1. Introduction
Limb loss, often resulting from trauma, disease, or congenital conditions, affects millions globally, with the World Health Organization estimating 57.7 million traumatic amputations in 2017 [
1,
2,
3]. Affordable and functional prostheses remain inaccessible for many, particularly in low-resource settings, necessitating cost-effective solutions like the one proposed here [
4]. In any case, this loss causes a significant deficit in a person’s quality of life, making it difficult to carry out everyday tasks and diminishing their sense of independence and self-esteem. Ref. [
5] highlight the importance of inclusive digital platforms for the customization of prostheses, facilitating access to personalized and low-cost devices. When a complication makes elective removal necessary, a surgical amputation is performed as a planned procedure [
6,
7]. According to [
7], a traumatic amputation is an emergency amputation that takes place after a traumatic event, such as a vehicle accident, or due to disease, infection, cancer, vascular disorders or congenital anomalies. Ref. [
8] explore the impact of human factors on prosthesis design, promoting social acceptance and integration. This process can be used for a variety of purposes, such as replacing amputated limbs, repairing fractured joints, replacing missing teeth and rebuilding a support structure. Recent advancements in electromyography (EMG) and additive manufacturing offer promising avenues for cost-effective solutions. EMG-controlled prostheses use bioelectric signals from muscle contractions to drive motorized movements, enhancing user autonomy [
9]. Meanwhile, 3D printing enables personalized designs at reduced costs [
10]. In contemporary medicine, prostheses are essential because they provide personalized solutions to improve the autonomy and quality of life of people who need to replace missing body parts. Most prostheses use Electromyography (EMG) sensors as sensors that identify electromyographic signals produced by muscle contractions and are used to operate myoelectric prostheses. These electric prostheses send precise instructions to electric motors via an external controller, such as external buttons, which causes the device to move [
4,
9,
11].
As a part of a broader project, this study introduces a novel combination of low-cost 3D-printed components, off-the-shelf electronics (e.g., Arduino Uno, BITalino sensors), and a simplified EMG control system to create a functional 1-DoF prosthetic hand. Unlike commercial systems prioritizing multi-DoF complexity, our approach focuses on accessibility, ergonomic design mirroring the intact limb (±2 mm accuracy), and a minimal learning curve, making it suitable for underserved populations (
Figure 1). The prototype achieves a cost of approximately USD 50, significantly lower than commercial alternatives like the Ottobock Bebionic [
12] (~USD 20,000).
This paper is structured as follows:
Section 2 reviews relevant literature;
Section 3 details materials and methods;
Section 4 presents results and discussion; and
Section 5 outlines conclusions and future directions. By addressing the global need for affordable prosthetics, this study contributes to rehabilitation technology and sets the stage for scalable, inclusive solutions.
2. Literature Review
Ref. [
13], analyze how additive manufacturing can reduce costs and improve integration between prostheses and users. This highlights the need for better prosthetic devices. Since the loss of an upper limb can have a major impact on daily activities and overall quality of life, the development of practical prosthetic devices needs to be a primary goal [
14].
According to [
15], surface or intramuscular electrodes can be used to capture EMG data, which are electrical impulses produced by muscle contractions. Before being processed further, the signals must first be filtered and amplified because they are often high frequency and low amplitude.
Research has indicated that the positioning and kind of electrodes have a major impact on signal quality. Although surface electrodes are often utilized and non-invasive, they could be affected by motion and noise artifacts [
16]. Although they offer more accurate signals, intramuscular electrodes are more intrusive and are not as frequently utilized in commercial applications [
17].
After being obtained, EMG signals need to be processed to derive useful characteristics that can be applied to the prosthesis control. Rectification, smoothing, and normalization are examples of common processing methods [
18]. Ref. [
5], present solutions for the digital personalization of prostheses, with a focus on inclusion and user-centered design. Prosthetic control precision and responsiveness have been improved by using more sophisticated algorithms, like machine learning and pattern recognition [
19].
Improvements in real-time performance and resilience of EMG-controlled systems have been the focus of recent developments in signal processing. Artificial neural networks and wavelet transforms are two methods that have demonstrated promise in managing the unpredictability of EMG signals [
20].
Direct control and pattern recognition-based control are the two main categories into which control systems for EMG prostheses fall. Direct control is adjusting the prosthesis’s movement proportionately by measuring the amplitude of the EMG signal [
17]. This approach is easy to use and intuitive, but its responsiveness and functionality might be restricted.
In contrast, machine learning algorithms are used in pattern recognition-based control to categorize various muscle activation patterns into distinct prosthetic movements [
21]. This method gives the user extra capability by enabling more intricate and organic movements. According to recent research, machine learning models like convolutional neural networks and support vector machines are good at increasing the accuracy of movement classification [
22].
The fluctuation of EMG signals resulting from electrode placement, muscle fatigue, and skin impedance is a major obstacle in the development of EMG-controlled prostheses. Research on ensuring reliable signal processing and acquisition under various circumstances is still essential [
23].
The user must undergo extensive training and adjust to the demands of an EMG-controlled prosthesis to utilize it successfully. Research has indicated that individuals require a certain amount of time to become proficient in producing steady EMG signals and adjust to the prosthesis’ feedback [
24]. These difficulties can be lessened by creating training protocols and adaptive control systems that are easy to use.
The study currently employs surface electrodes for EMG signal acquisition due to their non-invasiveness, ease of application, and cost-effectiveness, as seen in commercial systems like Ottobock’s Michelangelo hand [
14]. However, they are susceptible to motion artifacts and noise, potentially reducing signal reliability [
15]. In contrast, intramuscular electrodes, used in research-grade prostheses, offer higher precision by directly targeting muscle fibers but require invasive implantation, limiting practicality for widespread use [
16]. This trade-off prioritizes accessibility over precision in our design, aligning with the goal of affordability for low-resource settings. Compared to multi-DoF systems like the DEKA Arm [
25], which integrate advanced feedback and cost upwards of USD 50,000, this prototype’s single-DoF approach sacrifices functionality for simplicity and cost (~USD 50), positioning it as an entry-level solution.
