Gelatin Soft Actuators: Benefits and Opportunities
Abstract
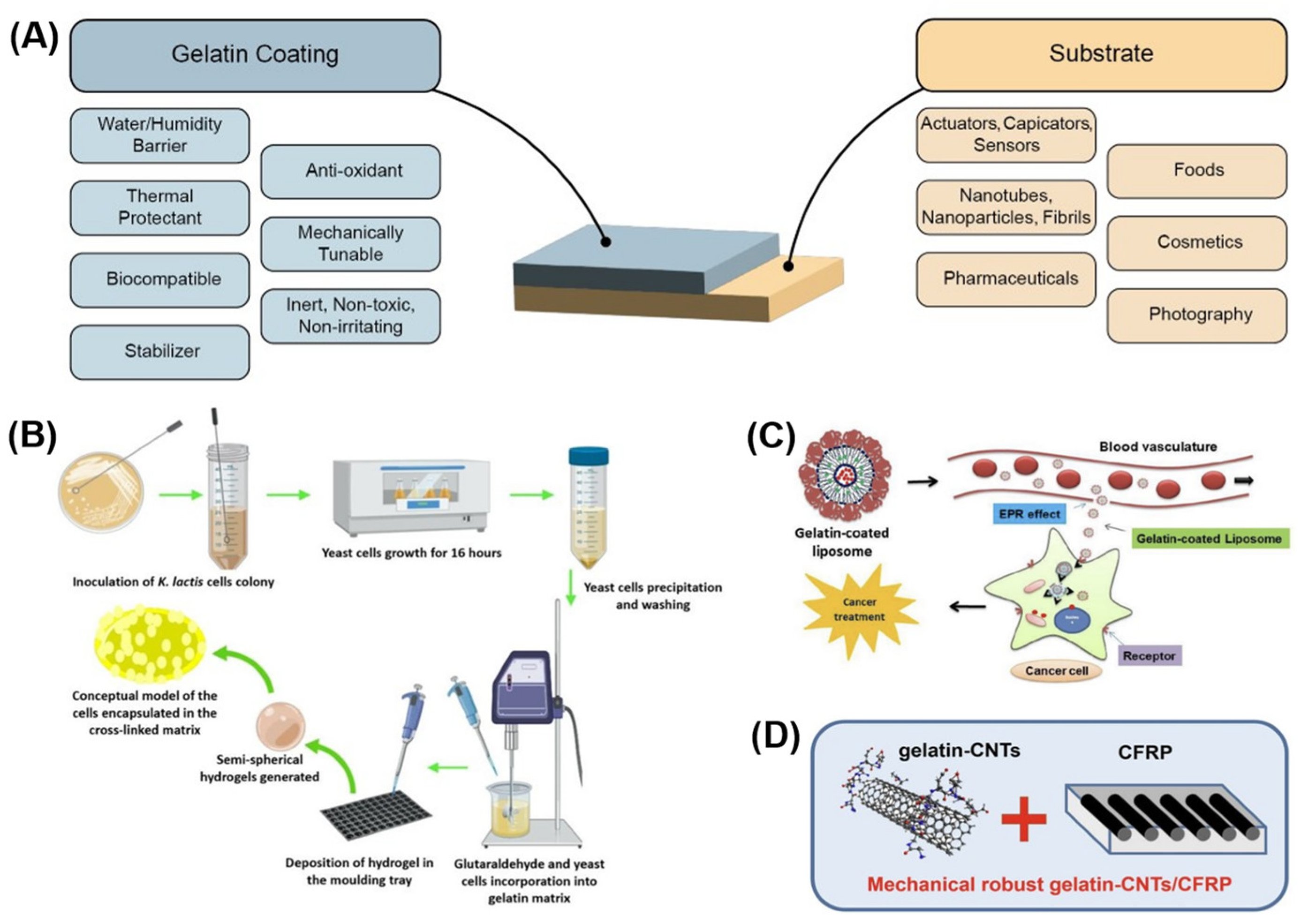
:1. Introduction
2. Versatile Actuation Using Gelatin
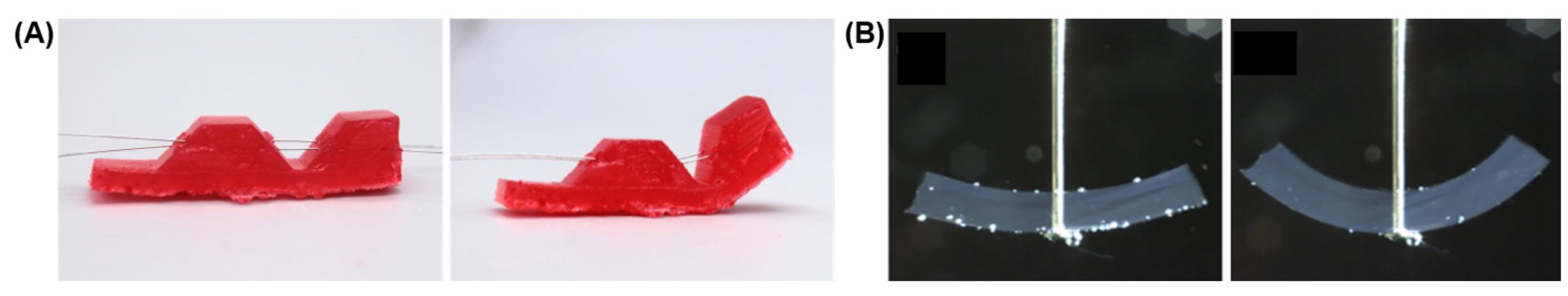
2.1. Linear Actuators
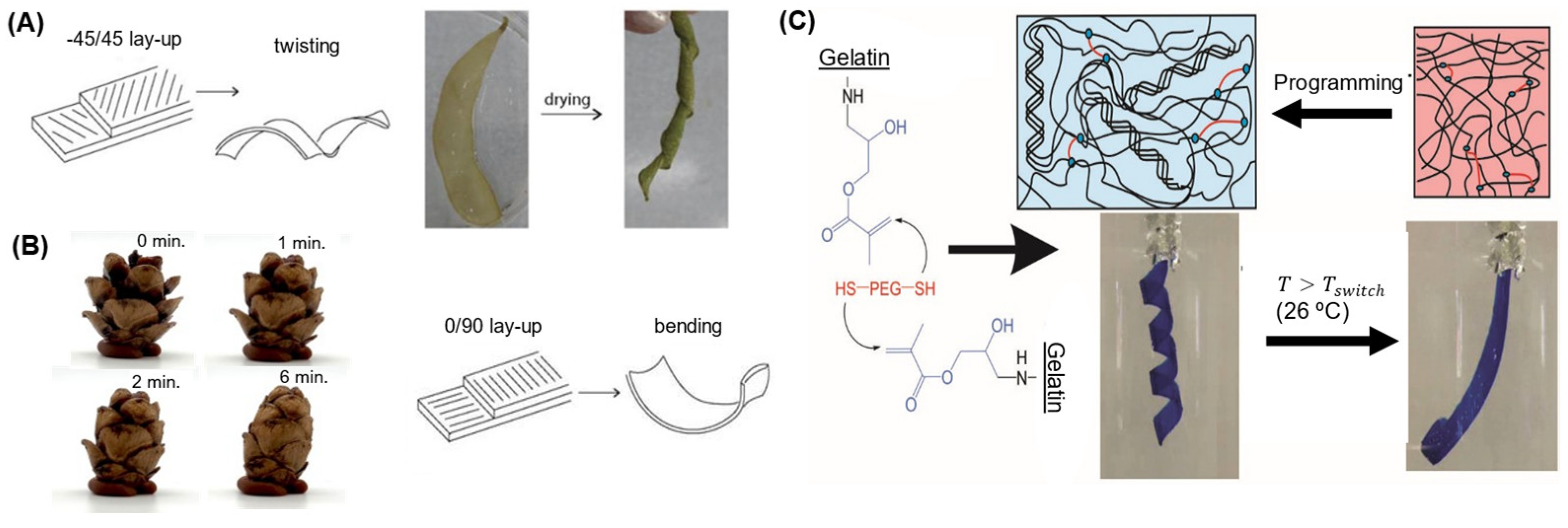
2.2. Bending Actuators
2.3. Twisting and Winding Actuators
3. Expanding the Use of Gelatin for Actuation
3.1. Reinforcing Gelatin with Fibers to Improve Material Characteristics
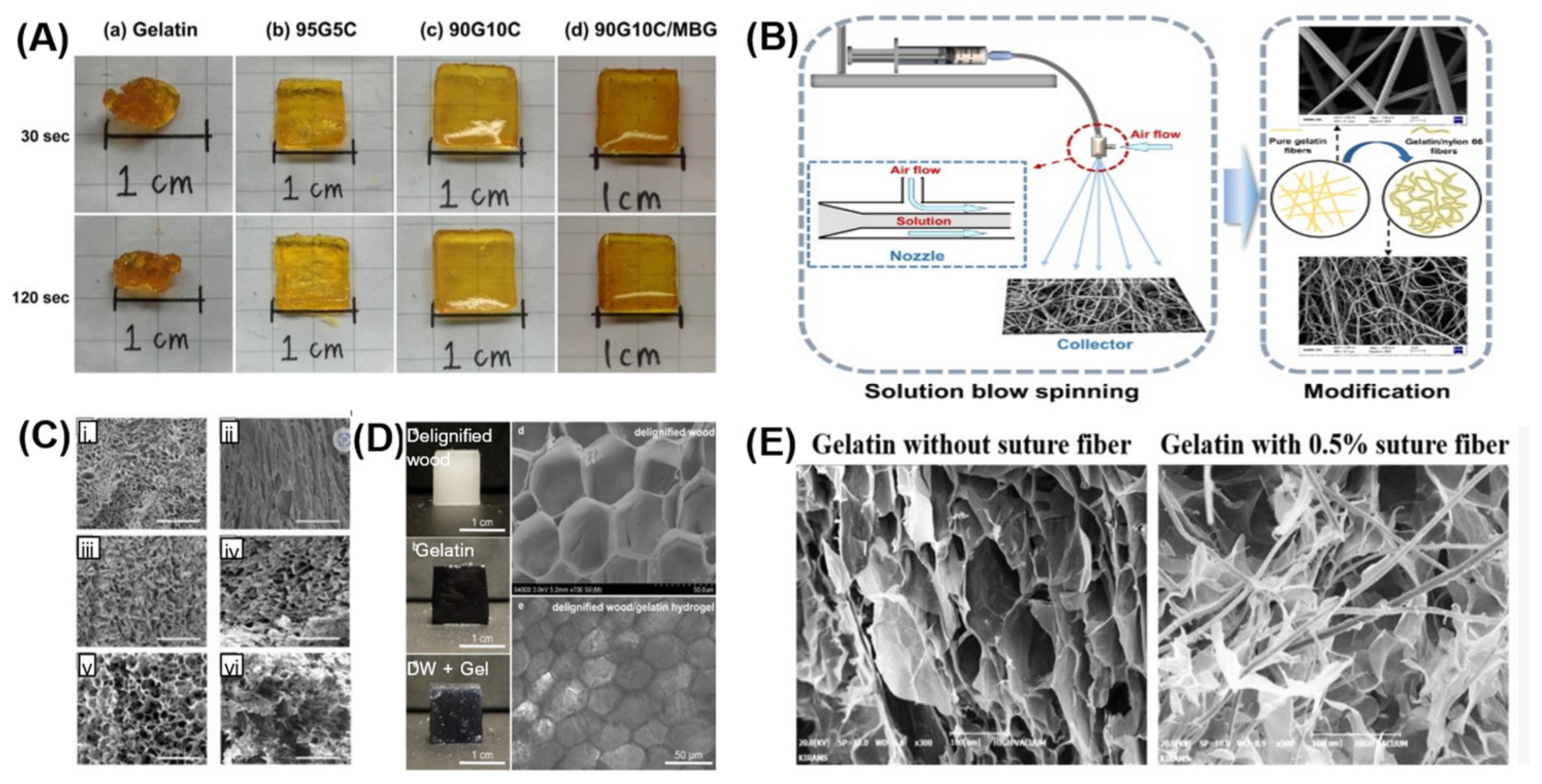
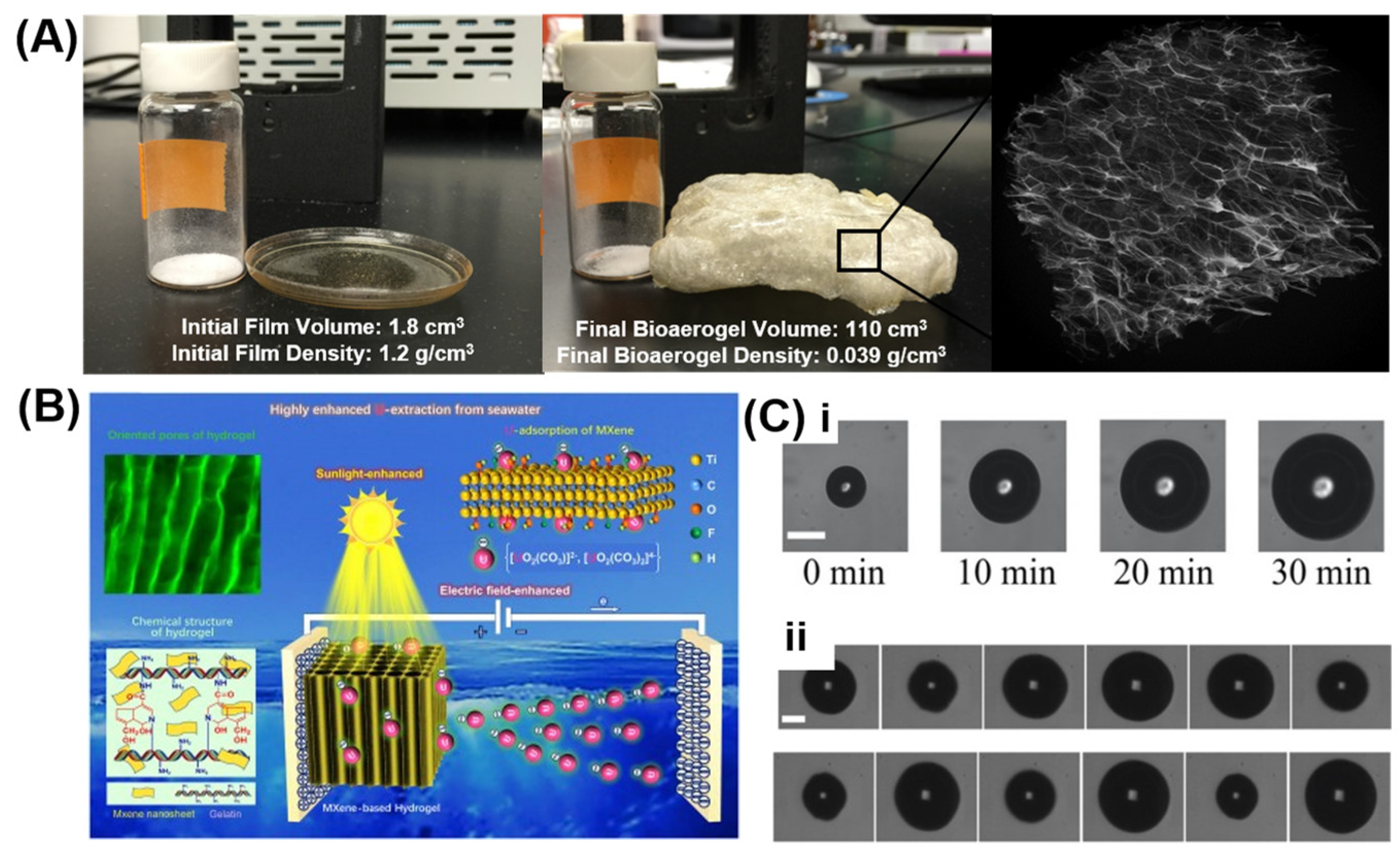
3.2. Gelatin Cellular Solids to Improve Material Properties
3.3. Using Gelatin as Coatings on Other Actuators
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, S.; Islam, M.; Islam, S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological Characterization of Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 819–863. [Google Scholar]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.R.; Mohamed, M.A.; Mohamed, H.; Emara, M.T. Application of alginate and gelatin-based edible coating materials as alternatives to traditional coating for improving the quality of pastirma. Food Sci. Biotechnol. 2018, 27, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Babu, R.J.; Annaji, M.; Alsaqr, A.; Arnold, R.D. Animal-Based Materials in the Formulation of Nanocarriers for Anticancer Therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–341. [Google Scholar]
- Battogtokh, G.; Joo, Y.; Abuzar, S.M.; Park, H.; Hwang, S.-J. Gelatin Coating for the Improvement of Stability and Cell Uptake of Hydrophobic Drug-Containing Liposomes. Molecules 2022, 27, 1041. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a Photosensitive Material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Y.; Hu, D.; Chao, X.; Zhou, Y.; Wang, J. An Essential Role of Gelatin in the Formation Process of Curling in Long Historical Photos. Polymers 2021, 13, 3894. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, X.; Xu, J.; Du, Y.; Yao, M.; Guo, S. Gel properties of SPI modified by enzymatic cross-linking during frozen storage. Food Hydrocoll. 2016, 56, 445–452. [Google Scholar] [CrossRef]
- Saxena, A.; Sachin, K.; Bohidar, H.; Verma, A.K. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf. B Biointerfaces 2005, 45, 42–48. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin Carriers for Drug and Cell Delivery in Tissue Engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Lall, C.; Rajesh, A.; Maglinte, D.T. Distinguishing Gelatin Bioabsorbable Sponge and Postoperative Abdominal Abscess on CT. Am. J. Roentgenol. 2005, 184, 475–480. [Google Scholar] [CrossRef]
