Ionic Polymer Microactuator Activated by Photoresponsive Organic Proton Pumps
Abstract
:1. Introduction
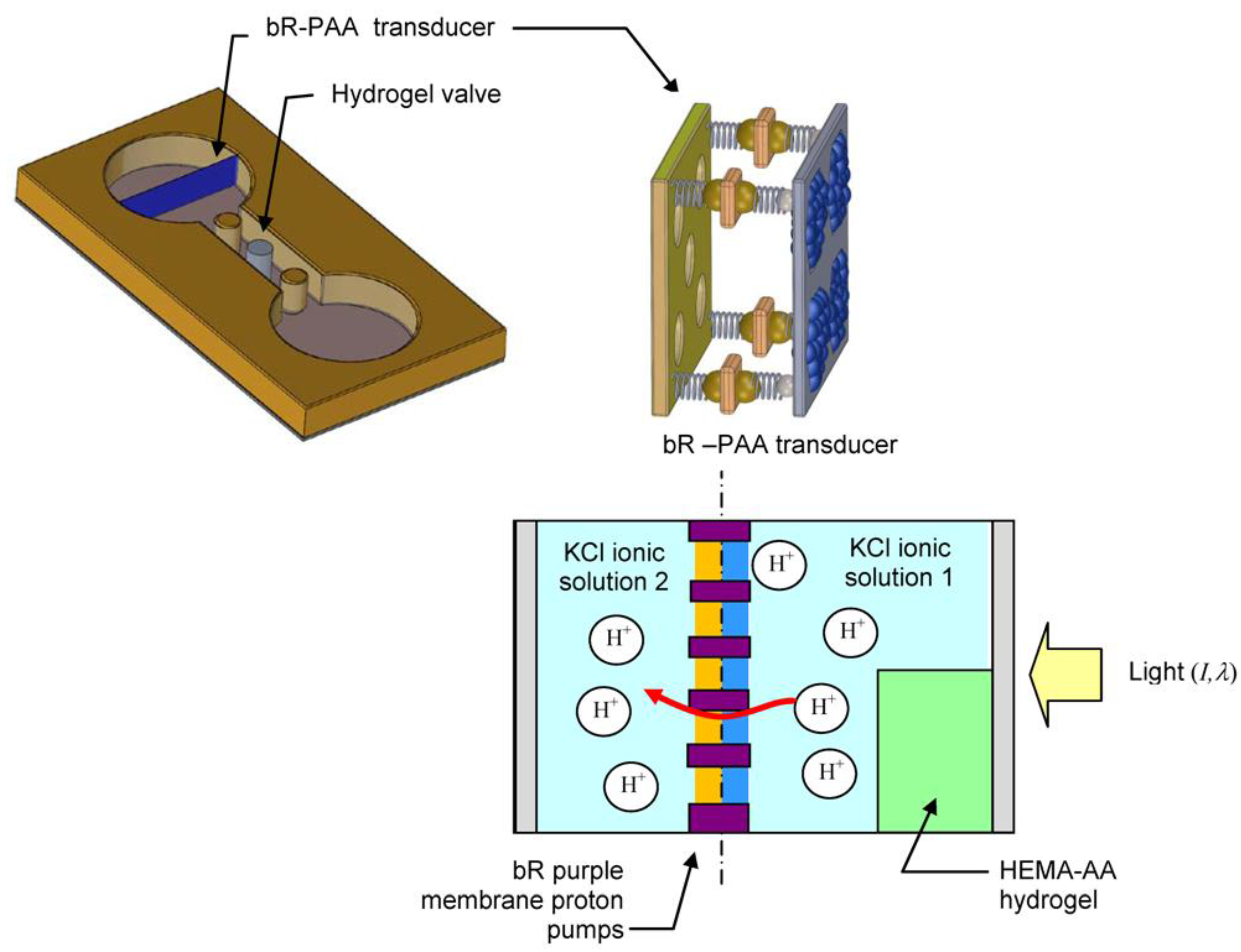
2. Light Driven Hydrogel Microactuator
2.1. Method of Actuation—Photoresponsive Proton Pumps
2.2. Microactuator Shell—Ionic Polymer Hydrogel
3. Microfabrication Processes
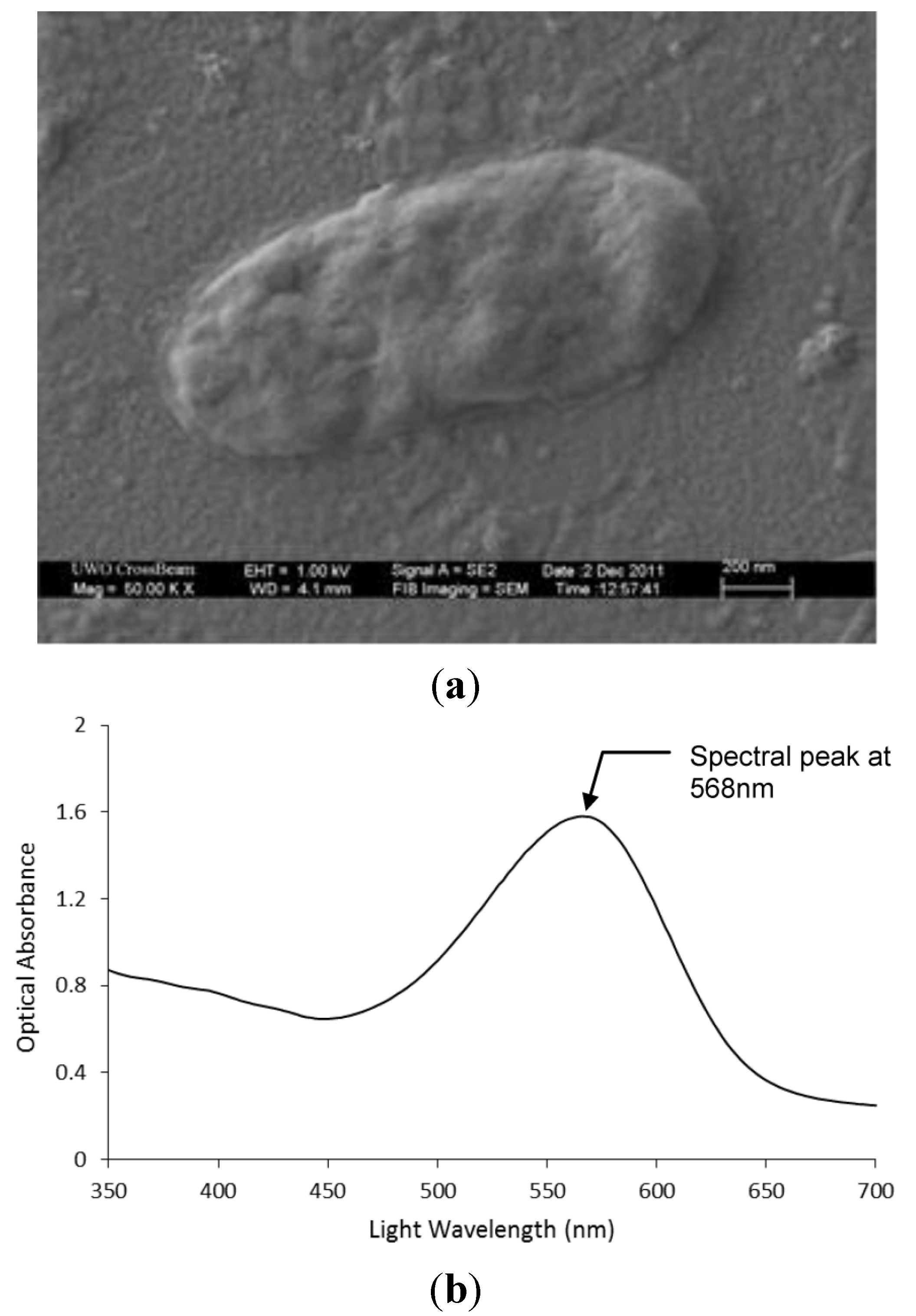