Hardware advancements in upper limb prostheses include lightweight actuators (e.g., Maxon DC motors, USD 15,000), which enhance durability but increase costs (Ottobock, n.d.). Control strategies range from direct threshold-based systems, like our prototype, to proportional control in the i-Limb Quantum (USD 5000), which integrate 3D-printed components but require proprietary electronics, limiting scalability in resource-constrained settings [
10]. Our approach bridges this gap by using open-source hardware (Arduino, ~USD 10) and simplified control, sacrificing multi-DoF functionality for affordability.
Future research to be developed should be focused on the potential integration of EMG-controlled prostheses with cutting-edge technologies like robotics, artificial intelligence, and brain–machine interfaces. According to [
26], these technologies have the potential to improve prosthetic device use, responsiveness, and functionality. So, it is based on the presented works that the study presented in this paper arises.
3. Materials and Methods
This section describes the resources and methodologies employed to design, develop, and test the proposed myoelectric control system for the device. By using electromyographic signals to control motorized components, the system bridges the gap between biological signals and mechanical actuation.
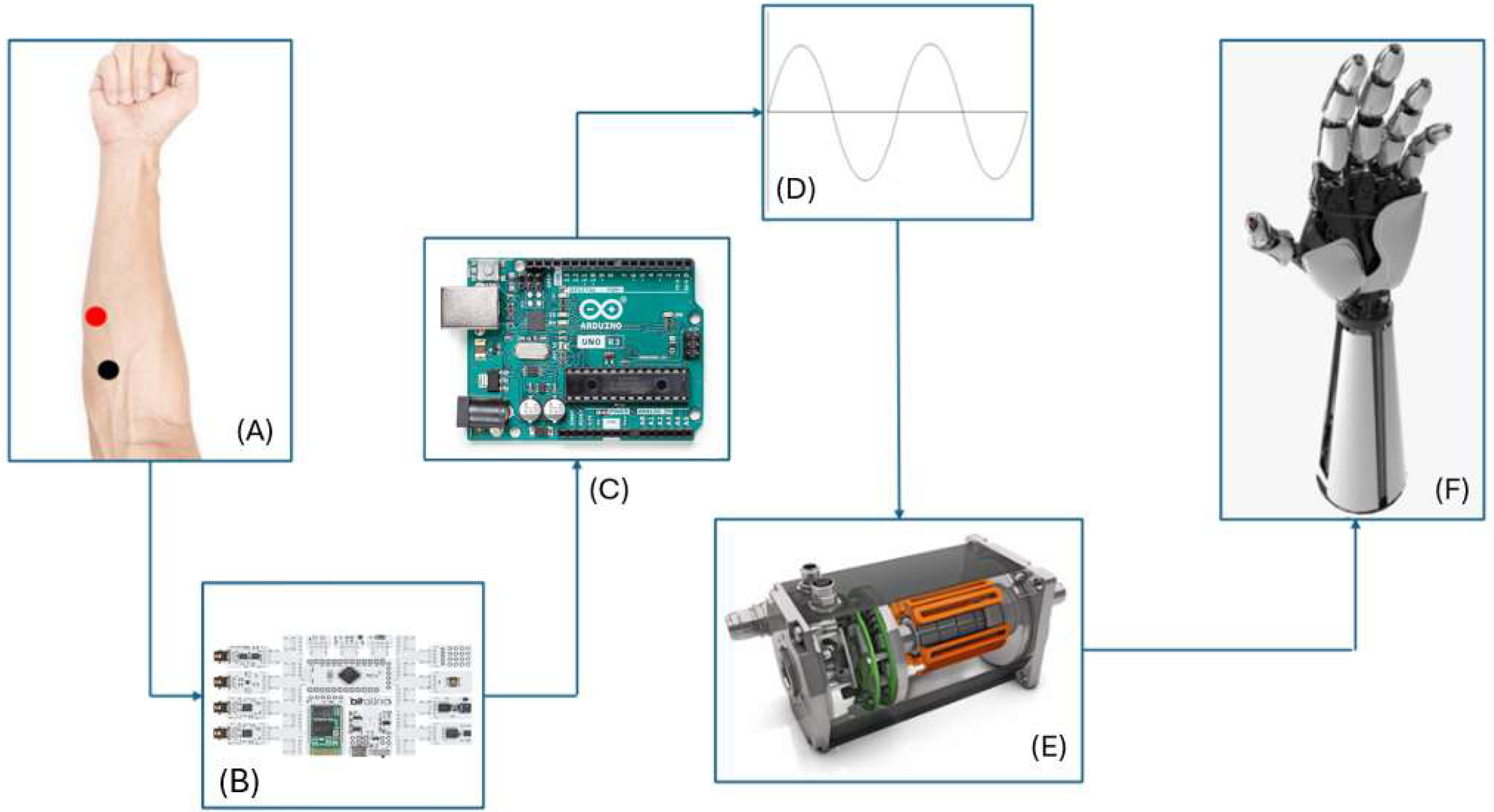
The development of this work followed a systematic approach, as represented by the flowchart shown in
Figure 1. The flowchart describes the essential steps for implementing the prosthesis, focusing on ergonomics, usability, and social inclusion.
The process begins with the placement of electrodes on the user’s muscles, enabling the capture of electrical signals generated during muscle contractions. These signals are transmitted to an EMG sensor, which amplifies and prepares them for further processing. The next step involves signal detection, where relevant patterns are identified to drive the desired movements. The detected signals are then processed by an Arduino Uno microcontroller, which interprets the data and converts them into actionable commands. This is complemented by an additional step of signal processing, ensuring that the commands are precise and suitable for subsequent stages. The movement of the prosthesis is controlled by an L293D motor driver, which receives processed commands and activates the motors. These motors perform the mechanical movements required for the operation of the prosthesis. The flowchart (
Figure 2) illustrates an integrated and sequential process, ensuring that each stage contributes directly to the functionality and efficiency of the prosthetic device. This methodology aims to optimize the interaction between the user and the device, fostering the development of technological solutions that meet the demands for ergonomics, functionality, and social inclusion.
