Abstract
Electromagnetic actuation represents a novel wireless control approach utilized for the manipulation of magnetic microrobots, particularly in the context of diverse minimally invasive therapeutic applications. This study presented contributions relating to the integration of a human operator into the control system of an electromagnetic actuation framework through haptic assistance. The intervention of a human operator serves multiple purposes, encompassing the safe piloting of the microrobot during the procedure and the utilization of the doctor’s expertise. Consequently, this human-in-the-loop approach not only ensures heightened safety but also enhances public acceptability, particularly in the realm of drug delivery within the human body. To facilitate these objectives, a haptic device was proposed to propel and orient the microrobots within blood vessels, thereby enabling their targeted delivery. Additionally, a novel magnetic guidance strategy was introduced, relying on the utilization of two magnetic forces to simplify and optimize the guidance process. The electromagnetic actuation system, developed in our research laboratory, offers a comprehensive workspace that has been obtained through analytical and quantitative modeling of the magnetic field generated by the system. With an accessible workspace encompassing a cubic volume of 70 mm in length, the system facilitates easy access from all four lateral sides. Such an architectural design allows for efficient manipulation of microparticles within a significantly larger 3D workspace, surpassing the limitations imposed by traditional systems primarily confined to a small central area, as observed in existing literature. Experimental evaluations encompassing both 2D and 3D scenarios were conducted to validate the efficacy of the magnetic navigation platform.
1. Introduction
In recent years, as microrobots have begun to represent hope for the treatment of a significant number of diseases, the manipulation of medical microrobots has been the focus of attention of many scientists and researchers, especially after experiencing a massive development which shows progress in obtaining high efficacy of the remedies. Basically, the process is to guide and drive these microrobots (drug carriers) inside the body using a magnetic field. This technique is so precise that it not only allows the targeting of infectious regions, tumors, or any malignant tissue [1,2] that requires complex surgery, without affecting healthy organs as is the case with the traditional therapies. Therefore, the use of microrobots is minimally invasive [3,4,5] and more efficient [6,7]. However, to successfully implement this procedure, it is necessary to have a powerful electromagnetic actuation system with sufficient working space. In addition, a precise manipulation method would be required to effectively control the microrobot.
During the past years, several configurations of electromagnetic coils have been proposed for wireless actuation of microrobots [8,9,10,11,12,13]. The use of two coils makes it possible to produce a modular magnetic field by varying the electric current to manipulate and control the microrobots in the workspace located between the two coils. This consists of Helmholtz coils to generate a uniform magnetic field or Maxwell coils to produce a uniform magnetic gradient. Three pairs of Maxwell or Helmholtz coils arranged perpendicularly generate, respectively, a uniform magnetic field or gradient in 3D space [8]. Various electromagnetic systems have been proposed in the literature [10,11,12,13,14] with different characteristics in terms of electromagnet configuration, working space, and magnetic field distribution. The OctoMag [12] combines eight coils to generate a magnetic field and gradient capable of driving an intraocular microrobot in 5-DOF. It was designed for ophthalmic procedures like retinal-vein cannulation, with a semispherical accessible space of diameter. An EMA system composed of five electromagnetic coils called the Mag-µBot was proposed in [15], offering an accessible space with a 3D volume of 100 mm × 100 mm × 100 mm. An electromagnetic system that contained eight air-cor electromagnets was designed in [16], providing an accessible space of 120 mm × 120 mm × 120 mm.
The use of these electromagnetic systems in automatic mode imposes some constraints. The first consists in obtaining a model of the dynamics of the microrobot in its environment and the latter is physiological, therefore very changing and variable; its modeling became very complex. Indeed, it is very difficult to obtain a precise model or exact physiological parameters, such as the blood velocity or the position of the blood vessel, for example. In automatic mode, it is also necessary to know in advance the trajectory that the microrobot must follow for the execution of automatic mode in a closed loop. The planning of this trajectory is very complex, especially in the context of navigation in the human vascular network. The other constraint in the execution of the automatic mode is access to the position of the microrobot in real time to ensure controller convergence. Indeed, obtaining the 3D position of the microrobot from clinical images would require robust image processing algorithms which generally require a very large calculation time. For these different constraints, automating the navigation of the microrobot in any drug targeting application may not be the best solution from an application point of view.