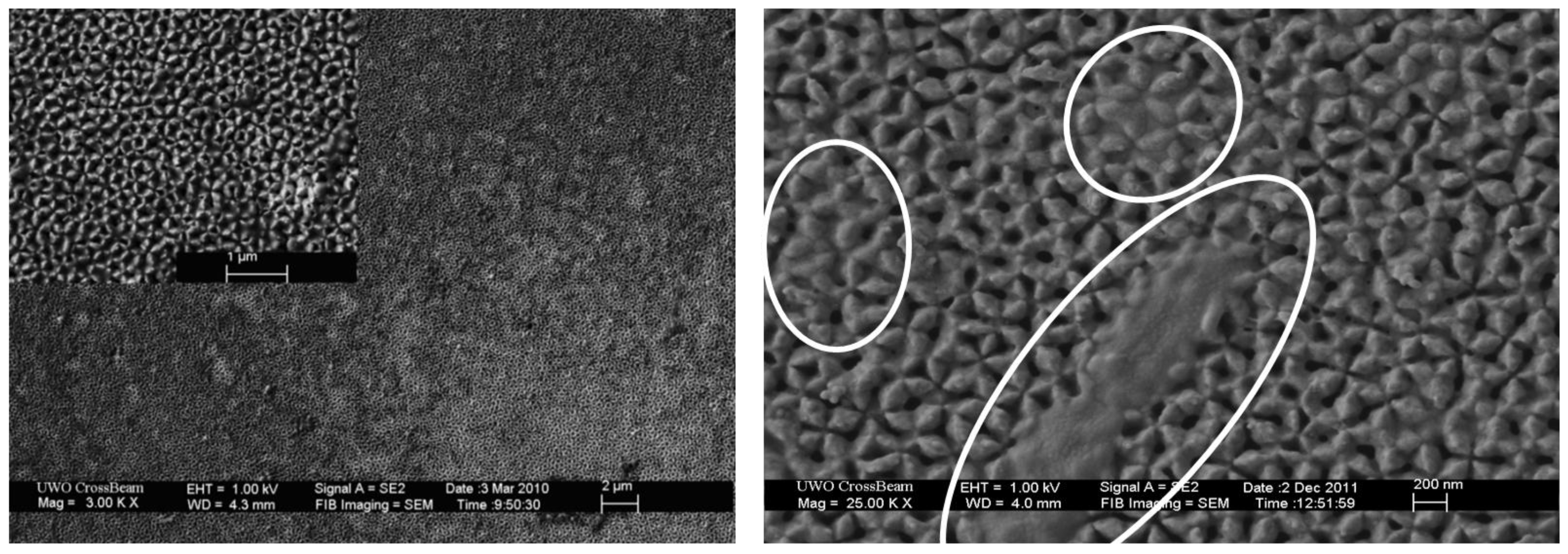
3.1. Light Driven bR-PAA Transducer
3.2. pH Sensitive HEMA-AA Microactuator Shell
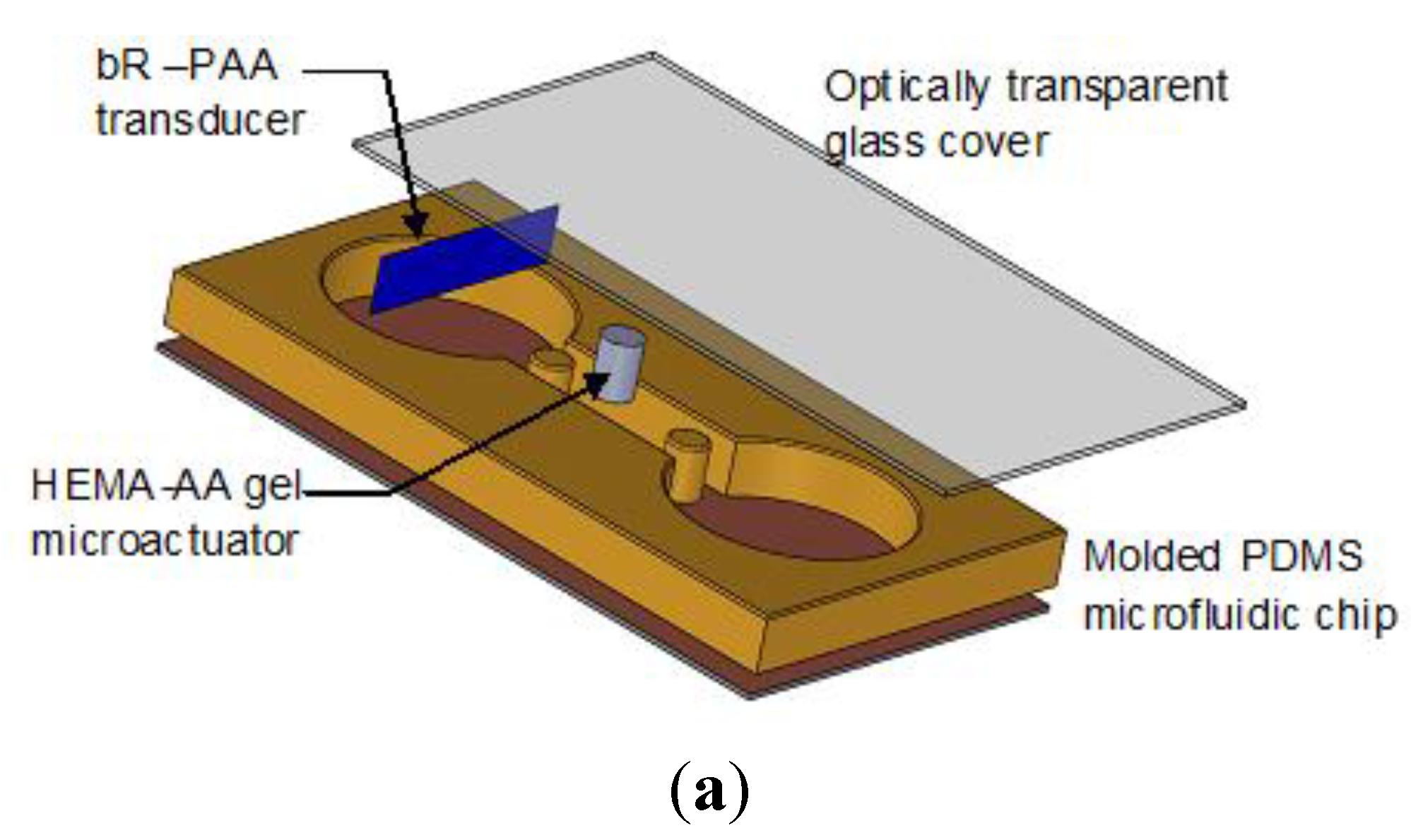
3.3. Polydimethylsiloxane (PDMS) Microfluidic Chip
3.4. Microfluidic Chip Assembly
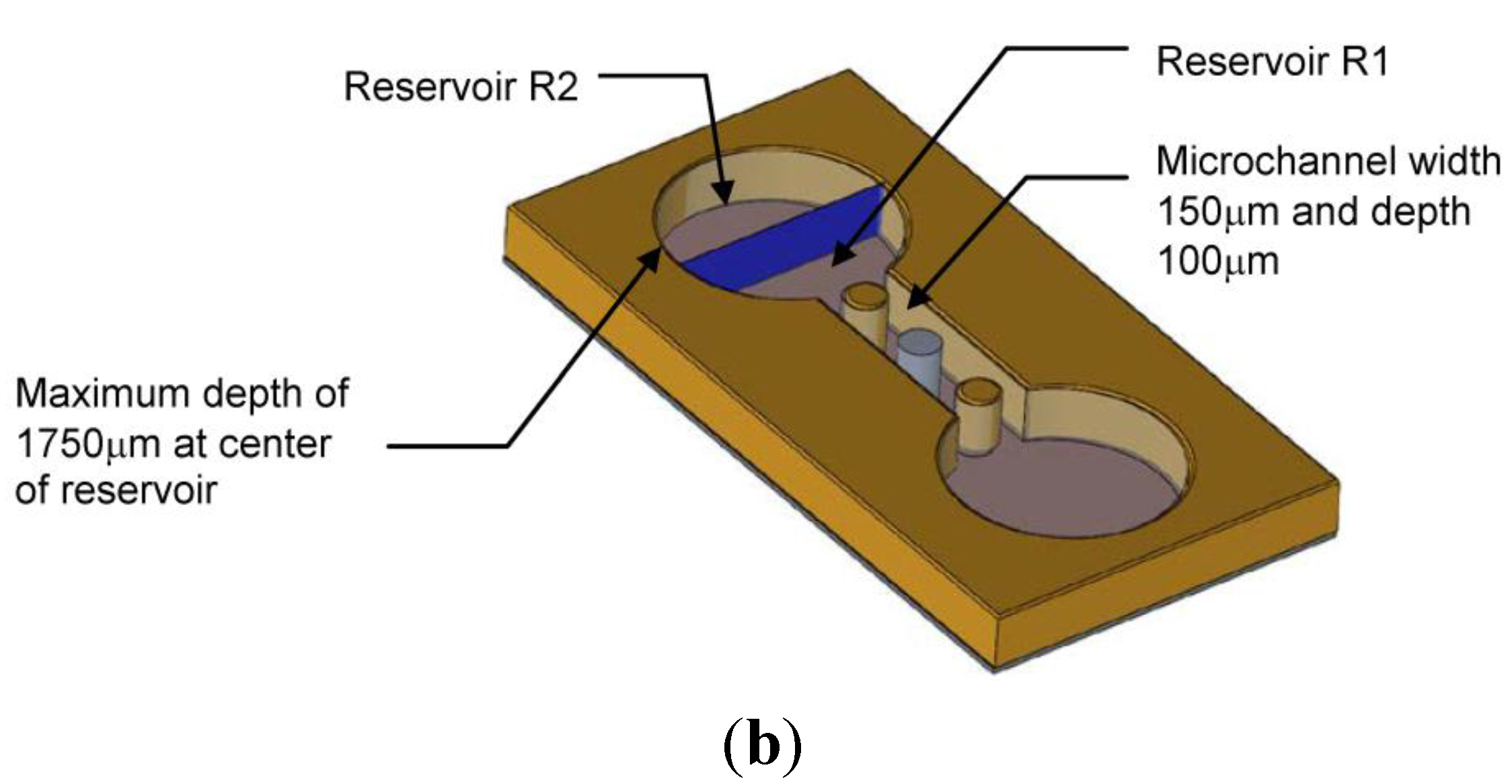
4. Experimental Results and Discussion
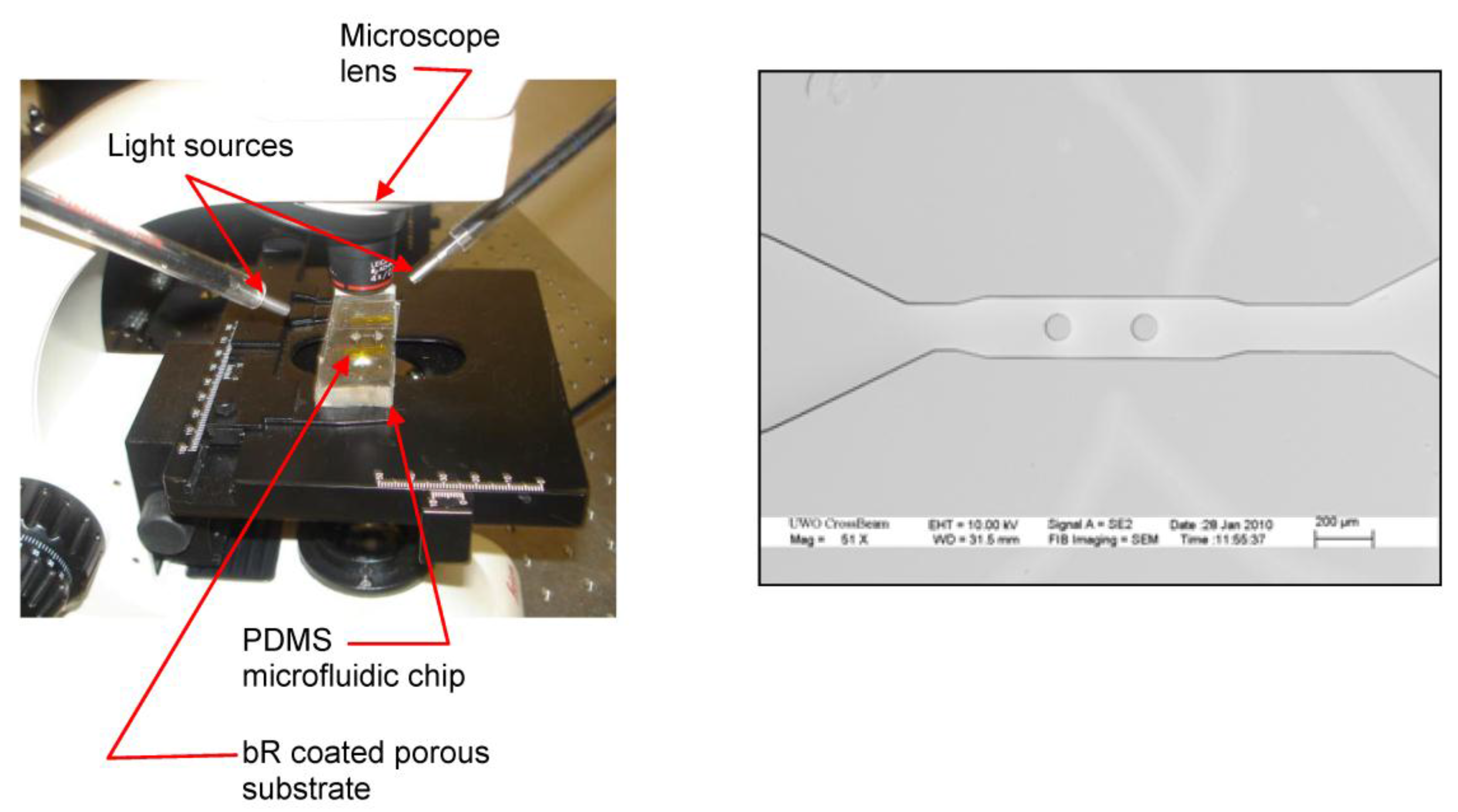