The selection of components, including sensors, microcontrollers, and actuators, was guided by the goals of affordability, efficiency, and accessibility. The methods section details the step-by-step process used to integrate these elements, from signal acquisition to motor control, ensuring a seamless workflow for translating muscle activity into actionable outputs (
Figure 3). This systematic approach underpins the reliability and effectiveness of the work.
3.1. Materials and Equipment
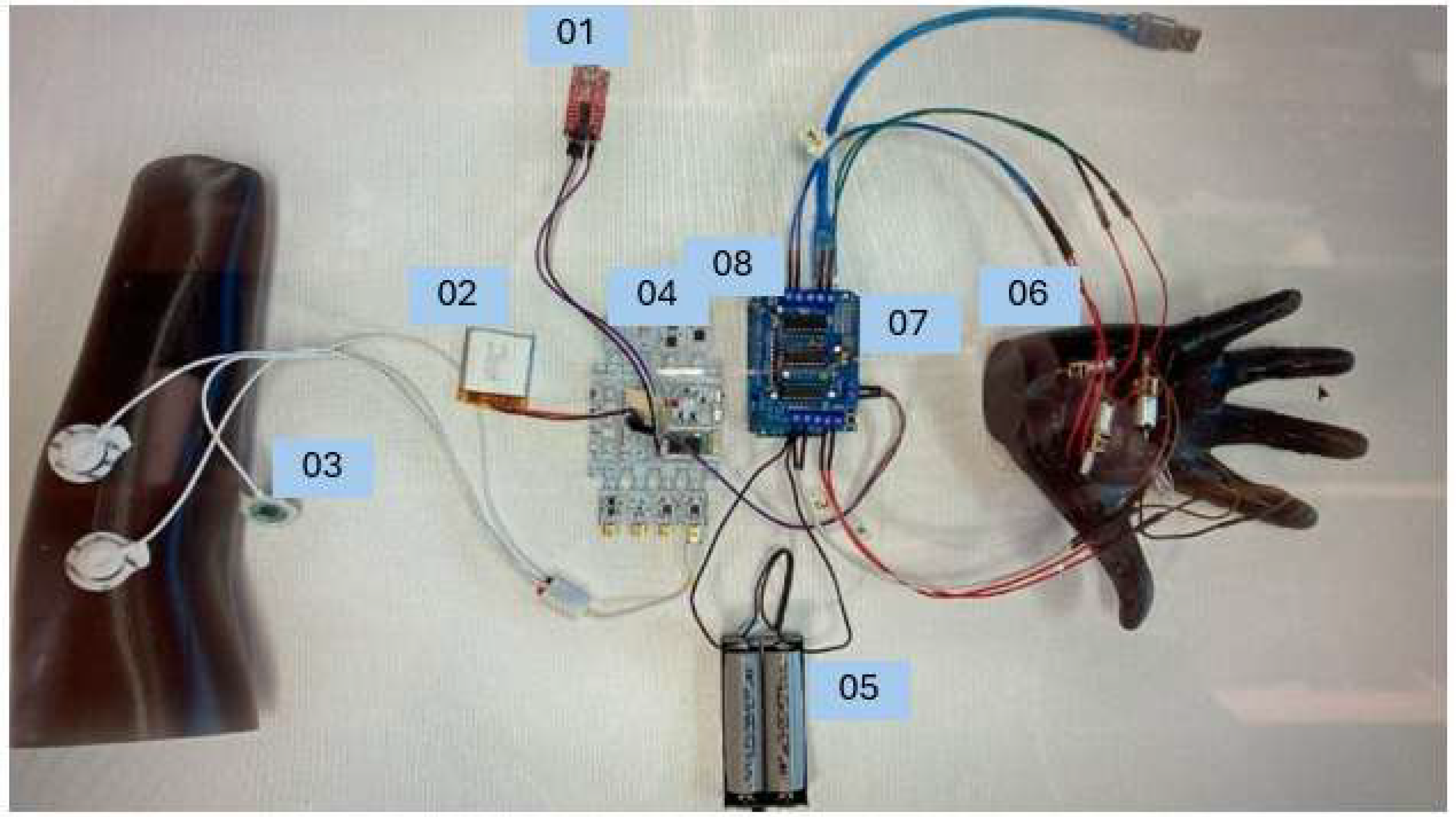
The following components represented in
Figure 4 were selected and tested to design and validate the functionality of the myoelectric control system. Each component played a critical role in the workflow, from signal acquisition to mechanical actuation, ensuring the seamless conversion of muscle activity into motor control.
These components were chosen based on their reliability, compatibility, and suitability for the intended application, forming the foundation of an effective and responsive system.
Arduino Uno (08): Central controller for processing EMG signals and managing motor actuation.
BITalino (r)evolution Board (04): Captures muscle activity via 3-lead pre-gelled Ag/AgCl surface electrodes (03), powered by a 3.7 V LiPo battery (02). Electrodes are placed over the muscle belly (signal), a distant site (reference), and a bony area (ground) for optimal signal quality (
Figure 5).
L293D Motor Driver (07): Controls the N20 micro motor (06, 6 V, 35 rpm) for smooth 1-DoF hand actuation.
External 12 V Power Supply (05): Powers the system and motors.
Programmer USB-serial (01): Facilitates Arduino programming.
The prototype’s estimated cost of ~USD 50 includes the Arduino Uno (USD 10), BITalino board (USD 20), L293D motor driver (USD 3), N20 motor (USD 5), 3D printing filament (USD 10), and electrodes (USD 2). This excludes labor, socket fabrication, and ancillary components like battery enclosures or wiring harnesses, which are planned for future iterations.