That is why we need a human operator in the control loop to eliminate all doubts that could cause the loss of the microrobot inside the human body. This intervention of a human operator is not only helpful to ensure secured steering of the microrobot during the process but it can also be beneficial to have all the expertise of a doctor, which would be a lot more responsible, safe, and even acceptable to the public as a system for drug delivery applications to a human body [17]. In such a case, integrating a haptic device in a teleoperated system can make the interaction with the distant environment more sensible and natural during manipulation. So, different from contact-based micro-manipulations, studies carried out to develop haptic feedback in non-contact micro-manipulations are rare. From this standpoint, a recent scientific investigation [18] aimed to elucidate a navigation approach by employing a haptic system for deflecting a microrobot at bifurcations and exploiting hydrodynamic force as the propulsive mechanism to guide the microrobot towards the branching point. A two-dimensional vascular network was employed as the experimental model, wherein a uniaxial actuation system encompassing two coils in the z-axis generated the magnetic force, and a haptic joystick facilitated the directional manipulation of the magnetic force, either upward or downward. Consequently, the authors’ proposed navigation strategy involved maneuvering the microrobot towards the bifurcation, thereby directing it towards the intended branch. Nonetheless, given that the conducted tests were conducted under simplified circumstances, it is crucial to assess the efficacy of this strategy within a complex vascular network representative of realistic conditions. Furthermore, although the utilization of hydrodynamic force is intriguing, employing magnetic flux lines would be more favorable for ensuring precise control of the microrobot while minimizing the required magnetic energy. The non-contact systems that already exist with haptic feedback are mostly on magnetic tweezers and optical tweezers [19,20,21,22]; however, the other non-contact systems that focus on drug delivery using magnetic microrobots function only on 1D or 2D. Equally important, to improve human performance in remotely manipulated tasks, there is a process of transferring a block of program code into internal memory to provide navigation information in teleoperation to assist the operator [23]. This process can be executed by virtual fixtures known as active constraints. In essence, “virtual fixtures” are software-generated force and position signals adjusted to human operators, such as doctors in our case, to improve the safety, precision, performance, and agility of microrobot assisted manipulation tasks [24,25,26]. Virtual fixtures are efficient and intuitive because they have an advantage from both the perfection of robotic systems and the intellect of human operators.
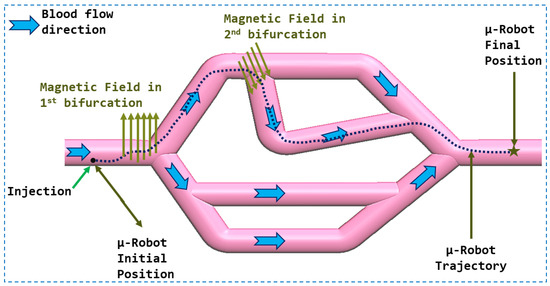
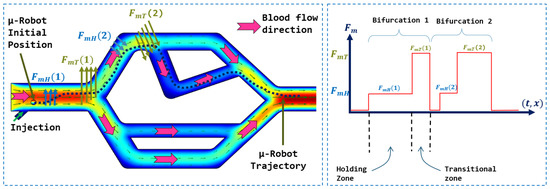
In this work, As presented in the Figure 1, we proposed a navigation strategy for microrobots that utilizes a haptic device to control the magnetic force generated by a magnetic actuation system. This approach leverages magnetic flux lines to facilitate navigation and reduce energy consumption. The aim was to integrate clinicians’ knowledge and expertise into the procedure, particularly their precise understanding of vascular anatomy. Their involvement enables accurate and safe navigation of microrobots within vascular networks. Our method avoids the use of closed-loop controls, eliminating the need for complex and unstable models associated with physiological parameters. By simplifying the navigation process, our approach offers a more practical and efficient implementation.
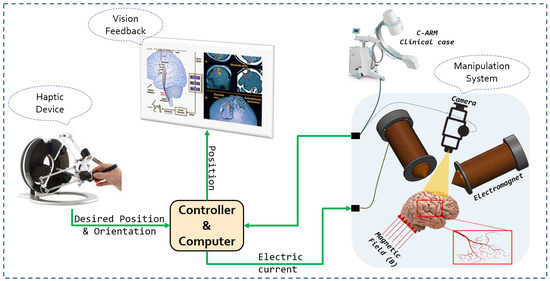
Figure 1.