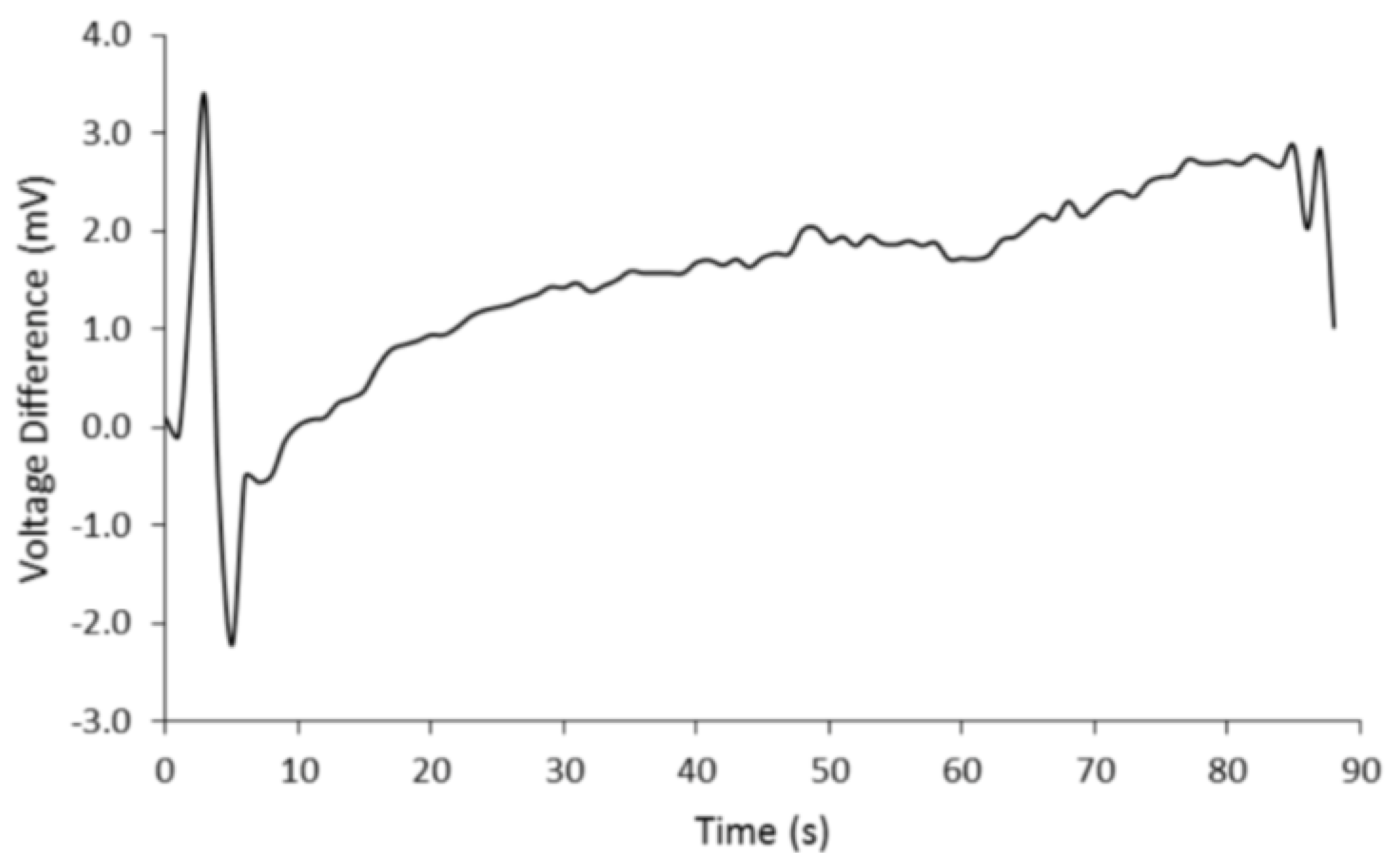
4.1. Photoelectric Analysis
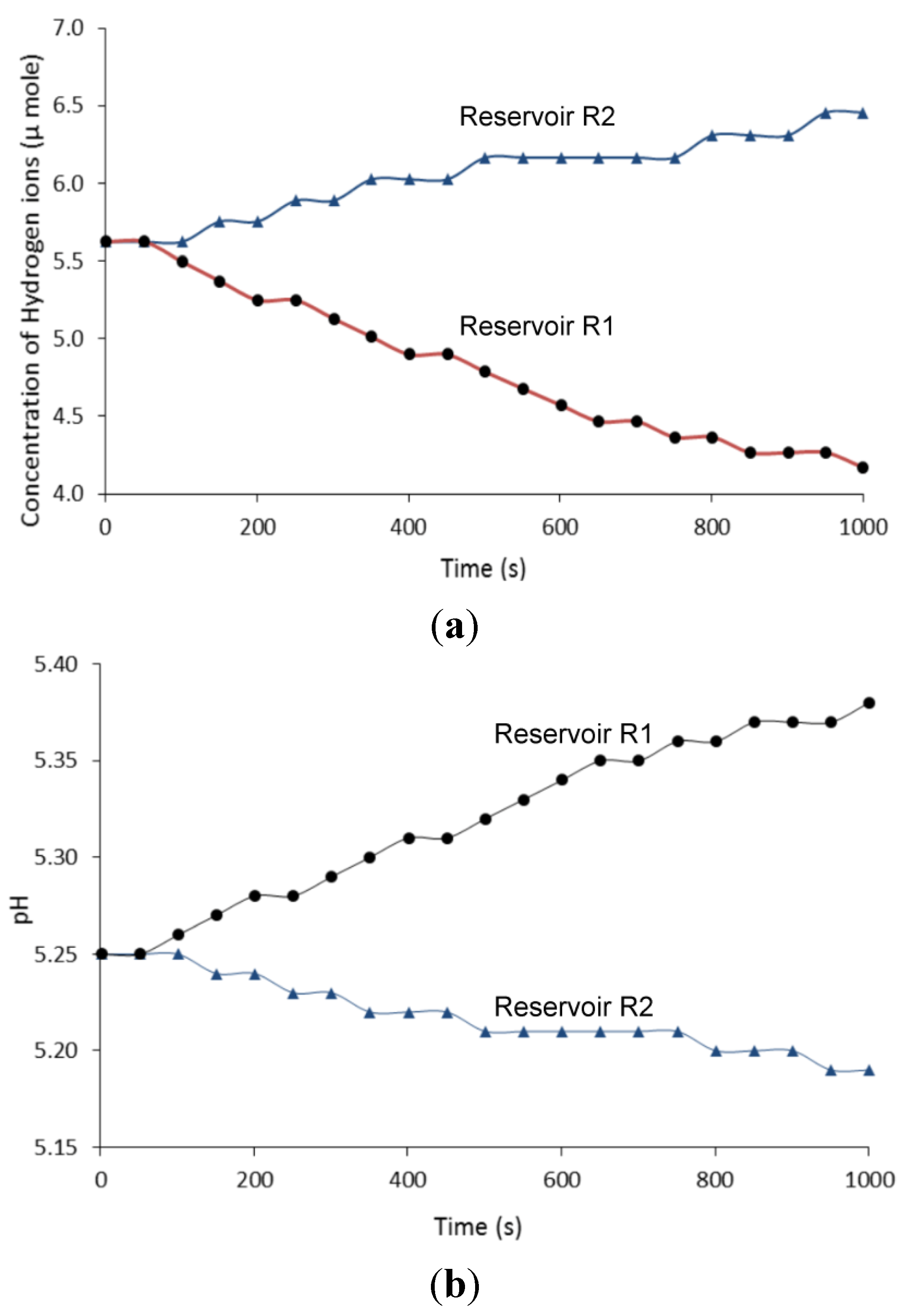
4.2. Light Driven pH Gradient Transducer
4.2.1. Ion Transfer under Fixed Light Intensity
4.2.2. Ion Transfer under Variable Light Intensity
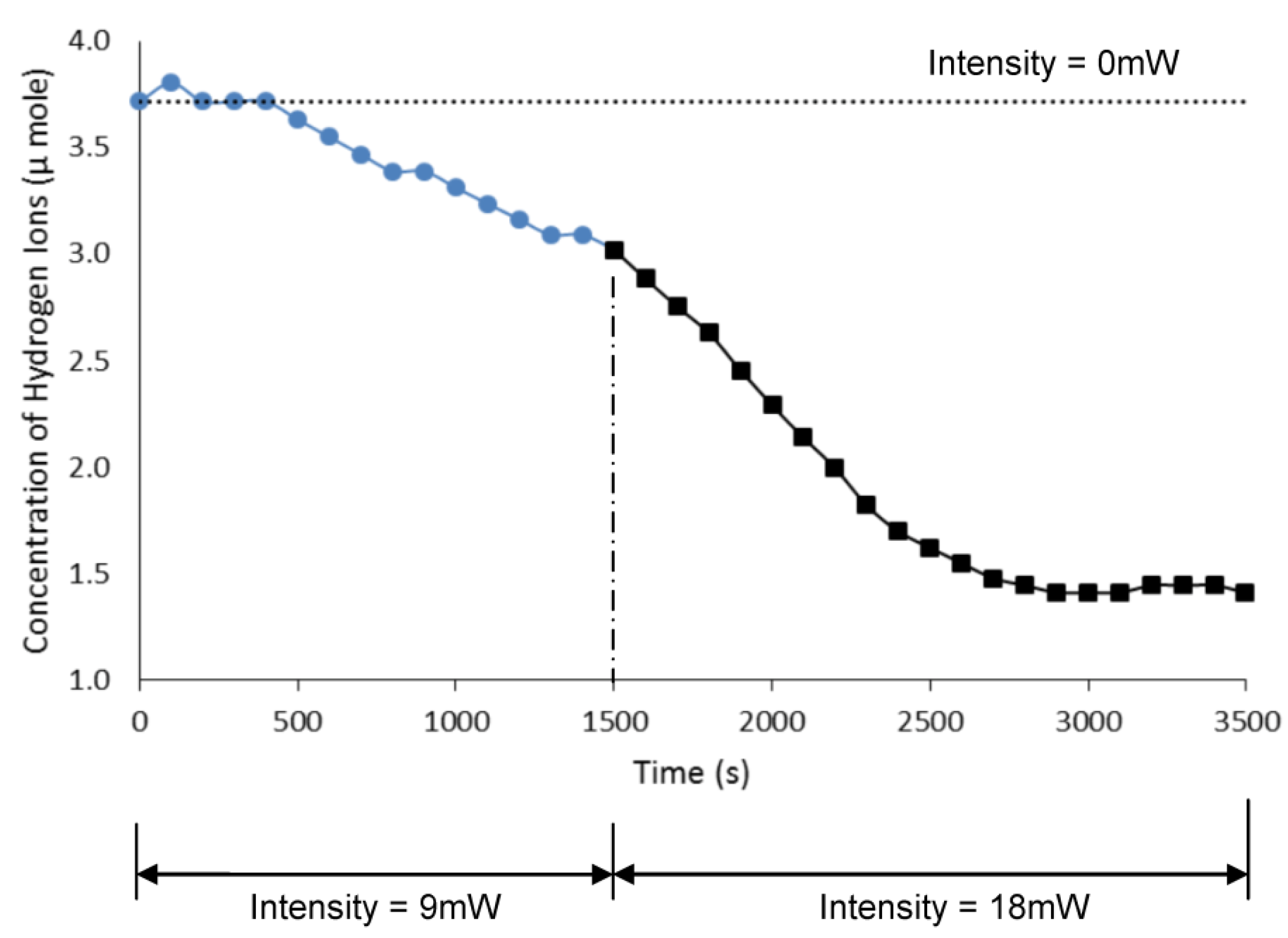
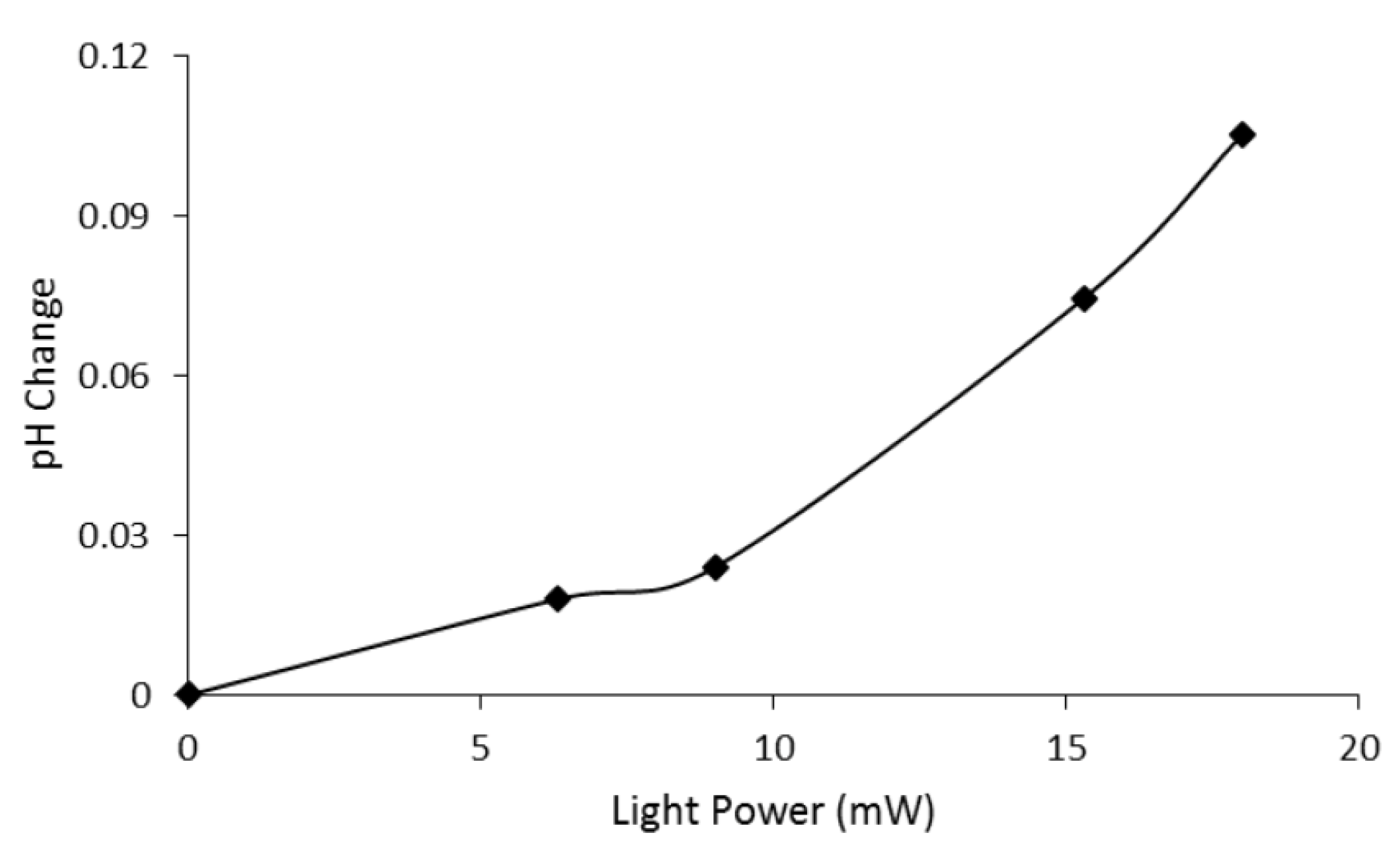
