Abstract
Degenerative joint diseases, such as osteoarthritis, are increasingly prevalent in aging populations, yet current treatments like stem cell injections face limitations in targeted delivery and efficacy. In this study, we proposed a biodegradable magnetically actuated microstructure for knee cartilage regeneration. The microstructure is composed of calcium-crosslinked alginate hydrogel embedded with magnetic nanoparticles (MNPs), allowing for precise control using an external magnetic field generated by an electromagnetic actuation (EMA) system. Fabricated via a centrifugal micro-nozzle process, the microstructures exhibited tunable sizes and uniform morphology. The proposed microstructures were characterized for their morphological, chemical, and magnetic properties, and their biodegradability and targeting ability in a phosphate-buffered saline (PBS) environment were experimentally analyzed. Experimental results demonstrated that smaller microstructures degraded more rapidly and that fewer microstructures resulted in improved targeting accuracy. In contrast, microstructures clustered at the lesion site degraded more slowly, supporting sustained therapeutic release. These results suggest that the proposed system can enhance delivery precision, minimize off-target accumulation, and reduce inflammation risks associated with residual materials. The biodegradable magnetically actuated microstructures present a promising platform for minimally invasive and site-specific cartilage therapy.
1. Introduction
The global increase in elderly populations has led to a corresponding rise in the prevalence of age-related diseases, particularly degenerative arthritis [1]. To address this clinical challenge, a variety of joint repair strategies have been developed [2,3]. Among them, stem cell injection therapy has emerged as a promising minimally invasive approach that promotes cartilage regeneration and tissue repair [4,5]. This technique involves harvesting bone marrow, isolating stem cells, and injecting them into the joint cavity to stimulate regeneration. While offering advantages such as reduced side effects and faster recovery, the efficacy of stem cell injection therapy is often limited by poor targeting efficiency, resulting in uncontrolled dispersion of therapeutic agents within the joint cavity. Consequently, drug delivery efficiency is reduced, ultimately diminishing the overall treatment effect. To compensate for this limitation, a large quantity of therapeutic agents is often required, leading to increased costs. Moreover, in cases where the minimally invasive procedure fails to achieve sufficient therapeutic outcomes, invasive procedures may become necessary, thereby prolonging the patient’s recovery time.
Magnetically actuated microstructures have recently gained attention as alternative delivery vehicles for therapeutic agents [6,7,8,9,10,11,12,13]. These systems exploit magnetic responsiveness to enable guided navigation to target sites under externally applied electromagnetic fields, such as those generated by an electromagnetic actuation (EMA) system. Upon arrival, the microstructures gradually degrade in vivo, enabling sustained, localized drug release. However, some microstructures may deviate from the target, leading to off-target accumulation.
In terms of materials, most previously reported microstructures have been fabricated using synthetic polymers such as polylactide-co-glycolide (PLGA) or polycaprolactone (PCL), which exhibit prolonged degradation periods ranging from several weeks to years [9,10,11,12,13]. This extended biodegradation may trigger adverse immune responses, including inflammation, at non-target sites. Although mechanical retrieval systems may be employed to remove non-targeted microstructures, such interventions are technically challenging and carry the risk of disturbing microstructures correctly positioned within the defect region.
Several prior studies have demonstrated the potential of magnetic-field-based systems for precise targeting. One study proposed a magnetic-field-driven microrobot system designed to deliver therapeutic cells directly to the site of cartilage injury [9]. Another utilized a bacterial-based magnetic guidance system in tumor environments [6], and a separate investigation introduced synthetic and biohybrid micropropellers to improve nanoparticle delivery through magnetically guided convection [11]. These efforts primarily focus on enhancing positional control and targeting efficiency; however, they provide limited discussion on biodegradability control, structural uniformity during fabrication, or personalized customization of microstructures based on the defect size and cartilage anatomy—key elements that are addressed in our proposed system.
In this study, we propose a magnetically actuated microstructure with enhanced biodegradability to overcome the limitations of microstructure-based approaches for knee cartilage regeneration. The microstructure is composed of alginate, a naturally derived biopolymer that confers intrinsic biodegradability and is embedded with magnetic nanoparticles (MNPs) to enable precise targeting under externally applied magnetic fields. Structural stabilization is achieved through ionotropic gelation, whereby alginate crosslinks with calcium ions (Ca2+) to form a robust hydrogel network [14]. Furthermore, the size of the Ca2+-hardened alginate microstructures can be precisely tuned via a micro-nozzle-based centrifugal fabrication process, allowing for delivery customization according to the extent of cartilage damage. Following minimally invasive arthroscopic administration, both targeted and non-targeted microstructures are exposed to phosphate-buffered saline (PBS) within the joint cavity. The ionic imbalance between the internal Ca2+ within the hydrogel and the surrounding PBS induces the dissociation of Ca2+–alginate crosslinks, initiating controlled degradation of the microstructure. This degradation mechanism supports sustained and localized therapeutic release at the lesion site while facilitating rapid clearance of non-targeted microstructures, thereby minimizing the risk of inflammatory side effects associated with residual materials in vivo.
The proposed procedure for knee cartilage regeneration employs a minimally invasive arthroscopic approach using magnetically actuated microstructures (Figure 1). Upon visual confirmation of the damaged cartilage area via arthroscopy, lesion-optimized microstructures are injected into the joint cavity through a medical catheter. These microstructures are precisely guided toward the defect site using external magnetic fields generated by the EMA system, with real-time tracking via the arthroscope. Upon arrival, the targeted microstructures are anchored to the defect region through magnetic attraction generated by a permanent magnet. Once localized, the microstructures gradually degrade in vivo, releasing therapeutic agents to promote cartilage regeneration. In contrast, non-targeted microstructures, which are not retained at the lesion site, degrade more rapidly and are subsequently cleared from the joint cavity. This selective degradation behavior, coupled with precision magnetic targeting and size-customized delivery, underscores the multifunctionality of the proposed platform. Overall, the system holds strong potential to enhance therapeutic efficacy while minimizing off-target accumulation and side effects, thereby improving the safety and outcomes of knee cartilage regeneration treatments.