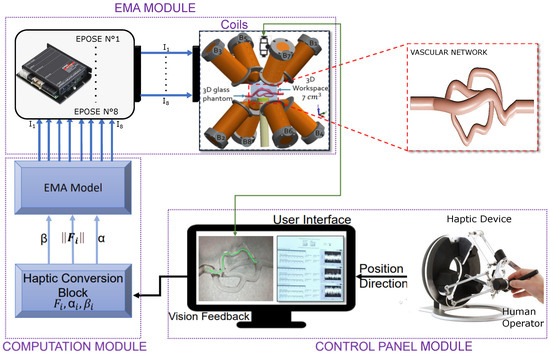
Conceptual diagram of the whole control system.
4. Experimental Evaluation
4.1. Haptic Control Validation
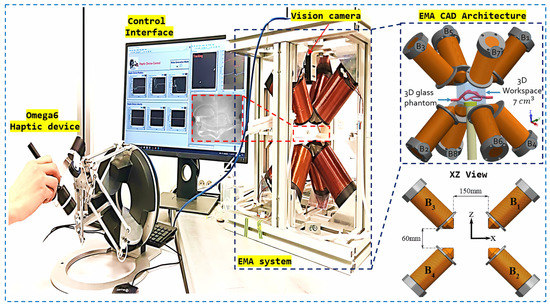
The fundamental objective of this experimentation was to validate the integration of the haptic device and demonstrate its utility in the precise manipulation of a microrobot. Indeed, the integration of the haptic device into the platform aims to enable the user to manipulate a microrobot through manual movements of the haptic joystick, while referring to the visual feedback from the vision system for synchronization. With this goal in mind, we positioned the microrobot in a free space and proceeded with manipulation using the effector of the haptic device to control the microrobot’s movements.
A thorough analysis of Figure 10 reveals a close correlation between the movements of the microrobot and those of the haptic system, attesting to the fact that the manipulation of the microrobot is entirely commanded by the user through the haptic device. Notably, the user is able to reach the four target points by controlling the effector of the haptic system. This close correspondence convincingly validates the successful integration of the haptic device and confirms its capability to provide intuitive and precise control of the microrobot during manipulation operations. Remarkably, the user, unaware of it, directs the haptic system towards a specific direction, and the microrobot faithfully follows that direction. In the background, the controller generates magnetic fields by injecting electrical currents into the coils to achieve the desired trajectory.
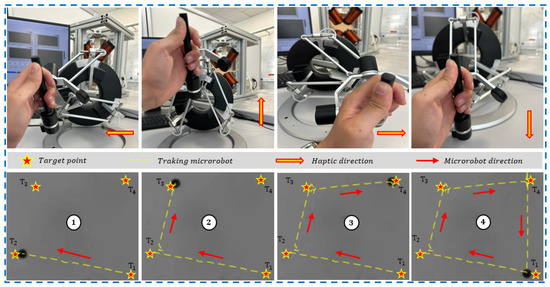
Figure 10.
Validation of the microrobot control in a free space using haptic device.
4.2. Navigation Strategy Validation
In this section, two experiments were conducted to validate the proposed navigation strategy outlined in Section 2. The first experiment aimed to compare the in-silico test (presented in Figure 5) with the in-vitro test in order to corroborate the modeling of the navigation strategy and ascertain the significance of integrating a joystick for navigation purposes. The second experiment entailed validating the performance of the guidance platform within a 3D vascular network for guiding soft ferromagnetic microrobots.
In our experiments, we used a soft magnetic microrobot consisting of a microsphere made of soft iron (AISI-35C) with an average radius of 250 μm. AISI-35C is a magnetic stainless steel containing between 16 and 18% chromium and between 0.95 and 1.2% carbon. It is known for its moderate resistance to corrosion, which is particularly advantageous for medical applications. However, it should be noted that a gold coating layer should be provided in a real-life context to prevent any potential toxicity risks.
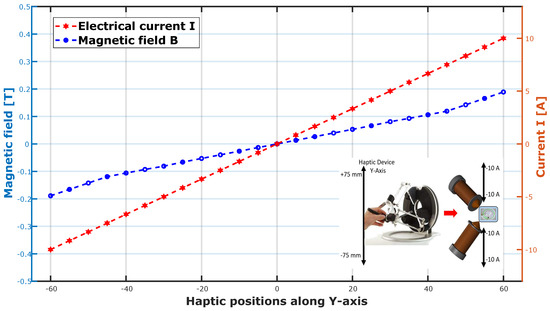
The spherical geometry imparts specific properties to the microrobot in terms of magnetic navigation. Note that our microrobot is of the soft ferromagnetic type and that its magnetization depends on the magnetic field generated by the magnetic actuation system. Since our system generates magnetic fields greater than 0.03 T, we can therefore consider its magnetization at saturation given by: . To emulate the blood environment, we used a mixed solution composed of 25% glycerin and 75% water. The primary objective of these experimental tests was to validate the navigation strategy within phantoms and substantiate the efficacy of a haptic platform guiding the microrobots within the vascular network.