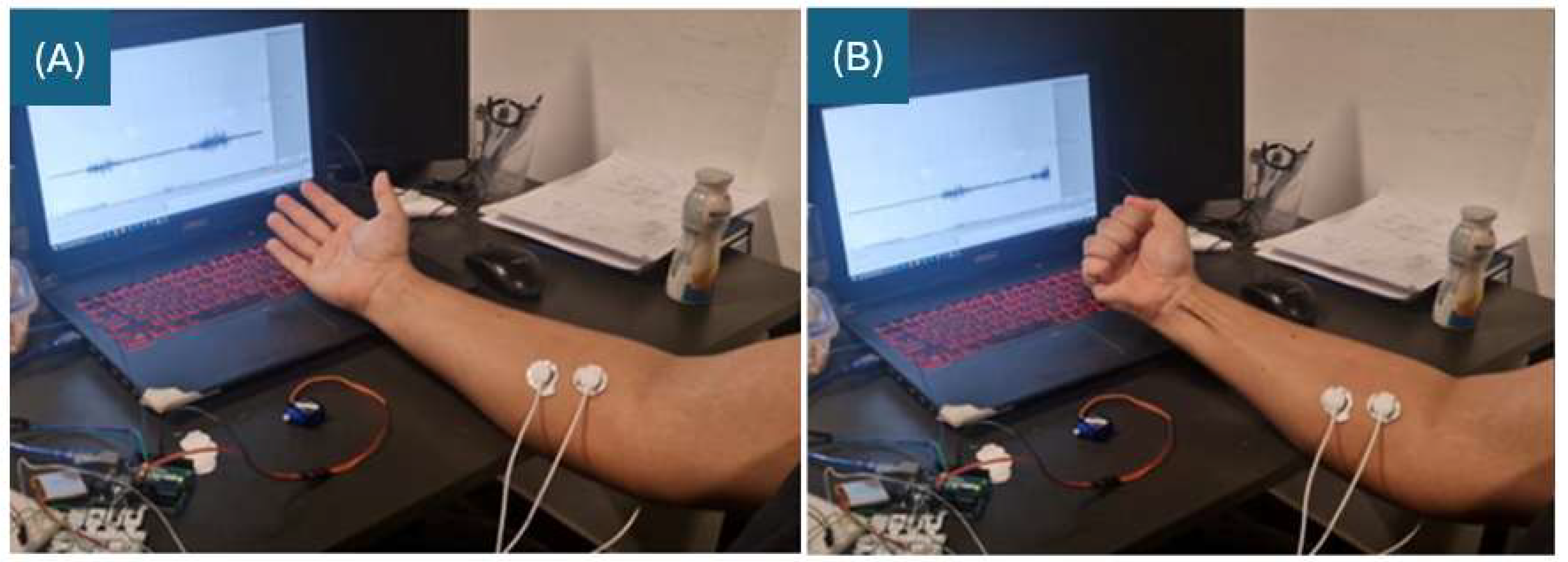
The prosthesis mirrors the user’s intact limb (±2 mm accuracy in length via 3D scanning) and is integrated into a breathable cotton sleeve, adjustable for seasonal comfort, enhancing wearability and aesthetics (see
Figure 5 [new figure of wearable design]).
The Arduino Uno, represented by number 08, is the main controller for receiving and analyzing the EMG signals measured by the BITalino Board as well as managing the electric motors that will provide movement of the prosthetic hand.
The BITalino revolution board, represented by number 04, records the electrical activity of muscles that are generated during the contraction of the arm from the user and includes a 3.7 V LiPo battery, represented by number 02.
The L298D Motor Driver Module, represented by the number 07, creates the possibility for the Arduino Uno to manage the direction and speed of the motors inside the prosthetic hand.
The system utilizes an L293D motor driver to control the DC motor. The prosthetic hand is actuated using an N20 micro motor with a 35 rpm reducer, ensuring smooth motion. The EMG signals are acquired using pre-gelled Ag/AgCl surface electrodes, selected for their reliable signal acquisition and minimal skin irritation.
Figure 2 was updated to reflect the correct motor driver.
The DC motors, N20 micro motor with a 35 rpm reducer, represented by the number 06, are responsible for converting electrical signals obtained by the EMG sensor into mechanical impulses that will provide the movement of the prosthesis.
The pre-gelled Ag/AgCl surface electrodes, represented by number 03, are applied directly to the skin to pick up muscle activity which is then fed into the EMG sensor.
Figure 5 represents the placement of the electrodes on the user:
Signal Electrode (Red Dot): Place directly over the muscle belly, along the muscle’s longitudinal axis.
Reference Electrode (Black Dot): Attach to a distant site to reduce electrical noise.
Ground Electrode (White Dot): Place on a nearby bone or non-muscular area to act as a neutral reference point.
The external 12 V Power Supply, represented by number 05, powers the system itself, the motors driver and the DC motors.
The prosthetic hand is designed as a one-degree-of-freedom (1-DoF) system where a DC motor controls the opening and closing of all fingers simultaneously.
3.2. Methods
According to the essential steps for implementing the prosthesis (
Figure 2), the workflow involves four main stages: A—signal acquisition, B—signal processing and calibration, C—decision-making, and D—actuation. These stages are described in the following.