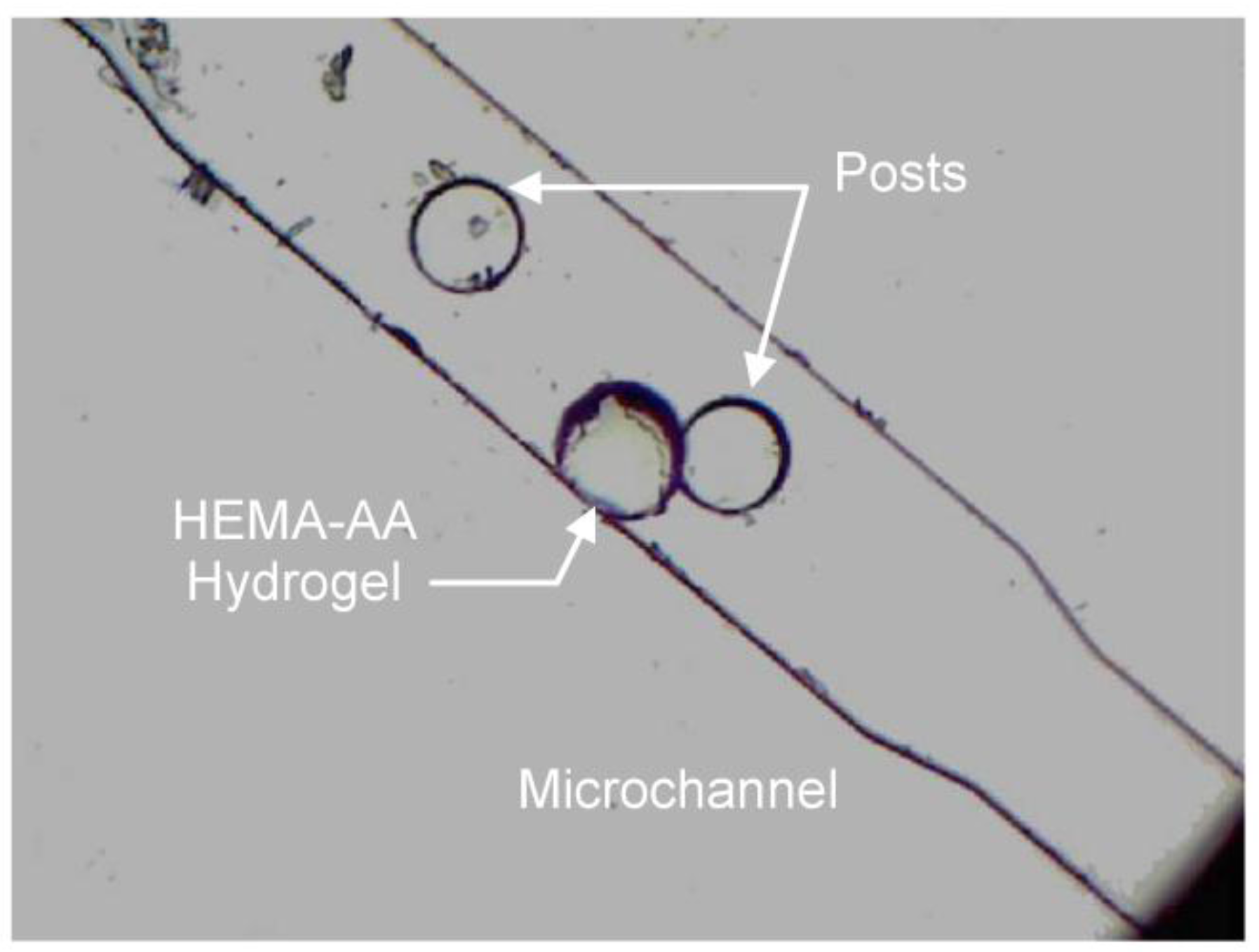
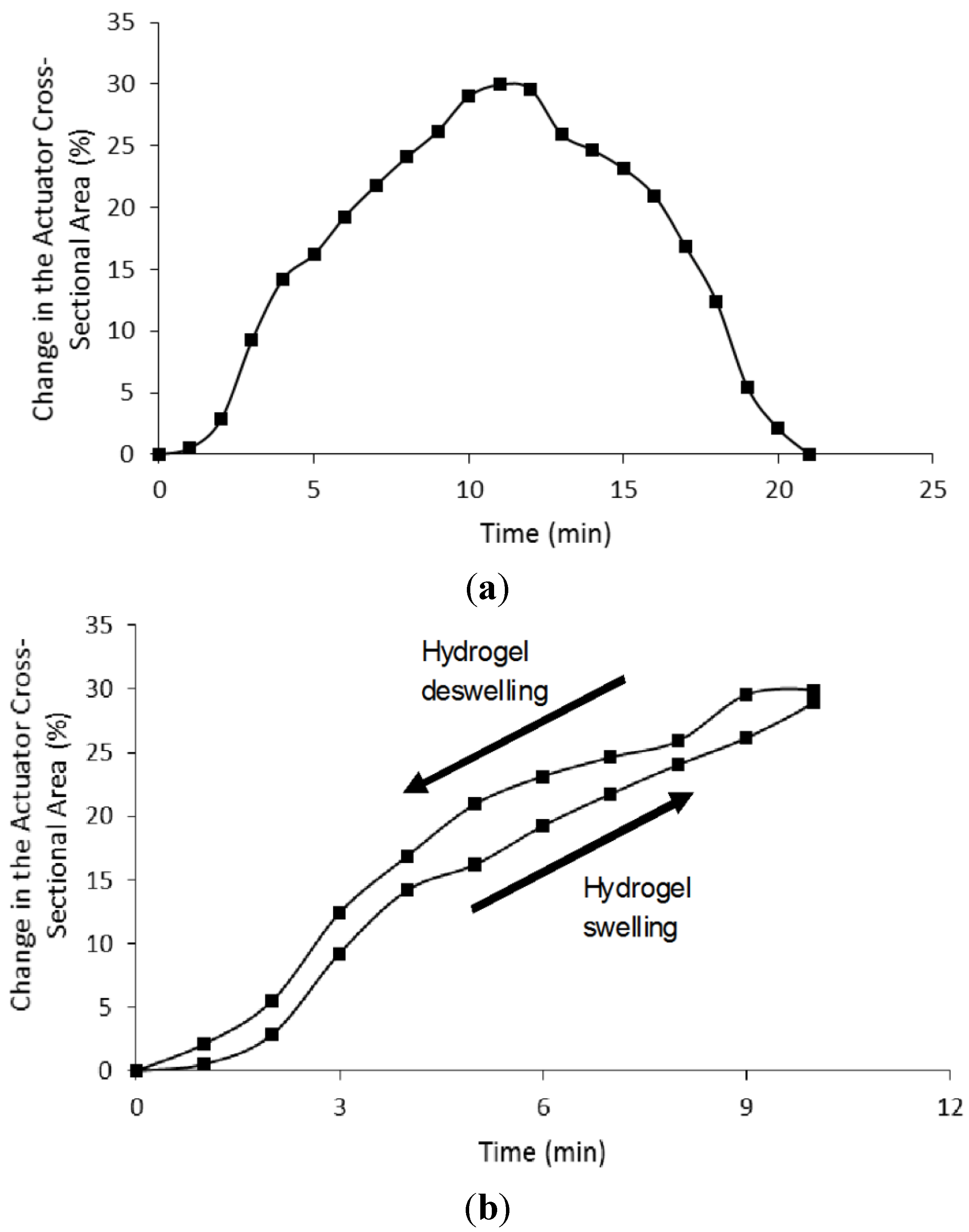
4.3. Microactuator Swelling Analysis
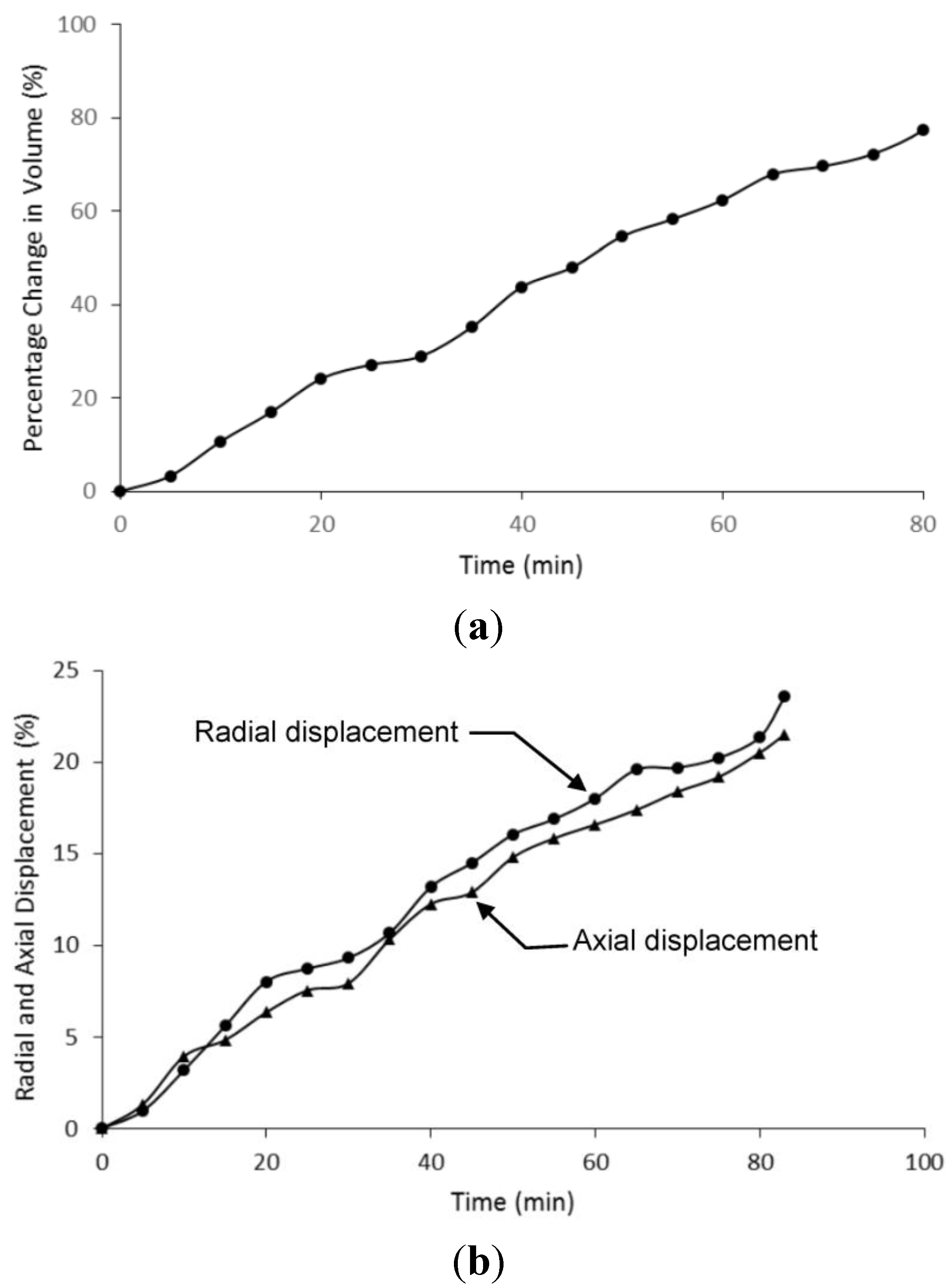
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abgrall, P.; Gué, A.-M. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15–R49. [Google Scholar] [CrossRef]