- Saw, M.M.; Chandler, B.; Ho, K.M. Benefits and Risks of Using Gelatin Solution as a Plasma Expander for Perioperative and Critically Ill Patients: A Meta-Analysis. Anaesth. Intensive Care 2012, 40, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Ichwan, A.M.; Karimi, M.; Dash, A.K. Use of gelatin–acacia coacervate containing benzocaine in topical formulations. J. Pharm. Sci. 1999, 88, 763–766. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.-K.; Xue, X.-T.; Wu, D.-Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Adv. Health Mater. 2018, 8, e1801054. [Google Scholar] [CrossRef]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Tripathi, A.; Zo, S.; Singh, D.; Han, S.S. Synthesis of composite gelatin-hyaluronic acid-alginate porous scaffold and evaluation for in vitro stem cell growth and in vivo tissue integration. Colloids Surf. B Biointerfaces 2014, 116, 502–509. [Google Scholar] [CrossRef]
- Hanzly, L.E.; Kristofferson, K.A.; Chauhan, N.; Barone, J.R. Biologically Controlled Gelatin Actuators. Green Mater. 2021, 9, 157–166. [Google Scholar] [CrossRef]
- Shintake, J.; Sonar, H.; Piskarev, E.; Paik, J.; Floreano, D. Soft Pneumatic Gelatin Actuator for Edible Robotics. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 6221–6226. [Google Scholar]
- Wang, H.; Xiang, J.; Wen, X.; Du, X.; Wang, Y.; Du, Z.; Cheng, X.; Wang, S. Multifunctional skin-inspired resilient MXene-embedded nanocomposite hydrogels for wireless wearable electronics. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106835. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Z.; Zheng, M.; Yue, O.; Hou, M.; Cui, B.; Su, R.; Wei, C.; Liu, X. Engineered gelatin-based conductive hydrogels for flexible wearable electronic devices: Fundamentals and recent advances. J. Sci. Adv. Mater. Devices 2022, 7, 100451. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Uto, K.; Aoyagi, T.; Kim, Y.-J.; Narain, R.; Idota, N.; Hoffman, J.M. Smart Hydrogels. In Smart Biomaterials; Springer: Tokyo, Japan, 2014; pp. 9–65. [Google Scholar]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Hardman, D.; Thuruthel, T.G.; Iida, F. Self-healing ionic gelatin/glycerol hydrogels for strain sensing applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Stevens, K.R.; Einerson, N.J.; Burmania, J.A.; Kao, W.J. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J. Biomater. Sci. Polym. Ed. 2002, 13, 1353–1366. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chang, W.-H.; Dong, G.-C.; Chen, K.-Y.; Chen, Y.-S.; Yao, C.-H. Cell Adhesion and Pro-liferation Enhancement by Gelatin Nanofiber Scaffolds. J. Bioact. Compat. Polym. 2011, 26, 565–577. [Google Scholar] [CrossRef]
- Gattazzo, F.; De Maria, C.; Rimessi, A.; Donà, S.; Braghetta, P.; Pinton, P.; Vozzi, G.; Bonaldo, P. Gelatin-Genipin-Based Biomaterials for Skeletal Muscle Tissue Engineering: Gelatin-Genipin-Based Biomaterials. J. Biomed. Mater. Res. 2018, 106, 2763–2777. [Google Scholar] [CrossRef]