3.2.1. A—Signal Acquisition
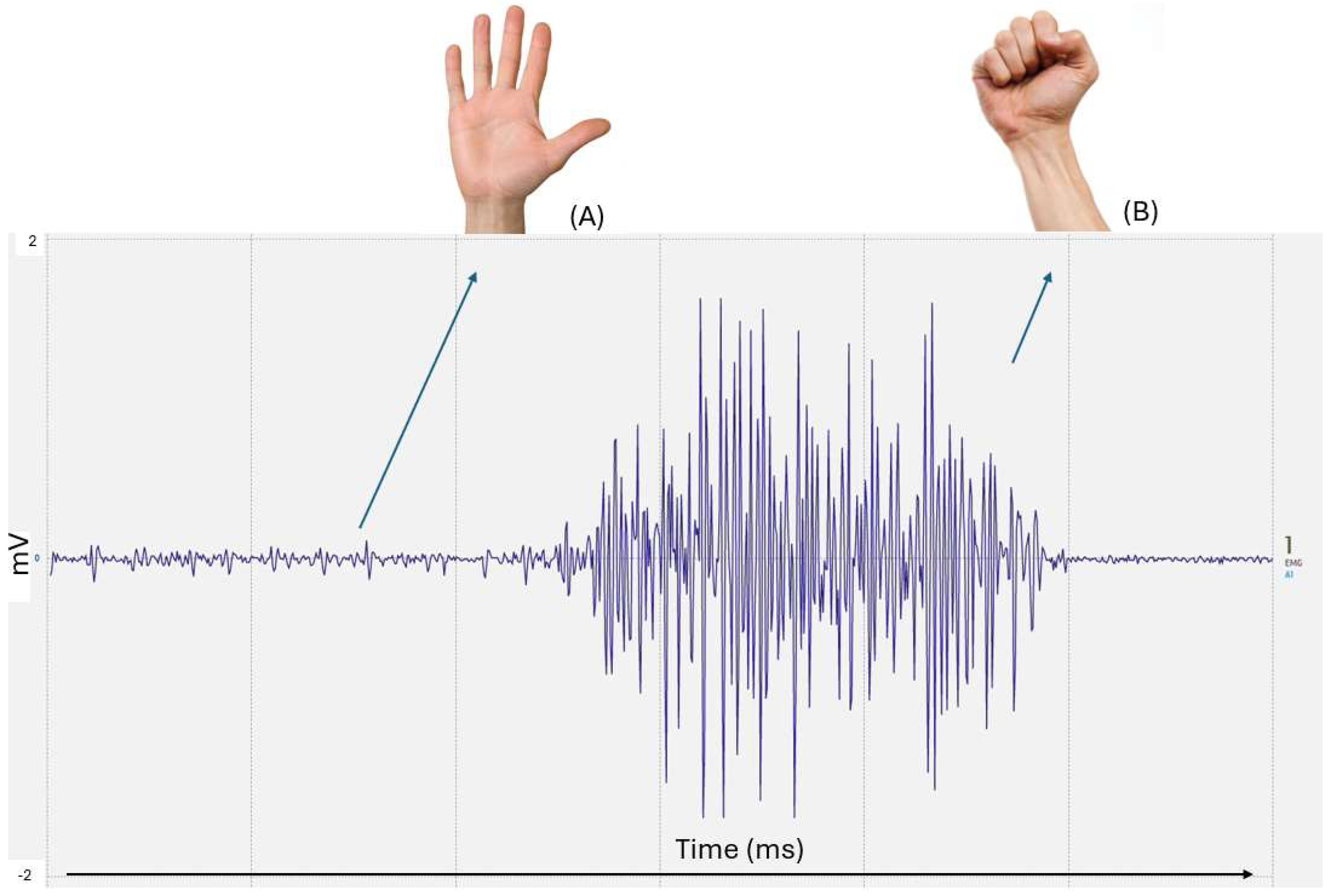
As previously referred, the EMG sensor is connected to the user through electrodes placed on the skin over the muscle group pretended. These electrodes are placed to ensure optimal detection of electrical signals generated during muscle contractions. The sensor captures the bioelectric signals produced by muscle activity (
Figure 6). These signals, which are of reduced SNR and complex, are amplified and conditioned by the EMG sensor to make them suitable for further processing. The sensor then converts the muscle activity into an analog signal that can then be used to control the prothesis. By transforming biological signals into usable electrical outputs, the EMG sensor functions as an interface between human physiology and technological systems.
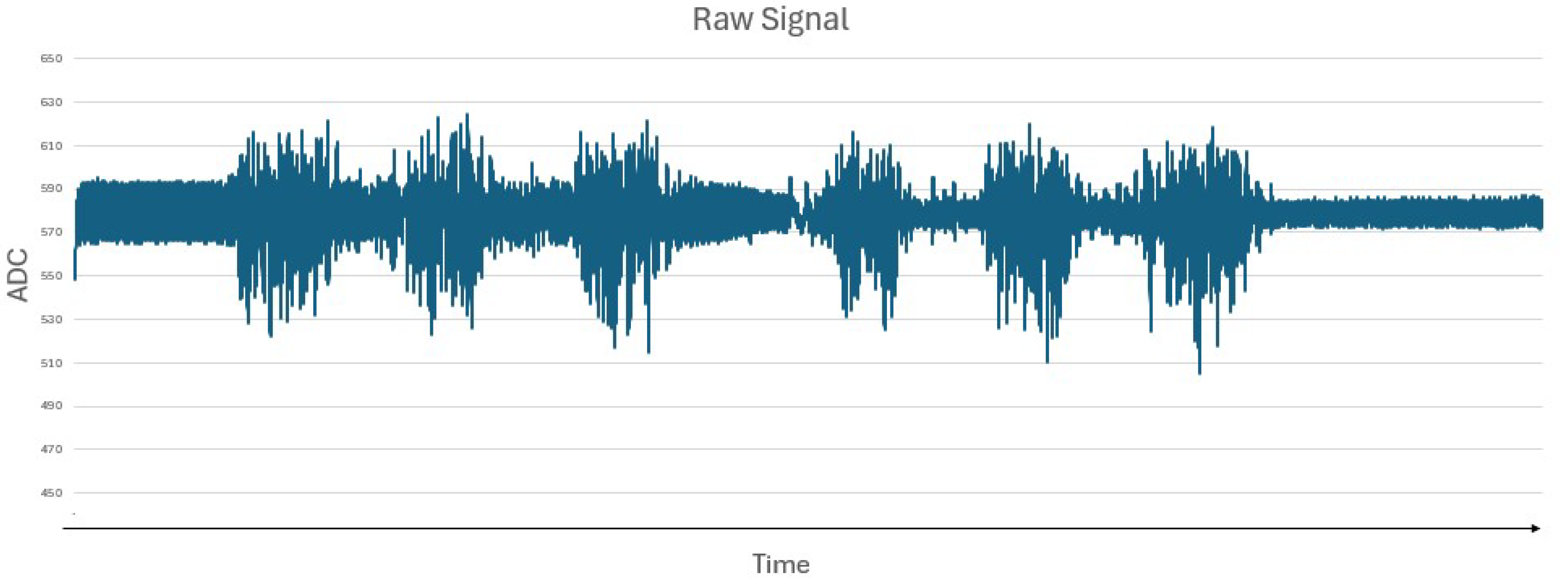
Figure 7 represents the raw signal obtained by the Bitalino Board when the user generates strength, indicating that higher peaks represent periods of greater muscle activity (contractions), and flatter intervals indicate periods of relaxation or absence of muscle activity.