4.2.1. Navigation Strategy Validation in a Vascular 2D Phantom
For the initial experiment, we employ a 2D vascular network consisting of an inlet artery receiving the flow, three bifurcations with different angles, as illustrated in Figure 11, and an outlet artery connected to the reservoir. The vascular network is designed using computer-aided design (CAD) and fabricated in glass.
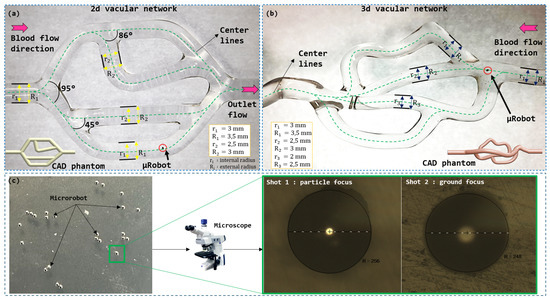
Figure 11.
Vascular phantom with multi-bifurcations for microrobot navigation: (a) 2D vascular network; (b) 3D vascular network; (c) microrobot and their measurement under microscope.
The primary objective of this elementary experiment was twofold. Firstly, we aimed to validate the navigation strategy employing two forces through the use of a joystick, as presented in the modeling section. Secondly, we sought to verify the magnetic system’s capability to generate a sufficiently strong force to guide the soft ferromagnetic microrobot. To accomplish this, we adopted navigation parameters similar to those utilized in the numerical simulation (see Table 1). The fluid’s viscosity and density were fixed by adjusting the glycerin and water mixture, while the pulsatile flow was ensured by the pump. Regarding the selection of the continuous flow velocity , careful control of the pump-delivered flow rate , the length of the conduit between the vessel inlet and the pump outlet , the radius of this conduit R, and the pressure drop associated with this length were necessary. These parameters contribute to the following formula for calculating the flow velocity:
Equation (12) was used to calculate the pressure drop between the pump outlet and the vascular network inlet, while Equation (13) was used to determine the velocity at the vessel inlet. For instance, a tube length of 10 and a flow rate of 72 yield a pressure drop of 0.6 , resulting in an applied velocity of 10 . In the experiment, the pump was calibrated to a flow rate of 72 .
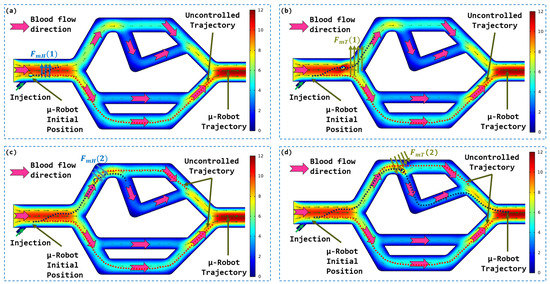
An analysis of Figure 12 provides us with a detailed view of the magnetic-guidance-free navigation of the microrobot within the 2D vascular network device. During this initial phase, the microrobot is subjected solely to hydrodynamic force, without the intervention of an external magnetic force. This approach allowed us to observe the microrobot’s uncontrolled trajectory based on its initial position. Notably, the microrobot’s trajectory in Figure 12 is opposite to that in Figure 12 due to its initial position within the inlet artery.
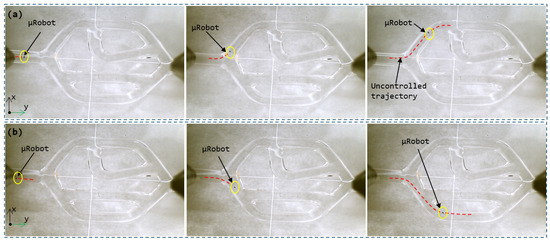
Figure 12.
The uncontrolled trajectory of the microrobot in the vascular network according to different initial positions: (a) first trajectory, (b) second trajectory.