- Sadeghi, A.H.; Shin, S.R.; Deddens, J.C.; Fratta, G.; Mandla, S.; Yazdi, I.K.; Prakash, G.; Antona, S.; Demarchi, D.; Buijsrogge, M.P.; et al. Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling. Adv. Health Mater. 2017, 6, 1601434. [Google Scholar] [CrossRef]
- Sundaram, C.P.; Keenan, A.C. Evolution of hemostatic agents in surgical practice. Indian J. Urol. 2010, 26, 374–378. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Mahmud, M.A.P.; Gharaie, S.; Bodaghi, M.; Kouzani, A.Z.; Kaynak, A. 3D/4D-Printed Bending-Type Soft Pneumatic Actuators: Fabrication, Modelling, and Control. Virtual Phys. Prototyp. 2020, 15, 373–402. [Google Scholar] [CrossRef]
- Xavier, M.S.; Tawk, C.D.; Zolfagharian, A.; Pinskier, J.; Howard, D.; Young, T.; Lai, J.; Harrison, S.M.; Yong, Y.K.; Bodaghi, M.; et al. Soft Pneumatic Actuators: A Review of Design, Fabrication, Modeling, Sensing, Control and Applications. IEEE Access 2022, 10, 59442–59485. [Google Scholar] [CrossRef]
- Xavier, M.S.; Fleming, A.J.; Yong, Y.K. Finite Element Modeling of Soft Fluidic Actuators: Overview and Recent Developments. Adv. Intell. Syst. 2021, 3, 2000187. [Google Scholar] [CrossRef]
- Czerner, M.; Fellay, L.S.; Suárez, M.P.; Frontini, P.M.; Fasce, L.A. Determination of Elastic Modulus of Gelatin Gels by Indentation Experiments. Procedia Mater. Sci. 2015, 8, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Bracq, A.; Haugou, G.; Bourel, B.; Maréchal, C.; Lauro, F.; Roth, S.; Mauzac, O. On the modeling of a visco-hyperelastic polymer gel under blunt ballistic impacts. Int. J. Impact Eng. 2018, 118, 78–90. [Google Scholar] [CrossRef]
- Bello, J.; Bello, H.R.; Vinograd, J.R. The mechanism of gelation of gelatin the influence of pH, concentration, time and dilute electrolyte on the gelation of gelatin and modified gelatins. Biochim. Biophys. Acta 1962, 57, 214–221. [Google Scholar] [CrossRef]
- Riedel, S.; Heyart, B.; Apel, K.S.; Mayr, S.G. Programing stimuli-responsiveness of gelatin with electron beams: Basic effects and development of a hydration-controlled biocompatible demonstrator. Sci. Rep. 2017, 7, 17436. [Google Scholar] [CrossRef] [Green Version]
- Sha, X.-M.; Hu, Z.-Z.; Ye, Y.-H.; Xu, H.; Tu, Z.-C. Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocoll. 2019, 92, 163–172. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.-C.; Lv, C.; Xia, H. Green nanoarchitectonics with PEDOT:PSS–gelatin composite for moisture-responsive actuator and generator. Smart Mater. Struct. 2021, 30, 125014. [Google Scholar] [CrossRef]
- Chungyampin, S.; Niamlang, S. The Soft and High Actuation Response of Graphene Oxide/Gelatin Soft Gel. Materials 2021, 14, 7553. [Google Scholar] [CrossRef]
