619. [Google Scholar]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measuring adhesion force of a cell sheet by the ninety-degree peel test using a multi hook type fixture. J. Biomech. Sci. Eng. 2013, 8, 129–138. [Google Scholar] [CrossRef]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef]
- Videler, J.J. Fish Swimming; Springer: Dordrecht, The Netherlands, 1993; Volume 10, ISBN 9780470015902. [Google Scholar]
- Houaria, B.; Chellali, B. Study of the mechanical behavior of a hyperelastic membrane. Sens. Transducers 2014, 168, 108–112. [Google Scholar]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking Effect on Polydimethylsiloxane Elastic Modulus Measured by Custom-Built Compression Instrument. J. Appl. Polym. Sci. 2014, 131, 41050. [Google Scholar] [CrossRef]
- Palmer, R.O.Y.E.; Brady, J.; Roos, P.; Roy, E.; Brady, A.J. Mechanical measurements using a pipette attachment from isolated system cardiac myocytes. Am. J. Physiol. Cell Physiol. 1996, 270, C697–C704. [Google Scholar] [CrossRef]
- Lin, G.; Pister, K.S.J.; Roos, K.P. Surface micromachined polysilicon heart cell force transducer. J. Microelectromechan. Syst. 2000, 9, 9–17. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Melnychenko, I.; Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 2004, 25, 1639–1647. [Google Scholar] [CrossRef]
- Xu, Q. Design and Implementation of Large-Range Compliant Micropositioning Systems; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781119131434. [Google Scholar]
- Vijayasai, A.P.; Sivakumar, G.; Mulsow, M.; Lacouture, S.; Holness, A.; Dallas, T.E. Haptic controlled three-axis MEMS gripper system. Rev. Sci. Instrum. 2010, 81, 105114. [Google Scholar] [CrossRef] [PubMed]

| Biological Component | Diameter | Performance | Reference | |
|---|---|---|---|---|
| Force | Normalizing | |||
| Insect dorsal vessel tissue | 217 µm | 42.7 µN (10% strain) 66.8 µN (20% strain) 79.6 µN (30% strain) | 1.15 kPa (10% strain) 1.81 kPa (20% strain) 2.15 kPa (30% strain) | This study |
| Insect dorsal vessel tissue | ~500 µm | 10.5 to 87.3 µN (20 % strain) | - | [29] |
| Cardiomyocyte | 20 to 30 µm | 11.5 µN | 22.3 kPa | [36] |
| Cardiomyocyte | ~30 µm | 12.6 µN | 23.7 kPa | [37] |
| Engineered heart tissue (Cardiomyocyte+ ECMs gel) | ~1 mm | - | 3 kPa | [38] |
| Size (mm) | Material | Environment Maintenance | Speed (µm/s) | Tissue Type | Reference |
|---|---|---|---|---|---|
| 2 | PDMS | Necessary | 5–10 | Cardiomyocyte cluster | [13] |
| 2 | PDMS | Necessary | 81 | Cardiomyocyte cluster | [13] |
| 6 | PDMS | Necessary | 400 | Cardiac cell tissue | [14] |
| 8 | PDMS | Necessary | 1500 | Cardiac cell tissue | [15] |
| 8.02 | PDMS | Unnecessary within 90 days [23] | 11.7/44.7 (theoretical) | Insect tissue | This study |
| 120 | PDMS | Necessary | 45,000 | Frog muscle tissue | [16] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalikun, Y.; Uesugi, K.; Hiroki, M.; Shen, Y.; Tanaka, Y.; Akiyama, Y.; Morishima, K. Insect Muscular Tissue-Powered Swimming Robot. Actuators 2019, 8, 30. https://doi.org/10.3390/act8020030
Yalikun Y, Uesugi K, Hiroki M, Shen Y, Tanaka Y, Akiyama Y, Morishima K. Insect Muscular Tissue-Powered Swimming Robot. Actuators. 2019; 8(2):30. https://doi.org/10.3390/act8020030
Chicago/Turabian StyleYalikun, Yaxiaer, Kaoru Uesugi, Minamida Hiroki, Yigang Shen, Yo Tanaka, Yoshitake Akiyama, and Keisuke Morishima. 2019. "Insect Muscular Tissue-Powered Swimming Robot" Actuators 8, no. 2: 30. https://doi.org/10.3390/act8020030
APA StyleYalikun, Y., Uesugi, K., Hiroki, M., Shen, Y., Tanaka, Y., Akiyama, Y., & Morishima, K. (2019). Insect Muscular Tissue-Powered Swimming Robot. Actuators, 8(2), 30. https://doi.org/10.3390/act8020030