Figure 1.
Conceptual overview of knee cartilage regeneration using the magnetically actuated microstructure. (a) The magnetically actuated microstructures, loaded with therapeutic agents, composed of magnetic nanoparticles and alginate-based hydrogel structure. (b) The treatment scenario using the prepared microstructures involves their arthroscopically guided injection, magnetic targeting, magnetic fixation, and gradual biodegradation while releasing therapeutic agents.
2. Materials and Methods
2.1. Materials
For the fabrication of magnetic microstructures, sodium alginate and calcium chloride (CaCl2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The MNPs used in this study are based on iron (II, III) oxide (Fe3O4) obtained via co-precipitation synthesis. The iron (II, III) oxide (Fe3O4) nanoparticles (50–100 nm particle size) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Fabrication of the Magnetically Actuated Microstructure
To prepare the magnetically actuated microstructure, we used alginate, a naturally derived polymer known for its biocompatibility and biodegradability [14]. Alginate forms a stable hydrogel structure through ionotropic gelation when it reacts with CaCl2. This property is widely utilized for encapsulating and protecting bioactive molecules, drugs, and cells. To enable precise magnetic control, magnetic nanoparticles were embedded into the microstructures. The detailed preparation of the microstructure was conducted as follows. A total of 500 µL each of a 6% alginate solution and a 6% MNP solution were mixed to create a 3% alginate–magnetic nanoparticle solution. An aliquot of 20 µL from this mixture was then dispensed into a 50 mL syringe barrel equipped with a needle. The prepared solution was dropped into a 10% CaCl2 solution via centrifugal force using a centrifuge to form bead-shaped microstructures, during which their size was controlled by adjusting the centrifuge rotation speed. The microstructures were further hardened in the 10% CaCl2 solution for 3 min. After hardening of the alginate hydrogel, the microstructures were retrieved from the CaCl2 solution and washed several times with deionized water (DW) to remove any residual ions or unreacted components. Finally, the microstructures were stored in DW before drug loading, targeting, and biodegradation tests.
2.3. Morphological, Chemical, and Magnetic Characterization of Microstructures
The images of the microstructures were obtained using a digital single-lens reflex (DSLR) camera (EOS 600D, CANON, Tokyo, Japan) and a Nikon inverted microscope (Eclipse Ti-E, NIKON, Tokyo, Japan) to observe the structures formed according to the centrifuge rotation speed. Morphologies of the microstructures were observed using a Scanning electron microscope (SEM, SU8010, Hitachi, Tokyo, Japan). O, C, and Fe signals of the microstructures were detected using energy-dispersive X-ray spectroscopy (EDX). In addition, the microstructures prepared by each size (200 µm, 400 µm, and 500 µm) were immersed in a 20 mg/mL doxorubicin (DOX) solution and then evaluated for loading of the therapeutic agent into the microstructure using a fluorescence microscope. The measurements were performed with 16 scans per sample in the range of 400–4000 cm−1, and the resolution was set to 4 cm−1. The magnetic response of the microstructures was evaluated using a neodymium permanent magnet (35 mm in width, 35 mm in length, and 10 mm in height), and the magnetic mobility of the microstructures was observed using a DSLR camera. Next, the magnetic properties were quantitatively measured using a vibrating sample magnetometer (VSM, Lake Shore Cryotronics 7404, Westerville, OH, USA) to analyze the magnetization of the microstructures and the MNPs. The main chemical components and chemical structures of the microstructure were analyzed using Fourier Transform–Infrared Spectroscopy (FT-IR) (Thermo Nicolet Corporation, Waltham, MA, USA).
2.4. Biodegradation Test of the Magnetically Actuated Microstructure
To evaluate the biodegradability of Ca2+-hardened alginate microstructures, we tested ~500 µm sized microstructures, which were the largest among those prepared, in phosphate-buffered saline (PBS) to mimic physiological conditions. Each Petri dish (60 mm in diameter and 15 mm in height) was filled with either 10 mL of DW or 10 mL of PBS. We monitored the degradation behavior of the microstructures using a DSLR camera. In addition, the biodegradability of microstructures of ~200 µm, ~400 µm, and ~500 µm sizes was confirmed for the application of arthroscopic-based knee cartilage surgery according to the extent of damaged cartilage. Each set of microstructures was placed in a separate Petri dish containing 10 mL of PBS, and their degradation was similarly recorded over time using a DSLR camera.
To verify the stable fixation and biodegradability of microstructures targeted to the damaged cartilage area, N35 neodymium permanent magnets (diameter 7 mm and height 3 mm) were used. As an experimental method, the microstructures with a size of 500 µm were placed in a chamber with a permanent magnet attached to the bottom. Subsequently, 10 mL of PBS was added to the chamber. The fixation and biodegradation of the microstructures were monitored using the DSLR camera.
2.5. Magnetic-Fields-Driven Mobility Test of the Magnetically Actuated Microstructure
Magnetically actuated microstructures can be aligned in a desired direction by an external magnetic field generated within the three-dimensional (3D) workspace of an electromagnetic actuation (EMA) system. When exposed to this field, the microstructures experience a magnetic torque (), which depends on the orientation and strength of the applied magnetic field. This relationship is described by the following equation:
where V, , and are the volume, magnetization, flux density, and gradient of the microstructure, respectively. For the magnetic mobility of the microstructure, the EMA system utilizes six orthogonally arranged rectangular electromagnetic coils, which generate a uniform rotating magnetic field within the 3D workspace [15]. Under the magnetic field, the microstructure undergoes rolling locomotion, driven by magnetic torque and supported by rolling friction against the bottom surface. The magnetic nanoparticles inside the microstructure align along the direction of the rotating magnetic field, generating magnetic torque that induces rotational force and drives the rolling locomotion of the structure.
where and are the magnetic flux generated by the electromagnetic coil and the rotational angular velocity ω of the microstructure, respectively. This rolling locomotion is governed by the balance between magnetic torque and the drag torque generated by rotational motion in the fluid.