- Helminger, M.; Wu, B.; Kollmann, T.; Benke, D.; Schwahn, D.; Pipich, V.; Faivre, D.; Zahn, D.; Cölfen, H. Synthesis and Characterization of Gelatin-Based Magnetic Hydrogels. Adv. Funct. Mater. 2014, 24, 3187–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable Protein-Based Rockets for Drug Transportation and Light-Triggered Release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, G.; Trase, I.; Han, X.; Mei, Y. Mechanical Self-Assembly of a Strain-Engineered Flexible Layer: Wrinkling, Rolling, and Twisting. Phys. Rev. Appl. 2016, 5, 017001. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.; Rus, D. Mechanically Programmable, Degradable & Ingestible Soft Actuators. In Proceedings of the 3rd IEEE International Conference on Soft Robotics (RoboSoft), New Haven, CT, USA, 15 May–15 July 2020; pp. 836–843. [Google Scholar]
- Heiden, A.; Preninger, D.; Lehner, L.; Baumgartner, M.; Drack, M.; Woritzka, E.; Schiller, D.; Gerstmayr, R.; Hartmann, F.; Kaltenbrunner, M. 3D Printing of Resilient Biogels for Omnidirectional and Exteroceptive Soft Actuators. Sci. Robot. 2022, 7, eabk2119. [Google Scholar] [CrossRef] [PubMed]
- Tawk, C.; Alici, G. A Review of 3D-Printable Soft Pneumatic Actuators and Sensors: Research Challenges and Opportunities. Adv. Intell. Syst. 2021, 3, 2000223. [Google Scholar] [CrossRef]
- Ehrenhofer, A.; Elstner, M.; Wallmersperger, T. Normalization of hydrogel swelling behavior for sensoric and actuatoric applications. Sens. Actuators B Chem. 2018, 255, 1343–1353. [Google Scholar] [CrossRef]
- Tattanon, T.; Pongprayoon, T.; Arpornmaeklong, P.; Ummartyotin, S. Development of Hydroxy-apatite from Cuttlebone and Gelatin-Based Hydrogel Composite for Medical Materials. J. Polym. Res. 2022, 29, 364. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crop. Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H. Gelatin Film and Fiber Reinforced Gelatin Composites. Gelatin: Production, Applications and Health Implications; Boran, G., Ed.; Nova Publishers: New York, NY, USA, 2013. [Google Scholar]
- Qiao, C.; Cao, X.; Wang, F. Swelling Behavior Study of Physically Crosslinked Gelatin Hydrogels. Polym. Polym. Compos. 2012, 20, 53–58. [Google Scholar] [CrossRef]
- Hajikarimi, A.; Sadeghi, M. Free radical synthesis of cross-linking gelatin base poly NVP/acrylic acid hydrogel and nanoclay hydrogel as cephalexin drug deliver. J. Polym. Res. 2020, 27, 57. [Google Scholar] [CrossRef]
- Barthélémy, F.; Santoso, J.W.; Rabichow, L.; Jin, R.; Little, I.; Nelson, S.F.; McCain, M.L.; Miceli, M.C. Modeling Patient-Specific Muscular Dystrophy Phenotypes and Therapeutic Responses in Reprogrammed Myotubes Engineered on Micromolded Gelatin Hydrogels. Front. Cell Dev. Biol. 2022, 10, 830415. [Google Scholar] [CrossRef]
- Gupta, D.; Santoso, J.; McCain, M. Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering 2021, 8, 6. [Google Scholar] [CrossRef]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically Cross-linked Gelatin–Laminin Hydrogels for Applications in Neuromuscular Tissue Engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef]