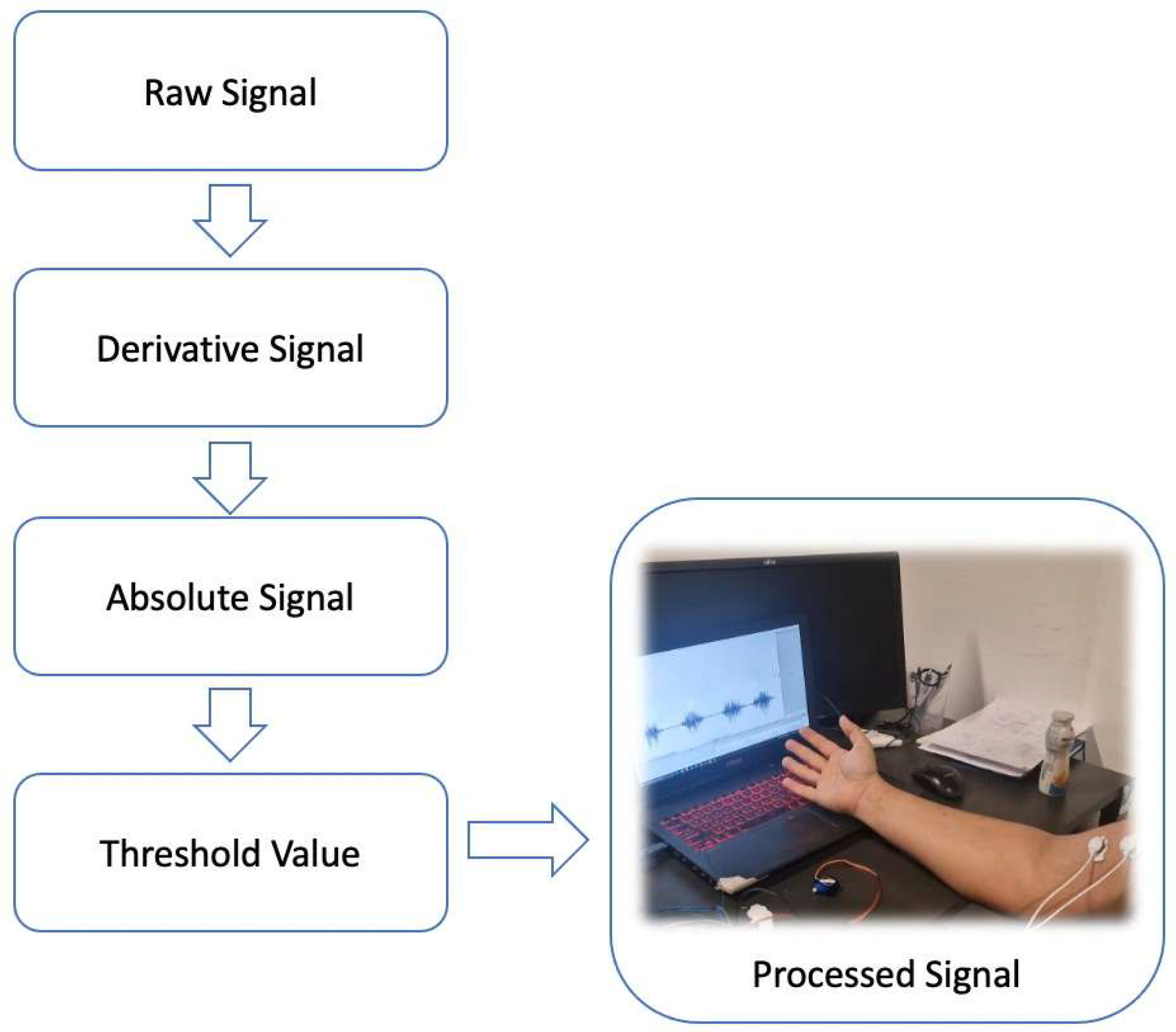
3.2.2. B—Signal Processing and Calibration
The output signal, which represents the electrical activity of the muscles, is transmitted to the analog input pin (A0) of the Arduino Uno, which serves as the central processing unit of the project, continuously monitoring the signal of the EMG sensor.
The EMG signal is transmitted to the Arduino Uno’s analog input (A0) at a 1000 Hz sampling rate. A central difference method (Δy/Δt = [y(t + 1) − y(t − 1)]/2) computes the signal derivative, reducing phase lag. A 20 Hz high-pass filter removes baseline drift, and 4× oversampling improves the signal-to-noise ratio by ~6 dB. The absolute value of the derivative ensures positive outputs for thresholding (
Figure 8).
3.2.3. C—Decision Making
The processed signal is then compared to a predefined threshold value, which acts as a benchmark for detecting muscle activation. If the signal intensity surpasses this threshold, it signifies active muscle engagement, triggering decisions within the system. This real-time processing capability enables the Arduino to effectively interpret muscle activity and translate it into movement for controlling the motors inside of the prosthetics.