The drag torque is defined as follows:
where represents the rotational friction coefficient and is the rotational angular velocity of the microstructure. As the rotational frequency increases, the frictional torque proportionally increases. When the frequency exceeds a certain threshold, known as the step-out frequency, the magnetic torque is no longer sufficient to overcome the drag torque, causing the microstructure to lose synchronization with the rotating magnetic field. As a result, the velocity of the microstructure initially increases with the rotational frequency but decreases beyond the step-out frequency, exhibiting a nonlinear velocity–frequency relationship. In this study, the mobility and frequency-dependent behavior of the microstructures were analyzed based on this torque balance model. By controlling the magnetic field generated from the EMA system, the microstructure can be guided to a desired position. As an experimental setup, the current of each electromagnetic coil of the EMA system is applied through the control of a motor driver controller (ESCON 70/10, Maxon Motor, Sachseln, Switzerland).
To perform targeting and degradation tests on damaged knee cartilage of microstructures, a 3D knee cartilage phantom was fabricated using a 3D printer (Objet30 Pro, Staratsys, Eden Prairie, MN, USA). The inside of the knee cartilage phantom was filled with PBS to create an environment similar to arthroscopic-based knee cartilage surgery. The design of the phantom model and the magnetic targeting setup was based on the magnetic microrobot-assisted cartilage regeneration model [8]. In particular, the targeting strategy within the phantom environment was partially adapted from a magnetic steering approach that demonstrated trajectory control of bacterial microswimmers using a phantom model prior to in vivo test [6]. The experimental setup was designed to simulate the delivery and precise positioning of microstructures during cartilage regeneration procedures.
3. Results
3.1. Characterization of the Magnetically Actuated Microstructure
The microstructures were fabricated by dropping the alginate–MNP solution into a CaCl2 solution using a centrifuge (Figure 2a,b). The size of the microstructures was adjusted according to the rotation speed of the centrifuge. As the rotation speed increased, the size of the alginate–MNP droplets formed at the syringe needle became smaller due to the increased centrifugal force, resulting in smaller microstructures (Figure 2c). The microstructure sizes corresponding to the rotation speeds were approximately ~500 µm at 600 rpm, ~400 µm at 800 rpm, and ~200 µm at 2000 rpm. The optical and SEM-EDS analyses of microstructures with different sizes are presented in Figure 2d, and the corresponding zoom-in SEM-EDS images are shown in Figure S1. The EDS elemental mapping results confirmed that the distribution of oxygen (O), carbon (C), and iron (Fe) did not show significant differences among the different sizes of microstructures. In particular, the Fe content remained relatively constant across all size groups, indicating that the MNPs were uniformly distributed within the microstructures regardless of their size. In addition, to confirm the drug loading capability of the microstructures, DOX, a fluorescent compound, was used. As a result, strong fluorescence signals were observed in the DOX-loaded microstructures, whereas no fluorescence was detected in the microstructure without DOX (Figure 2e). Furthermore, the drug loading capacity of microstructures with different sizes was analyzed (Figure S2). These results suggest that the higher drug loading efficiency observed in the 200 µm microstructures is due to their larger surface area relative to volume compared to the larger-sized microstructures, which provides more available surface for drug adsorption and loading. These results confirmed that the Ca2+-hardened alginate hydrogel formed within the microstructure is capable of encapsulating therapeutic agents. Furthermore, the DOX-loaded microstructures exhibited smaller sizes compared to the unloaded structures. This result was attributed to the formation of hydrogen bonds between the amino (-NH2) and phenolic hydroxyl (-OH) groups of DOX and the carboxyl (-COO−) groups of alginate, which led to the contraction of the hydrogel network due to intermolecular interactions [16,17].
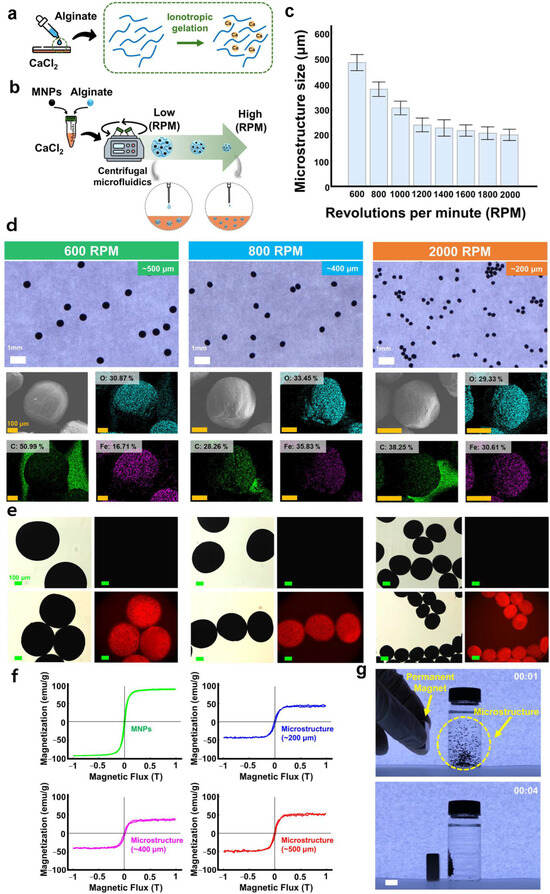
Figure 2.
Preparation and characterization of the magnetically actuated microstructure. (a) Schematic illustration of the hydrogel formation when alginate reacts with calcium chloride. (b) Schematic illustration of the preparation of the microstructure. (c) Size distribution of the microstructures according to revolutions per minute (RPM). (d) Optical and SEM-EDS images of microstructures. (e) Fluorescence images without (top) and with (bottom) DOX loading. Microstructures (~500 μm, ~400 μm, and ~200 μm). (f) Magnetization curves of the microstructures with different sizes (~500 µm, ~400 µm, and ~200 µm) and MNPs. (g) Magnetic response of the magnetically actuated microstructures using a permanent magnet. Scale bar, 10 mm. In all figures, the time is indicated on each figure in the minutes/seconds format.