- Li, J.; Chee, H.L.; Chong, Y.T.; Chan, B.Q.Y.; Xue, K.; Lim, P.C.; Loh, X.J.; Wang, F. Hofmeister Effect Mediated Strong PHEMA-Gelatin Hydrogel Actuator. ACS Appl. Mater. Interfaces 2022, 14, 23826–23838. [Google Scholar] [CrossRef]
- Wang, Y.; Bamdad, F.; Song, Y.; Chen, L. Hydrogel Particles and Other Novel Protein-Based Methods for Food Ingredient and Nutraceutical Delivery Systems: Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2012; pp. 412–450. [Google Scholar]
- Gomes, C.M.; Liu, C.; Paten, J.A.; Felton, S.M.; Deravi, L.F. Protein-Based Hydrogels That Actu-ate Self-Folding Systems. Adv. Funct. Mater. 2019, 29, 1805777. [Google Scholar] [CrossRef]
- Stroganov, V.; Al-Hussein, M.; Sommer, J.-U.; Janke, A.; Zakharchenko, S.; Ionov, L. Reversible Thermosensitive Biodegradable Polymeric Actuators Based on Confined Crystallization. Nano Lett. 2015, 15, 1786–1790. [Google Scholar] [CrossRef]
- Harris, H.; Radecka, A.; Malik, R.; Pineda Guzman, R.A.; Santoso, J.; Bradshaw, A.; McCain, M.; Kersh, M.; Golecki, H. Development and Characterization of Biostable Hydrogel Robotic Actuators for Implantable Devices: Tendon Actuated Gelatin. In Proceedings of the 2022 Design of Medical Devices Conference, American Society of Mechanical Engineers, Minneapolis, MN, USA, 11–14 April 2022; p. V001T05A004. [Google Scholar]
- Sardesai, A.N.; Segel, X.M.; Baumholtz, M.N.; Chen, Y.; Sun, R.; Schork, B.W.; Buonocore, R.; Wagner, K.O.; Golecki, H.M. Design and Characterization of Edible Soft Robotic Candy Actuators. MRS Adv. 2018, 3, 3003–3009. [Google Scholar] [CrossRef]
- Nagai, T.; Kurita, A.; Shintake, J. Characterization of Sustainable Robotic Materials and Finite Element Analysis of Soft Actuators Under Biodegradation. Front. Robot. AI 2021, 8, 760485. [Google Scholar] [CrossRef]
- Tungkavet, T.; Seetapan, N.; Pattavarakorn, D.; Sirivat, A. Graphene/Gelatin Hydrogel Compo-sites with High Storage Modulus Sensitivity for Using as Electroactive Actuator: Effects of Surface Area and Electric Field Strength. Polymer 2015, 70, 242–251. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Liu, Y.; Li, N.; Wang, W.; Gao, J. Preparation of Reduced Graphene Ox-ide/Gelatin Composite Films with Reinforced Mechanical Strength. Mater. Res. Bull. 2012, 47, 2245–2251. [Google Scholar] [CrossRef]
- Chambers, L.D.; Winfield, J.; Ieropoulos, I.; Rossiter, J. Biodegradable and Edible Gelatine Actuators for Use as Artificial Muscles. In Electroactive Polymer Actuators and Devices (EAPAD); Bar-Cohen, Y., Ed.; SPIE: San Diego, CA, USA, 2014; p. 90560B. [Google Scholar]
- Shin, S.R.; Bolagh, B.A.G.; Dang, T.; Topkaya, S.N.; Gao, X.; Yang, S.Y.; Jung, S.M.; Oh, J.H.; Dokmeci, M.R.; Tang, X.; et al. Cell-laden Microengineered and Mechanically Tunable Hybrid Hydrogels of Gelatin and Graphene Oxide. Adv. Mater. 2013, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Park, S.-Y.; Saeed, K.; Farmer, B. Swelling and electroresponsive characteristics of gelatin immobilized onto multi-walled carbon nanotubes. Sens. Actuators B Chem. 2007, 124, 517–528. [Google Scholar] [CrossRef]