Upon crossing the threshold, the Arduino generates a digital control signal and transmits it to the L293D motor driver module, identified by number 07 in
Figure 3.
The threshold for EMG signal activation was determined through empirical testing with multiple users. The initial trials involved setting a baseline of muscle activity at rest, followed by dynamic contractions at varying intensities. A threshold of 7 ADC units was chosen based on a statistical analysis of signal peaks during active contractions. The selection aimed to minimize false positives while ensuring responsiveness. Future iterations will incorporate adaptive thresholding techniques to personalize control sensitivity.
The motor driver module works as an intermediary between the Arduino and the connected motors, translating the control signals into precise electrical outputs that activate the motors. These actions involve starting, stopping, and changing the direction of the motors. By implementing this decision-making process, the system effectively converts muscle activity into mechanical responses, enabling real-time interaction with the prosthetics to achieve the desired functional outcomes.
3.2.4. D—Actuation
The L293D motor driver receives a control signal from the Arduino, interprets the signal and connects to the DC motors accordingly. Depending on the nature of the signal, the motor driver can command the DC motors to move forward, reverse their rotational direction, or come to a complete stop.
This interaction between muscle activity, detected by the EMG sensor, and motor actuation allows the system to convert biological signals into mechanical movements. By enabling intuitive and responsive control, this work enhances the user experience and broadens the potential for accessibility and independence in individuals requiring prostheses.
4. Results and Discussion
This section shows the outcomes of the myoelectric control system and offers an in-depth discussion of its performance, limitations, and implications.
4.1. I. Signal Acquisition and Processing
The EMG sensor successfully captured muscle activity, with the signals being transmitted to the Arduino Uno for processing. The system demonstrated consistent performance in detecting muscle activation when the subject contracted the muscle.
The obtained signal, represented by
Figure 9, captured by the EMG sensor, contains noise and unwanted variations. To achieve a clean signal, the derivative of the raw signal was calculated to highlight the changes in the wave signals,
Figure 10, to detect peaks or transitions. Then, to remove any negative values, the absolute value was applied to the derivative values,
Figure 11.
The analog signals were effectively converted into digital thresholds, allowing for accurate identification of muscle activity. Noise reduction methods, such as grounding and proper placement of electrodes, significantly improved signal clarity. The results observed confirm the findings of [
8], who emphasize how user-centered design can increase the social acceptance of prostheses.
4.2. II. Threshold Optimization
The threshold acts like a boundary or cut-off point, allowing the system to make decisions based on whether the signal exceeds or falls below the value 7 ADC.
If the EMG signal remains below the threshold (T < 7 ADC units), the prosthesis stays open. When muscle contraction is detected (T ≥ 7 ADC units), the motor engages to close the fingers. Once the desired grip is achieved, the motor stops to hold the position without additional power consumption. This ensures energy efficiency and prevents unwanted reopening of the hand.
Through iterative testing, the predefined threshold for muscle activation was optimized to minimize false positives and negatives. The results showed that a threshold of 7 ADC provided the best balance between sensitivity and specificity,
Figure 12, ensuring that motor actions were triggered only during intentional muscle contractions.
4.3. III. DC Motors Actuation
The DC motors responded promptly to the control signals, achieving forward, backward, and stop actions as programmed. The system exhibited a response time of approximately 150 ms from signal detection to actuation, with a false positive rate of 5% during 10-min trials with three non-amputee users, demonstrating efficiency for basic tasks.
4.4. IV. Signal Variation Analysis
Based on
Figure 13, the variation in the signal acquisition when the hand is closed and when it is open is distinctly identifiable from the rest of the signal. The different “waves” on the signal vary with the amount of strength that the user causes. The more force that the user makes, the more intensified the signal appears. This differentiation highlights the system’s capability to detect significant muscle activity patterns associated with gross motor movements,
Figure 14. Experimental validation included testing the prosthetic hand’s ability to respond to muscle contractions. However, occasional signal dropouts led to inconsistent movements, particularly in prolonged contractions. To prevent motor instability caused by temporary signal drops as seen in
Figure 12, a hysteresis mechanism can be implemented. The threshold of 7 ADC units (10-bit scale, 0–1023, ~34 mV at 5 V reference) balanced sensitivity and specificity. Oversampling at 4× increased resolution to a 12-bit equivalent, reducing quantization noise by 50%. A hysteresis mechanism (activation: 7 ADC, deactivation: 5 ADC) with a 5-sample moving average and 300 ms minimum duration mitigated motor stuttering (
Table 1).
In addition, ref. [
13] point out that additive manufacturing allows for greater geometric freedom and customization, improving rehabilitation.
4.5. V. User Feedback and Trials with Amputees
A limitation of the current study is the lack of real-world testing with amputees. Although preliminary trials with non-amputee users have demonstrated effective signal acquisition and motor control, the usability of the system in real-world scenarios remains to be verified. Future work will include clinical trials with amputees to assess usability, comfort and learning curve. In addition, sensory feedback systems such as the Funabot-Suit and Funabot-Sleeve will be considered to enhance the user experience [
27,
28].
4.6. VI. System Limitations
Despite the good results, the system still has several limitations, namely:
Noise Sensitivity: EMG signals were occasionally affected by external electrical noise, requiring improved shielding or filtering methods.
Electrode Dependency: The accuracy of signal acquisition heavily depended on the correct placement and adhesion of electrodes.
Scalability: The current design supports basic motor actions but would require additional development for complex, multi-joint movements.
Isolated Movement: The EMG sensor could not detect individual finger action. This limitation is likely due to smaller muscle groups involved in finger movements, resulting in weaker and almost non-existent EMG signals that cannot be distinguished from background noise.