As a characterization of the microstructures, we investigated the chemical composition and magnetic responsiveness of the microstructures. The chemical composition of the microstructures was analyzed using the FT-IR spectra (Figure S3). The spectral peaks of the microstructures matched the characteristic components of alginate (hydroxyl group (–OH) (–C=O) at 3410 cm−1, asymmetric stretching vibration of COO groups at 1635 cm−1, and elongation of C–O groups at 1050 cm−1) [18] and MNPs (Fe–O bond vibrations at 561 cm−1) [19,20] (Figure S3). These FT-IR spectra results reveal that the microstructures contain components without structural deformation by chemical bonding.
To investigate the magnetic response of the microstructures, the magnetization curve of the microstructures under an external magnetic field was assessed using a VSM. The microstructures with different sizes exhibited saturated magnetization in the narrow range of approximately 43–45 emu/g, resulting from the encapsulation of MNPs with high saturated magnetization (approximately 90 emu/g) (Figure 2f). These results indicate that the MNPs were uniformly distributed within the alginate mixture regardless of the rotation speed during the micro-nozzle-based centrifugal fabrication process. Next, the magnetic response of the microstructure was further evaluated using an N35 neodymium permanent magnet (35 mm width, 35 mm length, and 10 mm height) (Figure 2g and Supplementary Video S1). Upon application of an external magnetic field generated by a permanent magnet, the microstructures exhibited magnetization and were subsequently attracted toward the magnetic source. Based on these results, we confirmed that the microstructures possess sufficient magnetic response to be actuated by an external magnetic field.
3.2. Biodegradation Behavior of the Magnetically Actuated Microstructure
The biodegradation mechanism of the proposed microstructure follows the gradual breakdown of Ca2+-hardened alginate microstructures in environments with low Ca2+ concentrations (Figure 3a). Specifically, as the Ca2+ crosslinks within the hydrogel dissociate, Na⁺ ions replace them, leading to the degradation of the structure [21,22]. This degradation mechanism of the microstructure can be similarly applied in the PBS environment used during arthroscopic knee cartilage procedures.

Figure 3.
Biodegradation behavior of Ca2+-hardened alginate-based magnetically actuated microstructures. (a) Schematic illustration of the biodegradation mechanism of the microstructure. (b) Snapshots showing the degradation behavior of ~500 µm sized microstructures in DW and PBS. (c) Snapshots showing degradation behavior of the microstructures (~500 μm, ~400 μm, and ~200 μm) in PBS. (d) Snapshots showing the degradation behavior of ~500 µm microstructures in PBS without magnetic fixation and with magnetic fixation. In all figures, the time is indicated on each figure in the minutes/seconds format. (e) Summary of the degradation time of microstructures of different sizes, with and without drug loading.
We evaluated the biodegradability of ~500 µm sized microstructures in DW and PBS (Figure 3b and Supplementary Video S2). As a result, the shape of the microstructures in DW showed almost no change over time. In contrast, the microstructures in PBS exhibited dissociation of Ca2+ crosslinks within the alginate and subsequent release of MNPs within about 50 min. Additionally, microstructures of different sizes (~200 µm, ~400 µm, ~500 µm) were immersed in PBS to investigate their biodegradation (Figure 3c and Supplementary Video S3). The result indicates that microstructures with diameters of ~200 µm, ~400 µm, and ~500 µm were completely degraded in about 30 min, 37 min, and 47 min, respectively. In particular, we confirmed that smaller microstructures degraded more rapidly, which is likely due to more efficient ion exchange throughout the structure, leading to accelerated degradation.
Next, to examine the biodegradability of the microstructures delivered to the damaged cartilage, we investigated the biodegradability of the microstructures fixed by a permanent magnet. Here, PBS was filled into a chamber with a permanent magnet attached, and the degradation of 500 µm sized microstructures was observed in real time (Figure 3d and Supplementary Video S4). As a result, the microstructures dispersed in PBS without a permanent magnet underwent complete degradation in about 53 min. In contrast, the microstructures aggregated by the permanent magnet required about 1 h and 45 min for complete degradation of the Ca2+-hardened alginate in PBS. This result indicates that the microstructures fixed by the permanent magnet exhibit slower degradation due to limited PBS exchange during the degradation of Ca2+-hardened alginate, resulting in sustained high Ca2+ concentration within the densely packed microstructure region.
In addition, we have quantified the drug release profiles of the chemotherapeutic agent 5-fluorouracil (5-FU) from microstructures of different sizes and correlated them with their degradation behavior (Figure 3e and Figure S4). As shown in Figure S4a, the drug loading capacity of the microstructures was found to be size-dependent: 3.18 µg for the 200 µm structure, 7.58 µg for the 400 µm structure, and 9.01 µg for the 500 µm structure. The cumulative release profiles over time, presented in Figure S4b, indicate that the 200 µm and 400 µm structures released approximately 90% of their drug content within 30 min, whereas the 500 µm structure released only about 57% over the same period. These differences in release kinetics are consistent with the degradation profiles shown in Figure 3e and Figure S4c. The smaller microstructures (200 µm and 400 µm) underwent near-complete degradation within 30 min, facilitating rapid drug release. In contrast, the 500 µm structure degraded at a slower rate, leading to a more gradual release profile. These results demonstrate that the release kinetics of the microstructures are closely correlated with their degradation behavior, supporting the potential for tunable, size-dependent drug delivery in therapeutic applications. The size-dependent and magnetically tunable biodegradation behavior of the microstructures enables precise temporal control of therapeutic release in the joint cavity. Faster degradation of smaller, non-targeted structures minimizes off-target retention, while magnetically fixed microstructures at the cartilage lesion site ensure prolonged therapeutic action. This dual-mode degradation behavior can be leveraged to enhance both the safety and efficacy of arthroscopic cartilage-targeted drug delivery.