- Bigi, A. Relationship between Triple-Helix Content and Mechanical Properties of Gelatin Films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- Firouzeh, A.; Salerno, M.; Paik, J. Soft Pneumatic Actuator with Adjustable Stiffness Layers for Multi-DoF Actuation. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September 2015; pp. 1117–1124. [Google Scholar]
- Su, H.; Hou, X.; Zhang, X.; Qi, W.; Cai, S.; Xiong, X.; Guo, J. Pneumatic Soft Robots: Challenges and Benefits. Actuators 2022, 11, 92. [Google Scholar] [CrossRef]
- Na, H.; Kang, Y.-W.; Park, C.S.; Jung, S.; Kim, H.-Y.; Sun, J.-Y. Hydrogel-based strong and fast actuators by electroosmotic turgor pressure. Science 2022, 376, 301–307. [Google Scholar] [CrossRef]
- Dick, W.B. Cements and Uniting Bodies, Part 3. In Encyclopedia of Practical Receipts and Processes; Dick and Fitzgerald Publishers: New York, NY, USA, 1872. [Google Scholar]
- Mosadegh, B.; Polygerinos, P.; Keplinger, C.; Wennstedt, S.; Shepherd, R.; Gupta, U.; Shim, J.; Bertoldi, K.; Walsh, C.J.; Whitesides, G.M. Pneumatic Networks for Soft Robotics that Actuate Rapidly. Adv. Funct. Mater. 2014, 24, 2163–2170. [Google Scholar] [CrossRef] [Green Version]
- Greer, A.H.; King, E.; Lee, E.H.; Sardesai, A.N.; Chen, Y.; Obuz, S.E.; Graf, Y.; Ma, T.; Chow, D.Y.; Fu, T.; et al. Soluble Polymer Pneumatic Networks and a Single-Pour System for Improved Accessibility and Durability of Soft Robotic Actuators. Soft Robot. 2021, 8, 144–151. [Google Scholar] [CrossRef]
- Elhi, F.; Karu, K.; Rinne, P.; Nadel, K.-A.; Järvekülg, M.; Aabloo, A.; Tamm, T.; Ivaništšev, V.; Põhako-Esko, K. Understanding the Behavior of Fully Non-Toxic Polypyrrole-Gelatin and Polypyrrole-PVdF Soft Actuators with Choline Ionic Liquids. Actuators 2020, 9, 40. [Google Scholar] [CrossRef]
- Wan, C.; Frydrych, M.; Chen, B. Strong and Bioactive Gelatin–Graphene Oxide Nanocomposites. Soft Matter 2011, 7, 6159. [Google Scholar] [CrossRef]
- Choe, G.; Tang, X.; Wang, R.; Wu, K.; Jin Jeong, Y.; Kyu An, T.; Hyun Kim, S.; Mi, L. Printing of Self-Healable Gelatin Conductors Engineered for Improving Physical and Electrical Functions: Exploring Potential Application in Soft Actuators and Sensors. J. Ind. Eng. Chem. 2022, 116, 171–179. [Google Scholar] [CrossRef]
- Li, D.; Fan, D.; Zhu, R.; Lei, Q.; Liao, Y.; Yang, X.; Pan, Y.; Wang, Z.; Wu, Y.; Liu, S.; et al. Origami-Inspired Soft Twisting Actuator. Soft Robot. 2021. [Google Scholar] [CrossRef]
- Roche, E.T.; Wohlfarth, R.; Overvelde, J.T.B.; Vasilyev, N.V.; Pigula, F.A.; Mooney, D.J.; Bertoldi, K.; Walsh, C.J. A Bioinspired Soft Actuated Material. Adv. Mater. 2014, 26, 1200–1206. [Google Scholar] [CrossRef]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1712. [Google Scholar] [CrossRef] [Green Version]