Practical integration remains a challenge. The 3.7 V LiPo battery (500 mAh) lasts ~4 h under continuous use, necessitating a compact charging module and enclosure (est. USD 5–10). Wire routing requires flexible conduits embedded in the sleeve to prevent tangling, adding USD 2). Long-term durability tests under cold (0 °C), humid (90% RH), or dusty conditions are planned to ensure robustness.
4.7. VII. Comparison with Non-Electronic 1-DoF Systems
Non-electronic 1-DoF prostheses, such as body-powered hooks (e.g., Hosmer Model 5X, ~USD 500), rely on shoulder or elbow motion via cables, offering durability and low maintenance but requiring significant physical effort and limited precision. In contrast, our EMG-controlled system uses muscle signals for intuitive activation, reducing fatigue and improving user autonomy. However, it introduces battery dependency and potential signal noise, unlike the purely mechanical reliability of body-powered systems. Our approach trades simplicity for enhanced control, targeting users prioritizing ease of use over ruggedness.
5. Conclusions and Future Work
The objective of this work was to demonstrate the advancement and enhancement of electromyography (EMG)-controlled upper limb prostheses. It shows how bioelectric signals from muscles can be harnessed to create meaningful and transformative mechanical responses. The design and development of EMG-controlled upper limb prostheses have made significant strides, offering improved functionality and quality of life for amputees. The developed myoelectric control system successfully demonstrated the translation of muscle activity into mechanical motion. The findings emphasize the feasibility of creating low-cost, functional systems capable of significantly enhancing the quality of life for individuals requiring assistive technologies.
This preliminary study demonstrates a functional, low-cost (~USD 50) EMG-controlled prosthetic hand, integrating surface EMG sensors and 3D-printed components to achieve reliable 1-DoF movements. While not advancing EMG technology itself, it establishes a foundation for scalable, user-centered prosthetics, addressing accessibility gaps highlighted by WHO (2023). Using surface EMG sensors and a threshold-based system (7 ADC units, ~34 mV), the prototype reliably translates muscle contractions into 1-DoF movements with a ~150 ms response time, costing USD 50 compared to multi-DoF systems like the Ottobock Bebionic (USD 20,000). While effective for basic tasks, it lacks the sophistication of proportional control or AI-driven algorithms found in advanced prostheses.
The integration of 3D printing ensures customization, mirroring the intact limb’s proportions (±2 mm accuracy), and enhances affordability. However, limitations include noise sensitivity, single-DoF control, and untested amputee usability. This work highlights the feasibility of simple EMG interfaces for resource-limited settings, aligning with findings from [
10].
This study presented the design and implementation of the prototype using a simple threshold-based activation system. The results demonstrate that the system can effectively recognize muscle contractions and control basic prosthetic movements. While prior discussions have focused on control algorithms, it is also essential to compare existing prosthetic solutions. Modern myoelectric prostheses, such as the Ottobock Bebionic [
12] and the DEKA Arm [
25], integrate multi-degree-of-freedom (DoF) control and advanced sensor feedback. Compared to these commercial solutions, the prosthesis developed in this study is a low-cost alternative but currently lacks multi-DoF capabilities. Future improvements should aim to bridge this gap by incorporating additional control strategies.
Future enhancements will focus on: (1) AI integration, using convolutional neural networks to classify multi-DoF patterns [
22], targeting < 50 ms latency; (2) IoT connectivity for cloud-based analysis and tele-rehabilitation; and (3) wireless Bluetooth 5.0 sensors (<100 mW power consumption) to eliminate cabling. Clinical trials with amputees will assess comfort and learning curves, while adaptive thresholding and sensory feedback (e.g., Funabot-Suit) will improve precision and user experience. Challenges include managing latency, power efficiency, and cybersecurity in IoT systems. By addressing these, the prototype can evolve into a competitive, scalable alternative, fostering open-source collaboration for affordable myoelectric prosthetics.
The current system operates using a threshold-based activation approach, but machine learning offers significant potential to enhance control precision. Recent studies have applied convolutional neural networks (CNNs) and support vector machines (SVMs) to classify EMG patterns for finer motion control. While not implemented in the current prototype, future development will focus on training machine learning models to recognize complex movement patterns, enabling multi-degree-of-freedom (DoF) control for more natural prosthetic movement.
While the current implementation is not a technological breakthrough, it serves as a foundation for future development. The integration of adaptive thresholding, proportional control, and AI-driven pattern recognition in future iterations will enable more sophisticated control. Additionally, the study highlights the feasibility of using low-cost 3D-printed prostheses combined with simple EMG interfaces, making prosthetic solutions more accessible.
Despite the challenges related to signal variability, user training, and system robustness, ongoing research and technological advancements continue to drive progress in this field. The integration of artificial intelligence (AI), the Internet of Things (IoT), and wireless communication will significantly improve prosthetic functionality. AI will refine EMG signal classification, allowing for adaptive control mechanisms that learn user habits over time. IoT connectivity will enable remote monitoring and adjustments, facilitating tele-rehabilitation and clinician-guided optimization. Wireless EMG sensors will improve user comfort by eliminating the need for physical electrode connections. However, challenges such as latency, power consumption, and cybersecurity must be addressed in future research.
Future iterations will leverage low-cost microcontrollers with higher computational capacity (e.g., ESP32, ~USD 5) to implement convolutional neural networks for multi-DoF pattern recognition, as demonstrated by [
22]. This will enable finer control (e.g., individual finger movements) without significantly increasing costs. Additionally, integrating low-power Bluetooth 5.0 modules (<USD 3) will support wireless EMG sensors, enhancing portability while maintaining affordability.
Moreover, future work will focus on integrating a modular socket design with embedded wiring channels and a snap-fit electronics compartment, maintaining costs below USD 100. Battery life will be extended using low-power modes and higher-capacity cells (1000 mAh, ~USD 5), targeting 8-h operation.
Through these advancements, the system can evolve into a more robust and competitive alternative to high-cost commercial prosthetic solutions.