3.3. Magnetic Mobility of the Magnetically Actuated Microstructure
Before targeting the microstructures, magnetic mobility tests were conducted using microstructures of various sizes, considering their magnetic properties, which are influenced by external magnetic field strength, material magnetization, and size. In particular, we investigated the mobility of the microstructures under controlled magnetic field strength and direction using the EMA system. The EMA system consists of square-shaped electromagnetic coils arranged in pairs along the x-, y-, and z-axes (Figure 4a). This configuration allows for precise control of the magnetic field strength and direction in three-dimensional space, and it can generate a uniform magnetic field of up to 30 mT within the workspace. In the mobility test, we employed a rolling-based actuation method using a rotating magnetic field rather than a gradient magnetic field. Although spatial magnetic field gradients generally exist in coil-based magnetic field generation systems, the EMA system used in this study maintains a stabilized and uniform magnetic field gradient beyond a certain distance. Thus, the magnetic field gradient was not utilized as a control variable for locomotion of the microstructure. To induce the rolling motion of the microstructure, we applied the magnetic field strength and rotation frequency under a rotating magnetic field as the main control variables.
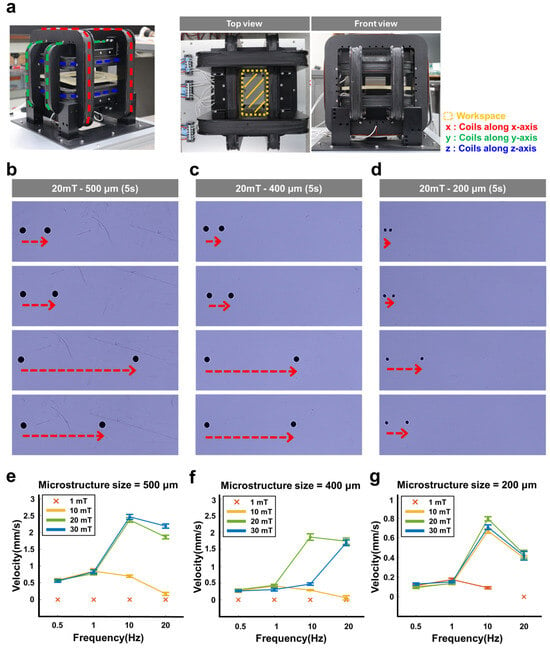
Figure 4.
Magnetic mobility of the magnetically actuated microstructure. (a) Experimental setup of an EMA system for generating an external magnetic field to evaluate the mobility of the microstructures. (b–d) Images and (e–g) graphs showing the velocity of the magnetically actuated microstructures with different sizes (~200 µm, ~400 µm, and ~500 µm) in five seconds according to the rotating magnetic field strengths (1, 10, 20, and 30 mT) and rotation frequencies (0.5, 1, 10, and 20 Hz). (b) ~500 µm, (c) ~400 µm, and (d) ~200 µm sized microstructures at 20 mT. ‘×’ markers indicate conditions under which the microstructures exhibited no observable movement (velocity = 0 mm/s).
The mobility of microstructures with various sizes (~200 µm, ~400 µm, and ~500 µm) was evaluated under different magnetic field strengths (1, 10, 20, and 30 mT) and rotation frequencies (0.5, 1, 10, and 20 Hz) (Figure 4b–d for 500 µm sized microstructure; Figure S5 for 400 µm and 200 µm sized microstructures). As shown in Figure 4e–g, at a magnetic field strength of 1 mT, all microstructures showed no mobility regardless of their size, which indicates that the external magnetic field strength is not sufficient for the magnetic mobility of the microstructures. Under a 10 mT magnetic field, the ~200 µm sized microstructure showed increasing speed across rotation frequencies from 0.1 to 10 Hz, followed by a decrease in speed beyond 10 Hz. The ~400 µm and ~500 µm sized microstructures exhibited increasing speed from 0.5 to 1 Hz, after which their speed declined at frequencies above 1 Hz.
Under a magnetic field strength of 30 mT, the ~200 µm and ~500 µm sized microstructures showed similar mobility patterns to those observed at 10 mT and 20 mT (Figure 4e–g). In contrast, the ~400 µm sized microstructure exhibited steadily increasing speed over the full frequency range from 0.5 to 20 Hz. As a result, at a magnetic field strength of 30 mT and a rotation frequency of 10 Hz, the ~200 µm, ~400 µm, and ~500 µm microstructures achieved maximum velocities of 2.4 mm/s, 1.8 mm/s, and 0.8 mm/s, respectively.
Overall, the velocity of the microstructures gradually increased with the rotation frequency of the magnetic field but began to decrease when the frequency exceeded the microstructure’s step-out frequency. Here, the step-out frequency represents the frequency response limit of a magnetic object under an external rotating magnetic field and depends on both the magnetization and the volume of the object [23,24]. Therefore, as the magnetic field strength increases, the magnetization of the microstructures also increases, leading to a higher step-out frequency. Similarly, larger microstructures exhibit higher step-out frequencies due to their increased volume.
Furthermore, the magnetic responsiveness and motility of the microstructures are governed not only by their physical dimensions but also by the concentration of MNPs. As the MNP concentration increases, the overall magnetization of the microstructures is enhanced, resulting in greater magnetic torque and, consequently, faster locomotion under identical magnetic field conditions (Figure S6). These findings indicate that both the microstructure size and the MNP concentration are key parameters in tuning magnetic actuation and mobility performance.
3.4. In Situ Targeting and Degradation Behavior of the Microstructures in a Knee Joint Phantom
Using the rolling locomotion of the microstructures driven by external magnetic field control from the EMA system, we evaluated the precise magnetic manipulability of the microstructures. A 500 µm sized microstructure was guided along a desired trajectory under a rotating external magnetic field with a strength of 30 mT and a frequency of 10 Hz without considering the magnetic field gradient. (Figure 5a and Supplementary Video S5). As a result, the microstructure was able to move while precisely controlling its speed, direction, and position under magnetic field modulation.

Figure 5.
Targeting and degradation behavior of the magnetically actuated microstructure. (a) Magnetic rolling locomotion of a single microstructure along a pre-selected pattern (‘CHOSUN’). (b–d) Snapshots showing the targeting and biodegradation of (b) one, (c) five, and (d) ten microstructures (~500 µm) within a 3D-printed knee joint phantom filled with PBS. (e) Summary of the degradation time of clustered microstructures (~500 µm) according to the number of microstructures. In all figures, the time is indicated on each figure in the minutes/seconds format.