- Lutz-Bueno, V.; Bolisetty, S.; Azzari, P.; Handschin, S.; Mezzenga, R. Self-Winding Gelatin–Amyloid Wires for Soft Actuators and Sensors. Adv. Mater. 2020, 32, 2004941. [Google Scholar] [CrossRef]
- Zhang, Y.; Le Ferrand, H. Bioinspired Self-Shaping Clay Composites for Sustainable Development. Biomimetics 2022, 7, 13. [Google Scholar] [CrossRef]
- Neffe, A.; Löwenberg, C.; Julich-Gruner, K.; Behl, M.; Lendlein, A. Thermally-Induced Shape-Memory Behavior of Degradable Gelatin-Based Networks. Int. J. Mol. Sci. 2021, 22, 15892. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, C.; Dong, L.; Wang, A.; Yao, H.; Guo, Z.; Mi, S. Fluid-driven hydrogel actuators with an origami structure. Iscience 2022, 25, 104674. [Google Scholar] [CrossRef]
- Sasaki, N.; Umeda, H.; Okada, S.; Kojima, R.; Fukuda, A. Mechanical Properties of Hydroxyapatite-Reinforced Gelatin as a Model System of Bone. Biomaterials 1989, 10, 129–132. [Google Scholar] [CrossRef]
- Lin, F.-H.; Yao, C.-H.; Sun, J.-S.; Liu, H.-C.; Huang, C.-W. Biological effects and cytotoxicity of the composite composed by tricalcium phosphate and glutaraldehyde cross-linked gelatin. Biomaterials 1998, 19, 905–917. [Google Scholar] [CrossRef]
- Avila-Ramirez, A.; Catzim-Ríos, K.; Guerrero-Beltrán, C.E.; Ramírez-Cedillo, E.; Ortega-Lara, W. Reinforcement of Alginate-Gelatin Hydrogels with Bioceramics for Biomedical Applications: A Comparative Study. Gels 2021, 7, 184. [Google Scholar] [CrossRef]
- Bhowmik, S.; Islam, J.; Debnath, T.; Miah, M.; Bhattacharjee, S.; Khan, M. Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications. Polymers 2017, 9, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, J.S.; dos Reis, K.C.; Menezes EG, T.; Pereira, F.V.; Pereira, J. Effect of Cellulose Nano-crystals and Gelatin in Corn Starch Plasticized Films. Carbohydr. Polym. 2015, 115, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogaru, B.-I.; Stoleru, V.; Mihalache, G.; Yonsel, S.; Popescu, M.-C. Gelatin Reinforced with CNCs as Nanocomposite Matrix for Trichoderma harzianum KUEN 1585 Spores in Seed Coatings. Molecules 2021, 26, 5755. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, S.; Bracci, B.; Gualandi, C.; Focarete, M.L.; Treossi, E.; Kouroupis-Agalou, K.; Rubini, K.; Bosia, F.; Brely, L.; Pugno, N.M.; et al. Structural reinforcement and failure analysis in composite nanofibers of graphene oxide and gelatin. Carbon 2014, 78, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Shubhra, Q.T.H.; Alam, A.M.; Khan, M.A.; Saha, M.; Saha, D.; Khan, J.A.; Quaiyyum, M.A. The Preparation and Characterization of Silk/Gelatin Biocomposites. Polym. Technol. Eng. 2010, 49, 983–990. [Google Scholar] [CrossRef]