It is worth noting that, in accordance with our predictions based on numerical simulation, the microrobot’s uncontrolled trajectory is closely tied to key factors such as its initial position relative to the central axis of the artery and the orientation of blood flow lines. These parameters significantly influence the direction and speed of the microrobot’s movement as it navigates through the artificial vascular network. As a result, each initial configuration yields a unique trajectory. These experimental observations confirm and validate the findings obtained from the numerical model, further underscoring the significance and relevance of the proposed navigation strategy. This strategy entails combining hydrodynamic forces with magnetic guidance to achieve precise and controlled microrobot navigation within the vascular network.
We are now harnessing the magnetic force generated by the magnetic actuation system to guide the microrobot along a desired trajectory, distinct from its uncontrolled path. In the case where the microrobot is positioned at a distance of −1.4 mm from the central axis, its uncontrolled trajectory is depicted in Figure 12. To achieve the trajectory illustrated in Figure 13, it is imperative to guide the microrobot in the two bifurcations by magnetically directing it towards the first zone and the second zone, respectively, for bifurcations 1 and 2. To accomplish this, the operator manipulates the haptic joystick to generate a force of magnitude N in order to bring the microrobot back into the secure zone and prepare it for passage into the desired branch. As the microrobot reaches the entrance of the bifurcation, the operator generates a magnetic force of magnitude N to ensure a smooth transition into the desired branch. The transition between these two forces occurs continuously, with a gradual increase in magnetic force in the same direction, utilizing the haptic system’s effector.
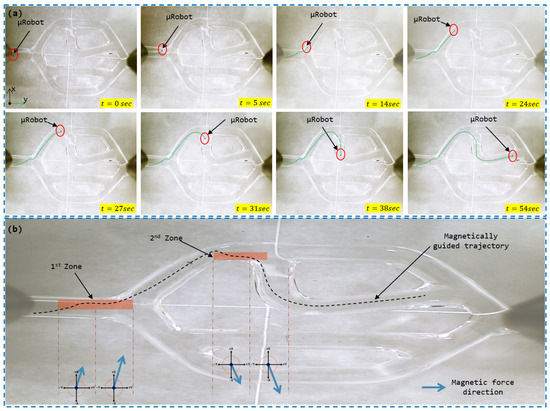
Figure 13.
Microrobot trajectory in the vascular vessel subjected to the control signal applied by the user via the joystick: (a) Magnetically guided trajectory of the microrobot; (b) Directions of the magnetic forces to guide the microrobot in the two bifurcations.
4.2.2. Navigation Strategy Validation in a Vascular 3D Phantom
The objective of this experiment was to demonstrate the navigation capability of a soft ferromagnetic microrobot through a 3D vascular model. The model used, as depicted in Figure 11, simulates the dimensions of small arteries and arterioles, with diameters ranging from 2 to 3 mm.
The experiment (shown in Figure 14) aims to validate the performance of our magnetic actuation platform for 3D guidance. Initially, we assessed the microrobot’s uncontrolled trajectory within the vascular network, as illustrated in Figure 14. Based on these findings, we planned a magnetic guidance trajectory to control the microrobot through two successive bifurcations.
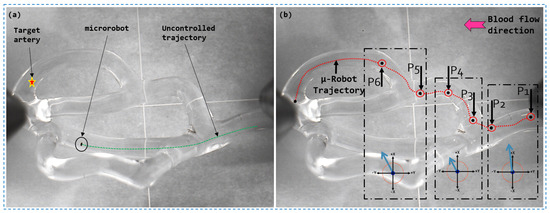
Figure 14.
Magnetic guidance result of a soft ferromagnetic microrobot in a 3D vascular network: (a) Non-magnetic trajectory; (b) Magnetic guidance trajectory.
In accordance with the previously established navigation strategy, we employed a magnetic force applied in the plane to deviate the microrobot from its natural trajectory. In the subsequent phase, a transition force was generated in the plane, allowing the microrobot to precisely penetrate into the desired first branch. To reach the final destination, a magnetic force was applied to facilitate the smooth transition of the microrobot through the second branch, thereby counteracting its tendency to continue along its uncontrolled trajectory.
The magnetic guidance platform, based on the analysis of control signals and the results of this experiment, provides the capability to generate a three-dimensional magnetic force within the range of N for a soft ferromagnetic particle with a diameter of 250 . The navigation strategy employing two forces, and , facilitated by the use of a haptic joystick, enhances navigation simplicity and effectiveness.