As a proof of concept, we verified the applicability of the proposed microstructures within a damaged knee joint by performing targeting and degradation tests inside a 3D-printed knee joint phantom. The knee cartilage phantom was filled with PBS to simulate an environment similar to that of arthroscopic knee cartilage procedures. We guided one, five, and ten 500 µm sized microstructures to the damaged cartilage site inside the knee joint phantom under an external rotating magnetic field with a strength of 30 mT and a frequency of 10 Hz (Figure 5b–d and Supplementary Video S6). Simultaneously, we observed the degradation of the microstructures after reaching the target site within the phantom. As a result, when a single microstructure was used, it reached the target site in approximately 11 s and fully degraded in about 53 min (Figure 5b and Supplementary Video S6). In contrast, when five microstructures were used, the time required to reach the target increased to 36 s, and the degradation time also increased to approximately 1 h and 30 min (Figure 5c and Supplementary Video S6). When ten microstructures were used, the delivery time to the target site further increased to about 2 min and 2 s, and even after 2 h, complete degradation had not yet occurred (Figure 5d and Supplementary Video S6).
From a targeting perspective, an increase in the number of microstructures resulted in prolonged targeting time, and some structures failed to accurately reach the intended site (Figure 5e). Despite being fabricated under identical conditions, minor variations in surface friction, MNP loading, and particle size led to differences in locomotion speed, ultimately affecting targeting performance. These findings suggest that minimizing the travel distance of microstructures is a key strategy for enhancing targeting efficiency. Therefore, we expect that positioning the catheter as close as possible to the cartilage lesion may facilitate short-range targeting, thereby improving delivery efficiency while reducing the risk of adverse effects from non-targeted structures.
Furthermore, in terms of biodegradation, an inverse relationship was observed between the number of microstructures and the overall degradation rate. This delayed degradation appears to result from limited ion exchange with PBS when multiple microstructures are densely packed, leading to sustained high Ca2+ concentrations within the microstructures. This behavior is consistent with the results from degradation tests performed under magnetic fixation. Interestingly, non-targeted microstructures, being spatially dispersed within the phantom, undergo more active ion exchange with the surrounding PBS, enabling faster degradation. This characteristic may help reduce adverse effects such as inflammation caused by residual materials. In contrast, the targeted microstructures that aggregate at the lesion site degrade more slowly, potentially allowing sufficient time for sustained therapeutic release.
4. Discussion
We demonstrated the feasibility of using magnetically actuated alginate-based microstructures as a biodegradable and targetable platform for knee cartilage regeneration. The system leveraged Ca2+-hardened alginate hydrogels embedded with MNPs, enabling precise magnetic guidance under an EMA system and controlled degradation through ionic exchange in physiological environments.
A key finding of this work is the tunable biodegradation behavior of the microstructures, which was found to be strongly dependent on both particle size and spatial density. Smaller microstructures exhibited accelerated degradation due to enhanced surface-to-volume ratios and more efficient ion exchange with PBS, whereas densely packed clusters of microstructures showed delayed degradation. This phenomenon, also observed under magnetic fixation conditions, is likely attributed to reduced PBS penetration and sustained localized Ca2+ concentrations within the aggregated region. Importantly, such dual-mode degradation characteristics can be strategically exploited: rapidly degrading, non-targeted microstructures are effectively cleared from the joint cavity, minimizing the risk of inflammation and off-target effects, while aggregated microstructures at the lesion site ensure prolonged therapeutic release.
In terms of in vivo biodegradation of MNPs, iron oxide-based nanoparticles gradually degrade in vivo, releasing iron ions (Fe2+ and Fe3+), which are subsequently processed through natural iron metabolism pathways, such as transferrin and ferritin [25]. The magnetic nanoparticles used in this study are also based on iron oxide, and it is therefore expected that the released iron ions would similarly be managed via these metabolic routes. In addition, the safety of iron oxide-based magnetic microrobot systems for cartilage regeneration has been validated in previous research [9]. The iron oxide used in this study was employed at substantially lower amounts compared to the clinical dosage for iron deficiency treatment. Accordingly, the risk of iron accumulation and potential side effects is considered to be minimal. Importantly, the magnetic nanoparticles in this system were designed to allow the aggregated iron oxide structures to degrade easily within physiological conditions, thereby promoting smooth integration into iron metabolism pathways. This biodegradation mechanism is intended to minimize long-term retention and reduce the possibility of adverse biological responses.
In this study, a concentration of 3% MNPs was chosen as the optimal condition, taking into account the balance between size, mobility, and biodegradation. At 1% MNPs, the instability in microstructure formation resulted from the inability of the droplets to retain a stable spherical shape during the centrifugal fabrication process (Figure S6). On the other hand, at higher concentrations of 5% and 10% MNPs, the locomotion speed increased excessively, which hindered precise control of the microstructures. Additionally, as shown in Figure S7, the degradation time did not show significant differences among the tested concentrations, suggesting that 3% MNPs provides the most appropriate balance between effective locomotion and metabolic safety.
The EMA system used in this study was designed to demonstrate the feasibility of microstructure guidance within a phantom model under controlled, uniform conditions. While this setup is suitable for initial validation, several challenges are anticipated when translating the system for in vivo applications. First, the electrical and magnetic properties of biological tissues can attenuate or distort externally applied magnetic fields, potentially compromising the precision of microstructure guidance and locomotion. Second, in vivo environments introduce additional complexities, such as joint fluid dynamics, blood flow, and irregular tissue structures, which may alter or disrupt the expected trajectories of the microstructures. Specifically, the complex three-dimensional topology and the heterogeneous surface of knee cartilage may hinder accurate delivery and reduce targeting efficiency. To address these limitations, further validation using more physiologically relevant in vitro models is planned. For instance, constructing a flow system that mimics the viscosity and composition of synovial fluid or integrating stem cell-loaded microstructures to observe biological responses could serve as intermediate steps toward in vivo application. This phased approach would allow for the identification and mitigation of technical barriers in advance, ultimately facilitating safer and more effective clinical translation of the EMA system.