- Watanabe, T.; Akiyama, M.; Totani, K.; Kuebler, S.M.; Stellacci, F.; Wenseleers, W.; Braun, K.; Marder, S.R.; Perry, J.W. Photoresonsive hydrogel microstructure fabricated by two-photon initiated polymerization. Adv. Funct. Mater. 2002, 12, 611–614. [Google Scholar] [CrossRef]
- Liu, R.H.; Yu, Q.; Beebe, D. Fabrication and characterization of hydrogel-based microvalves. J. Microelectron. Syst. 2002, 11, 45–53. [Google Scholar] [CrossRef]
- Baldi, A.; Gu, Y.; Loftness, P.E.; Siegel, R.A.; Ziaie, B. A hydrogel-actuated environmentally sensitive microvalve for active flow control. J. Microelectromech. Syst. 2003, 12, 613–621. [Google Scholar] [CrossRef]
- Al-Aribe, K.; Knopf, G.K.; Bassi, A.S. Photo-responsive hydrogel for controlling flow on a microfluidic chip. Proc. SPIE 2006, 6343. [Google Scholar] [CrossRef]
- Beebe, D.; Mensing, G.; Walker, G. Physics and Applications of Microfluidics in Biology Silicon based replication technology of 3D-microstuctures by conventional CD-injection molding techniques. In Proceedings of Transducers’97, Chicago, IL, USA, 16–19 June 2002; pp. 1415–1418.