In order to ensure the comprehensive evaluation of the magnetic navigation platform’s performance, it is important to investigate the effect of flow velocity variation on the navigation efficacy. With this objective in mind, we conducted the same magnetic guidance test as depicted in Figure 14 at different flow velocities. These velocities were determined by employing Equations (12) and (13). The pump generated flow rates to simulate velocities ranging from 30 to 150 . The results depicted in the Figure 15 reveal that the magnetic navigation performance is significantly influenced by the flow velocity. Specifically, at lower flow velocities in the range of 1 to 4 , the success rate reaches 100%. However, as the flow velocity is increased to between 8 and 12 , the success rate declines to a range of 80% to 20%. Beyond 14 , complete control over the microrobot is lost.
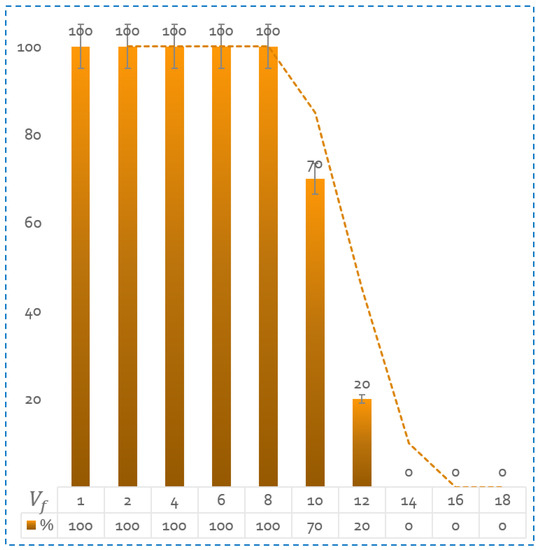
Figure 15.
Success rate of microrobot guidance at different flow velocity values. The results are calculated on an average of 10 tests per speed.
5. Conclusions
This article described the development of a navigation strategy for a soft magnetic microrobot, implemented by means of a haptic system integrated with a magnetic actuation platform designed in our research laboratory. We demonstrated, through numerical simulations carried out on an in silico model, the feasibility of exploiting the hydrodynamic forces of the flow to guide the microrobot by combining them with the magnetic forces. Indeed, these hydrodynamic forces, long perceived as an obstacle to navigation, can help us transport the microrobot and reduce the need to use magnetic forces, except at bifurcations, to guide the microrobot towards the target artery. With this in mind, our simulations also demonstrated that the navigation of the microrobot strongly depends on its initial position in the main artery.
To generate magnetic forces, we used our magnetic guidance platform, composed of electromagnetic coils, which has proven its effectiveness for the navigation of microrobots in various environments of the human body. This platform has been adapted by integrating a haptic system in order to involve the user in the control loop, thus making it possible to benefit from the expertise of the surgeon and to facilitate navigation. To demonstrate the efficiency of the proposed magnetic actuation system, we performed microrobot control experiments in two vascular networks. The first network was in 2D and made it possible to validate in-silico navigation results with and without magnetic actuation force. The second network, in 3D, demonstrated the effectiveness of the navigation procedure in more complex bifurcations. In the first network, the microrobot was successfully guided through two consecutive bifurcations in 10 trials, achieving a 100% success rate for flow velocities below 8 cm/s. This demonstrates the effectiveness of our method in successfully navigating vascular network bifurcations. For the complex 3D vascular network with pronounced curvatures, navigation results showed good control of the microrobot in traversing the bifurcations. However, when conducting varied tests with flow velocities ranging from 1 to 10 and repeating each test 10 times, the observed success rate was 59%. These results highlight the challenges associated with navigation in complex vascular networks, but still demonstrate adequate control of the microrobot under low-flow conditions. As specified in the navigation strategy section, our method was designed with the understanding that microrobots will primarily operate in arteries with relatively low-flow velocities. This is made possible by using endovascular catheters that facilitate access to these areas. Thus, our magnetic-guidance method specifically addresses the limitations of conventional endovascular methods, making it a relevant strategy in the clinical context. It provides a complementary solution for precise navigation in complex vascular networks and for reaching specific target areas.