Building on these findings, we investigated the magnetic mobility and targeting efficiency of the microstructures under varying magnetic field conditions. The experimental results confirmed that the microstructures could be precisely navigated within a phantom joint environment using a rotating magnetic field of 30 mT and 10 Hz, with their locomotion behavior modulated by size, MNP content, and rotational frequency. Notably, an increased number of microstructures resulted in a decline in targeting accuracy, likely due to minor fabrication-induced variations in particle size, surface properties, and magnetic loading, which induced speed heterogeneity during locomotion. These findings highlight the importance of minimizing the delivery path and optimizing catheter positioning to enhance targeting fidelity, especially under minimally invasive conditions.
Compared to existing microcarrier systems composed of synthetic polymers such as PLGA and PCL [9,10,11,12,13], the proposed alginate-based microstructures offer significant advantages in terms of biodegradability, biocompatibility, and temporal control over therapeutic release. The use of naturally derived alginate, combined with Ca2+-mediated ionic gelation and degradation, provides a tunable and safer alternative for intra-articular applications.
Furthermore, the centrifugal microfabrication process employed in this study offers both scalability and flexibility, enabling the production of microstructures in a range of sizes tailored to the extent and geometry of cartilage defects. This customizable fabrication strategy allows the microstructures to be matched precisely to the specific anatomical features and dimensions of the damaged cartilage area in individual patients. Such patient-specific customization is expected to enhance targeting efficiency, improve fixation stability at the lesion site, and optimize therapeutic outcomes while minimizing off-target diffusion and undesired drug accumulation. For future clinical applications, the ability to adjust the size and number of microstructures according to individual variations in cartilage thickness, defect size, and anatomical structure will be crucial for achieving personalized and effective regenerative treatments. However, the current centrifuge-based small-batch fabrication process has inherent limitations in terms of scalability, uniformity, and reproducibility. In particular, centrifugal droplet formation can lead to uneven particle distribution, especially under high rotational speeds, resulting in size variability and reduced targeting accuracy. To address these challenges, we are exploring alternative fabrication methods such as droplet-based microfluidic systems, high-speed spray-solidification processes, and continuous production platforms incorporating automated magnetic separation. Among these, microfluidic systems offer precise control of fluid flow within microchannels, enabling stable and consistent generation of uniform-sized microstructures by adjusting variables such as flow rate, viscosity, and channel dimensions. Although the initial setup costs for these systems may be relatively high, they provide significant advantages, including higher production rates, improved size uniformity, and greater scalability compared to the centrifugation method. In the long term, these approaches represent practical and scalable manufacturing strategies that could not only reduce production costs but also enhance the consistency and targeting performance of the microstructures, thereby facilitating their clinical translation.
Despite the promising results, several limitations should be addressed in future studies. The current evaluation was conducted in a controlled in vitro environment, and in vivo studies are required to validate the therapeutic efficacy, immune response, and degradation kinetics under physiological conditions. We are currently planning animal studies to assess magnetic targeting efficiency and biodegradation kinetics in more physiologically relevant settings. Furthermore, we are actively pursuing collaborative efforts with hospitals and biomedical research institutions to obtain stem cells and establish protocols for in vivo cartilage regeneration studies based on cell transplantation. These future steps will build upon the findings of the current work and help advance the clinical translation of the proposed system. Furthermore, advanced magnetic control strategies, such as spatially varying field gradients or closed-loop feedback systems, were studied to enable individualized control of multiple microstructures in complex anatomical settings.
5. Conclusions
In this study, we proposed a biodegradable, magnetically actuated microstructure for targeted knee cartilage regeneration. The microstructures were prepared using alginate hydrogel crosslinked with Ca2+ and embedded with MNPs, enabling precise control under external magnetic fields generated by the EMA system. The proposed microstructures exhibited tunable sizes via centrifugal micro-nozzle processing and underwent degradation in PBS through ion exchange, where Ca2+ crosslinks were replaced by Na+. Biodegradation tests confirmed that smaller microstructures degraded more rapidly due to enhanced ionic exchange, while clustered microstructures showed delayed degradation. Targeting tests in a knee joint phantom demonstrated that the number of microstructures affected both delivery efficiency and degradation behavior. Single microstructures reached the target site quickly and degraded within an hour, whereas multiple microstructures required more time to localize and degraded more slowly, likely due to limited ion exchange in dense clusters. Importantly, the densely packed targeted microstructures degraded slowly at the lesion site, allowing for sustained drug release, while the non-targeted microstructures disperse and degrade rapidly, minimizing the risk of inflammatory side effects. These results highlight the potential of the proposed system as a minimally invasive site-specific drug delivery platform for cartilage repair. Future work will focus on further improving delivery precision and therapeutic outcomes by optimizing the injection route and improving the magnetic control strategy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/act14050232/s1, Video S1: Magnetic response of the magnetically actuated microstructures; Video S2: Degradation behavior of the magnetically actuated microstructures in DW and PBS; Video S3: Size-dependent degradation behavior of magnetically actuated microstructures in PBS; Video S4: Degradation behavior of magnetically actuated microstructures by magnetic fixation; Video S5: Magnetic locomotion of the magnetically actuated microstructure along a pre-selected pattern (‘CHOSUN’); Video S6: Targeting and degradation tests of the magnetically actuated microstructures in a knee joint phantom; Figure S1: SEM-EDS zoom-in images of microstructures with different sizes. Figure S2: (a) The standard curve showing the relationship between DOX concentration and UV absorbance at a wavelength of 480 nm. (b) The DOX loading capacity of microstructures according to their size. Figure S3: FT-IR analysis of MNPs, alginate, and microstructures. Figure S4: (a) The 5-FU loading capacity of microstructures according to their size. (b) Drug release of microstructures with different sizes over time. (c) Degradation behavior of drug-loaded microstructures depending on their size. Figure S5: Images showing the velocity of the magnetically actuated microstructures with different sizes (~200 µm, ~400 µm, and ~500 µm) in five seconds according to the rotating magnetic field strengths ((a) 10 and (b) 30 mT) and rotation frequencies (0.5, 1, 10, and 20 Hz). Figure S6: (a) Optical images of microstructures fabricated with different MNP concentrations (1%, 3%, 5%, and 10%). (b) Locomotion speed of 500 µm microstructures measured under a rotating magnetic field of 30 mT and 10 Hz, with different MNP concentrations (3%, 5%, and 10%). Figure S7: Degradation behavior of microstructures with different MNP concentrations (3%, 5%, and 10%). Scale bar, 10 mm.