- Yu, Q.; Bauer, J.M.; Moore, J.S.; Beebe, D.J. Responsive biomimetic hydrogel valve for microfluidics. Appl. Phys. Lett. 2001, 78, 2589–2591. [Google Scholar] [CrossRef]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halbacterium. Nature 1971, 233, 149–152. [Google Scholar]
- Hampp, N. Bacteriorhodopsin as a photochromic retinal protein for optical memories. Chem. Rev. 2000, 100, 1755–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Knopf, G.K.; Bassi, A. Photoelectric properties of a detector based on dried bacteriorhodopsin film. Biosens. Bioelectron. 2005, 21, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Varo, G.; Lanyi, J. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry 1991, 30, 5016–5022. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Bacteriorhodopsin—the movie. Nature 2000, 406, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, I.; Aydemir, A.; Turker, L.; Yucel, M. Photoresponse of bacteriorhodopsion immobilized in polyacrylamide gel membranes. J. Membr. Sci. 1994, 86, 171–179. [Google Scholar] [CrossRef]
- Liu, S.Y.; Kono, M.; Ebrey, T.G. Effect of pH buffer molecules on the light-induced currents from oriented purple membrane. Biophys. J. 1991, 60, 204–216. [Google Scholar] [CrossRef]
- Schranz, M.; Noll, F.; Hampp, N. Oriented purple membrane monolayers covalently attached to gold by multiple thiole linkages analyzed by single molecule force spectroscopy. Langmuir 2007, 23, 11134–11138. [Google Scholar] [CrossRef] [PubMed]
- Al-Aribe, K.; Knopf, G.K.; Bassi, A.S. Photoelectric monolayers based on self-assembled and oriented purple membrane patches. J. Microelectron. Syst. 2011, 20, 800–810. [Google Scholar] [CrossRef]
- Henderson, R.; Stubb, J.; Whytock, S. Specific labelling of the protein and lipid on the extracellular surface of purple membrane. J. Mol. Biol. 1978, 123, 259–274. [Google Scholar] [CrossRef]
- Chen, D.; Lu, Y.; Sui, S.; Xu, B.; Hu, K. Oriented assembly of purple membrane on solid support mediated by molecular recognition. J. Phys. Chem. B 2003, 107, 3598–3605. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, Y.; Han, L.; Zhao, H. A nanomechanical device based on light-driven proton pumps. Nanotechnology 2006, 17, 1778–1785. [Google Scholar] [CrossRef]
- Chiu, Y.; Varanasi, P.P.; McGlade, M.J.; Varanasi, S. pH-induced swelling kinetics of polelectrolyte hydrogels. J. Appl. Polymer Sci. 1995, 58, 2161–2176. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Al-Aribe, K.; Knopf, G.K.; Bassi, A.S. Fabrication of an optically driven pH-gradient generator based on self-assembled proton pumps. Microfluid. Nanofluid. 2012, 12, 325–335. [Google Scholar] [CrossRef]
- Feng, J.-T.; Zhao, Y.-P. Influence of different amount of Au on the wetting behavior of PDMS membrane. Biomed. Microdevices 2008, 10, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Peterson, E.T.K.; Papautsky, I. Long-term stability of plasma oxidized PDMS Surfaces. In Proceedings of IEEE EMBS, San Francisco, CA, USA, 1–4 September 2004; pp. 5013–5016.
- Horn, C.; Steinem, C. Photocurrents generated by bacteriorhodopsin adsorbed on nano-black lipid membranes. Biophys. J. 2005, 89, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Tabib-Azar, M. Microactuators; Electrical, Magnetic, Thermal, Optical, Mechanical, Chemical and Smart Structures, 1st ed.; Kluwer Academic Publishers: New York, NY, USA, 1998; pp. 219–274. [Google Scholar]
- Richter, A.; Kuckling, D.; Howitz, S.; Gehring, T.; Arndt, K. Electronically controllable microvalves based on smart hydrogels: Magnitudes and potential applications. J. Microelectromech. Syst. 2003, 12, 748–753. [Google Scholar] [CrossRef]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.; Hans-Jürgen, P.; Adler, H.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Jang, J.; Jhaveri, S.J.; Rasin, B.; Koh, C.Y.; Ober, C.K.; Thomas, E.L. Three dimensionally-patterned submicrometer-scale hydrogel/air networks that offer a new platform for biomedical applications. Nano Lett. 2008, 8, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Aribe, K.M.; Knopf, G.K.; Bassi, A.S. Ionic Polymer Microactuator Activated by Photoresponsive Organic Proton Pumps. Actuators 2015, 4, 237-254. https://doi.org/10.3390/act4040237
Al-Aribe KM, Knopf GK, Bassi AS. Ionic Polymer Microactuator Activated by Photoresponsive Organic Proton Pumps. Actuators. 2015; 4(4):237-254. https://doi.org/10.3390/act4040237
Chicago/Turabian StyleAl-Aribe, Khaled M., George K. Knopf, and Amarjeet S. Bassi. 2015. "Ionic Polymer Microactuator Activated by Photoresponsive Organic Proton Pumps" Actuators 4, no. 4: 237-254. https://doi.org/10.3390/act4040237
APA StyleAl-Aribe, K. M., Knopf, G. K., & Bassi, A. S. (2015). Ionic Polymer Microactuator Activated by Photoresponsive Organic Proton Pumps. Actuators, 4(4), 237-254. https://doi.org/10.3390/act4040237