Through these experimental tests, the guidance strategy with a haptic system has demonstrated its ability to offer simple and intuitive magnetic navigation to reach specific areas of the vascular network. Our future studies will include improving the current magnetic platform in several ways, such as developing a human-scale magnetic actuation system to increase workspace and enable in-vivo testing, taking into account medical constraints. We also plan to improve the haptic system by integrating force feedback to interact with the walls (in particular to control the force). As for limiting the navigation of microrobots to velocities above 100 , the approach proposed in this article can be easily implemented in low-velocity arteries such as liver and kidney, where flow velocities are well below 100 . From the same perspective, we aim to maximize the use of haptic devices for full integration of the clinician in the procedure. The envisioned improvement is the utilization of force feedback in the haptic device, enabling the operator to feel the magnetic force during microrobot’s manipulation. We plan to incorporate magnetic force limitation modules to prevent undesirable interactions with surrounding tissues. For future developments, we intend to create a virtual environment that integrates the vascular network based on medical images and is fed in real-time by physiological data of blood circulation. This approach would reduce reliance on real-time medical images, thereby minimizing X-ray exposure time for both patients and operating room personnel.
Author Contributions
Conceptualization, All authors; methodology, All authors; software, A.C.; formal analysis, All authors; investigation, A.C.; resources, K.B.; data curation, A.C.; writing original draft preparation, All authors; writing review and editing, All authors; visualization, All authors; supervision, K.B.; project administration, K.B.; funding acquisition, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by µ-Mag project, founded by Junia-HEI campus centre, in France.
Data Availability Statement
Data are available from the corresponding author on request.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.; Herbst, R.S.; Onn, A. Targeted drug delivery strategies to treat lung metastasis. Expert Opin. Drug Deliv. 2009, 6, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Udayakumar, K.; Barrett, D.; McLurkin, J.; Franck, D.; Shectman, A. Tomorrow’s surgery: Micromotors and microrobots for minimally invasive procedures. Minim. Invasive Ther. Allied Technol. 1998, 7, 343–352. [Google Scholar] [CrossRef]
- Ullrich, F.; Bergeles, C.; Pokki, J.; Ergeneman, O.; Erni, S.; Chatzipirpiridis, G.; Pané, S.; Framme, C.; Nelson, B.J. Mobility experiments with microrobots for minimally invasive intraocular surgery. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2853–2863. [Google Scholar] [CrossRef]
- Hamdi, M.; Ferreira, A. Guidelines for the design of magnetic nanorobots to cross the blood–brain barrier. IEEE Trans. Robot. 2013, 30, 81–92. [Google Scholar] [CrossRef]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. The behaviors of ferromagnetic nano-particles in and around blood vessels under applied magnetic fields. J. Magn. Magn. Mater. 2011, 323, 651–668. [Google Scholar] [CrossRef]
- Jing, X.; Guo, W. Modeling and Configuration Design of Electromagnetic Actuation Coil for a Magnetically Controlled Microrobot. Chin. J. Mech. Eng. 2019, 32, 63. [Google Scholar] [CrossRef]
- Petruska, A.J.; Mahoney, A.W.; Abbott, J.J. Remote manipulation with a stationary computer-controlled magnetic dipole source. IEEE Trans. Robot. 2014, 30, 1222–1227. [Google Scholar] [CrossRef]
- Go, G.; Choi, H.; Jeong, S.; Lee, C.; Ko, S.Y.; Park, J.O.; Park, S. Electromagnetic navigation system using simple coil structure (4 coils) for 3-D locomotive microrobot. IEEE Trans. Magn. 2014, 51, 1–7. [Google Scholar]
- Jeong, S.; Choi, H.; Choi, J.; Yu, C.; Park, J.o.; Park, S. Novel electromagnetic actuation (EMA) method for 3-dimensional locomotion of intravascular microrobot. Sens. Actuators A Phys. 2010, 157, 118–125. [Google Scholar] [CrossRef]
- Kratochvil, B.E.; Kummer, M.P.; Abbott, J.J.; Borer, R.; Ergeneman, O.; Nelson, B.J. Octomag: An electromagnetic system for 5-dof wireless micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar]