Author Contributions
Conceptualization, G.M., S.Z. and G.G.; formal analysis, G.M.; investigation, G.M. and S.Z.; writing—original draft preparation, G.M., S.Z. and G.G.; writing—review and editing, G.G.; visualization, G.M. and S.Z.; supervision, G.G.; project administration, G.G.; funding acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research fund from Chosun University, 2024.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank the Medical Microrobot Center at Chonnam National University for providing facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tang, S.A.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.-M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Creamer, P.; Hochberg, M. Why does osteoarthritis of the knee hurt–sometimes? Rheumatology 1997, 36, 726–728. [Google Scholar] [CrossRef][Green Version]
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem cells in articular cartilage regeneration. J. Orthop. Surg. Res. 2016, 11, 42. [Google Scholar] [CrossRef]
- Nöth, U.; Steinert, A.F.; Tuan, R.S. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat. Clin. Pract. Rheumatol. 2008, 4, 371–380. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magnetic Steering of a Cluster of Bacterial Microswimmers along Pre-defined Trajectories. Nat. Commun. 2014, 5, 3119. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Ge, Z.; Yu, H. Magnetically Controlled Millipede Inspired Soft Robot for Releasing Drugs on Target Area in Stomach. IEEE Robot. Autom. Lett. 2024, 99, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Cheng, B.; Zhao, S.; Li, Y.; Kang, H.; Chen, S. Magnetic nanocarriers as a therapeutic drug delivery strategy for promoting pain-related motor functions in a rat model of cartilage transplantation. J. Mater. Sci. Mater. Med. 2021, 32, 37. [Google Scholar] [CrossRef]
- Go, G.; Jeong, S.-G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.; Nguyen, K.T.; Jin, Z.; Kim, C.-S.; Seo, Y.R. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020, 5, eaay6626. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.-H.; Sun, D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef]
- Schuerle, S.; Soleimany, A.P.; Yeh, T.; Anand, G.M.; Häberli, M.; Fleming, H.E.; Mirkhani, N.; Qiu, F.; Hauert, S.; Wang, X.; et al. Synthetic and Living Micropropellers for Convection-Enhanced Nanoparticle Transport. Sci. Adv. 2019, 5, eaav4803. [Google Scholar] [CrossRef]
- Pacheco, M.; Mayorga-Martinez, C.C.; Viktorova, J.; Ruml, T.; Escarpa, A.; Pumera, M. Microrobotic carrier with enzymatically encoded drug release in the presence of pancreatic cancer cells via programmed self-destruction. Appl. Mater. Today 2022, 27, 101494. [Google Scholar] [CrossRef]
- Szczęch, M.; Orsi, D.; Łopuszyńska, N.; Cristofolini, L.; Jasiński, K.; Węglarz, W.P.; Albertini, F.; Kereïche, S.; Szczepanowicz, K. Magnetically responsive polycaprolactone nanocarriers for application in the biomedical field: Magnetic hyperthermia, magnetic resonance imaging, and magnetic drug delivery. RSC Adv. 2020, 10, 43607–43618. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Go, G. Wireless Hybrid-Actuated Soft Miniature Robot for Biomedical Applications. Actuators 2024, 13, 341. [Google Scholar] [CrossRef]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Havrdova, M.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Horakova, J. In vitro cytotoxicity analysis of doxorubicin-loaded/superparamagnetic iron oxide colloidal nanoassemblies on MCF7 and NIH3T3 cell lines. Int. J. Nanomed. 2015, 10, 949–961. [Google Scholar] [CrossRef]
- Zoppellaro, G.; Kolokithas-Ntoukas, A.; Polakova, K.; Tucek, J.; Zboril, R.; Loudos, G.; Fragogeorgi, E.; Diwoky, C.; Tomankova, K.; Avgoustakis, K. Theranostics of epitaxially condensed colloidal nanocrystal clusters, through a soft biomineralization route. Chem. Mater. 2014, 26, 2062–2074. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Santos, E.C.; Dos Santos, T.C.; Guimarães, R.B.; Ishida, L.; Freitas, R.S.; Ronconi, C.M. Guanidine-functionalized Fe3O4 magnetic nanoparticles as basic recyclable catalysts for biodiesel production. RSC Adv. 2015, 5, 48031–48038. [Google Scholar] [CrossRef]
- Eisavi, R.; Ahmadi, F. Fe3O4@SiO2-PMA-Cu Magnetic Nanoparticles as a Novel Catalyst for the Synthesis of 1,2,3-Triazoles. Sci. Rep. 2022, 12, 15980. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Chen, H.; Law, J.; Wang, Y.; Chen, Z.; Du, X.; Fang, K.; Wang, Z.; Duan, F.; Sun, Y.; Yu, J. Active microgel particle swarms for intrabronchial targeted delivery. Sci. Adv. 2025, 11, eadr3356. [Google Scholar] [CrossRef]
- Diller, E.; Giltinan, J.; Sitti, M. Independent control of multiple magnetic microrobots in three dimensions. Int. J. Robot. Res. 2013, 32, 614–631. [Google Scholar] [CrossRef]
- Mahoney, A.W.; Nelson, N.D.; Peyer, K.E.; Nelson, B.J.; Abbott, J.J. Behavior of rotating magnetic microrobots above the step-out frequency with application to control of multi-microrobot systems. Appl. Phys. Lett. 2014, 104, 144101. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).