- Niu, F.; Ma, W.; Chu, H.K.; Ji, H.; Yang, J.; Sun, D. An electromagnetic system for magnetic microbead’s manipulation. In Proceedings of the 2015 IEEE International Conference on Mechatronics and Automation (ICMA), Beijing, China, 2–5 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1005–1010. [Google Scholar]
- Pawashe, C.; Floyd, S.; Sitti, M. Modeling and experimental characterization of an untethered magnetic micro-robot. Int. J. Robot. Res. 2009, 28, 1077–1094. [Google Scholar] [CrossRef]
- Floyd, S.; Pawashe, C.; Sitti, M. An untethered magnetically actuated micro-robot capable of motion on arbitrary surfaces. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, Pasadena, CA, USA, 19–23 May 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 419–424. [Google Scholar]
- Diller, E.; Giltinan, J.; Lum, G.Z.; Ye, Z.; Sitti, M. Six-degree-of-freedom magnetic actuation for wireless microrobotics. Int. J. Robot. Res. 2016, 35, 114–128. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Magdanz, V.; Medina-Sánchez, M.; Schmidt, O.G.; Prattichizzo, D.; Misra, S. Intuitive control of self-propelled microjets with haptic feedback. J. Micro-Bio Robot. 2015, 10, 37–53. [Google Scholar] [CrossRef]
- Hamdipoor, V.; Afzal, M.R.; Le, T.A.; Yoon, J. Haptic-based manipulation scheme of magnetic nanoparticles in a multi-branch blood vessel for targeted drug delivery. Micromachines 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- van West, E.; Yamamoto, A.; Higuchi, T. The concept of “Haptic Tweezer”, a non-contact object handling system using levitation techniques and haptics. Mechatronics 2007, 17, 345–356. [Google Scholar] [CrossRef]
- Pacoret, C.; Regnier, S. Invited article: A review of haptic optical tweezers for an interactive microworld exploration. Rev. Sci. Instruments 2013, 84, 081301. [Google Scholar] [CrossRef]
- Basdogan, C.; Kiraz, A.; Bukusoglu, I.; Varol, A.; Doğanay, S. Haptic guidance for improved task performance in steering microparticles with optical tweezers. Opt. Express 2007, 15, 11616–11621. [Google Scholar] [CrossRef]
- Mehrtash, M.; Tsuda, N.; Khamesee, M.B. Bilateral macro–micro teleoperation using magnetic levitation. IEEE/ASME Trans. Mechatronics 2011, 16, 459–469. [Google Scholar] [CrossRef]
- Okamura, A.M. Methods for haptic feedback in teleoperated robot-assisted surgery. Ind. Robot. Int. J. 2004, 31, 499–508. [Google Scholar] [CrossRef]
- Ammi, M.; Ferreira, A. Robotic assisted micromanipulation system using virtual fixtures and metaphors. In Proceedings of the Proceedings 2007 IEEE International Conference on Robotics and Automation, Rome, Italy, 10–14 April 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 454–460. [Google Scholar]
- Bolopion, A.; Xie, H.; Haliyo, D.S.; Régnier, S. Haptic teleoperation for 3-D microassembly of spherical objects. IEEE/ASME Trans. Mechatron. 2010, 17, 116–127. [Google Scholar] [CrossRef]
- Ghanbari, A.; Horan, B.; Nahavandi, S.; Chen, X.; Wang, W. Haptic microrobotic cell injection system. IEEE Syst. J. 2012, 8, 371–383. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Nelson, B.J.; Choi, H. Fabrication and targeted particle delivery using microrobots. In Proceedings of the 2015 12th International Conference on Ubiquitous Robots and Ambient Intelligence (URAI), Goyang, Republic of Korea, 28–30 October 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 524–525. [Google Scholar]
- Mellal, L.; Belharet, K.; Folio, D.; Ferreira, A. Optimal structure of particles-based superparamagnetic microrobots: Application to MRI guided targeted drug therapy. J. Nanopart. Res. 2015, 17, 1–18. [Google Scholar] [CrossRef]
- Chah, A.; Kroubi, T.; Belharet, K. A new electromagnetic actuation system with a highly accessible workspace for microrobot manipulation. In Proceedings of the 2020 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Boston, MA, USA, 6–9 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 723–728. [Google Scholar]
- Chah, A.; Elfakir, H.; Meziane, L.; Belharet, K. Soft ferromagnetic microrobot navigation in the cochlea using haptic assistance. In Proceedings of the 2022 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Sapporo, Japan, 11–15 July 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 626–631. [Google Scholar]
- Ceccacci, S.; Generosi, A.; Leopardi, A.; Mengoni, M.; Mandorli, F. The role of haptic feedback and gamification in virtual museum systems. J. Comput. Cult. Herit. (JOCCH) 2021, 14, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).