- Shubhra, Q.T.; Alam, A.; Beg, M.D.H. Mechanical and degradation characteristics of natural silk fiber reinforced gelatin composites. Mater. Lett. 2011, 65, 333–336. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.-K.; Brown, E. Development and Characterization of Genipin Cross-Linked Gelatin Based Composites Incorporated with Vegetable-Tanned Collagen Fiber (VCF). J. Am. Leather Chem. Assoc. 2017, 112, 410–419. [Google Scholar]
- Wei, S.-M.; Pei, M.-Y.; Pan, W.-L.; Thissen, H.; Tsai, S.-W. Gelatin Hydrogels Reinforced by Absorbable Nanoparticles and Fibrils Cured In Situ by Visible Light for Tissue Adhesive Applications. Polymers 2020, 12, 1113. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, C.; Zou, Y.; Wu, D.; Zhang, H.; Chen, K. Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film. Foods 2021, 10, 2339. [Google Scholar] [CrossRef]
- Campodoni, E.; Montanari, M.; Dozio, S.M.; Heggset, E.B.; Panseri, S.; Montesi, M.; Tampieri, A.; Syverud, K.; Sandri, M. Blending Gelatin and Cellulose Nanofibrils: Biocomposites with Tunable Degradabil-ity and Mechanical Behavior. Nanomaterials 2020, 10, 1219. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Zhou, Q. High strength and low swelling composite hydrogels from gelatin and delignified wood. Sci. Rep. 2020, 10, 17842. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, K.; Park, S.J.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Suture Fiber Reinforcement of a 3D Printed Gelatin Scaffold for Its Potential Application in Soft Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 11600. [Google Scholar] [CrossRef]
- Singh, C.; Wong, C.S.; Wang, X. Medical Textiles as Vascular Implants and Their Success to Mimic Natural Arteries. J. Funct. Biomater. 2015, 6, 500–525. [Google Scholar] [CrossRef] [Green Version]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Kuijpers, A.; van Wachem, P.; van Luyn, M.; Engbers, G.; Krijgsveld, J.; Zaat, S.; Dankert, J.; Feijen, J. In vivo and in vitro release of lysozyme from cross-linked gelatin hydrogels: A model system for the delivery of antibacterial proteins from prosthetic heart valves. J. Control. Release 2000, 67, 323–336. [Google Scholar] [CrossRef]
- Hassan, M.L.; Fadel, S.M.; El-Wakil, N.A.; Oksman, K. Chitosan/Rice Straw Nanofibers Nano-composites: Preparation, Mechanical, and Dynamic Thermomechanical Properties. J. Appl. Polym. Sci. 2012, 125, E216–E222. [Google Scholar] [CrossRef]
- Jiang, Y.; Xv, X.; Liu, D.; Yang, Z.; Zhang, Q.; Shi, H.; Zhao, G.; Zhou, J. Preparation of cellulose nanofiber-reinforced gelatin hydrogel and optimization for 3D printing applications. Bioresources 2018, 13, 5909–5924. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Teng, A.; Liu, A. Mechanical Reinforcement of Gelatin Hydrogel with Nanofiber Cellulose as a Function of Percolation Concentration. Int. J. Biol. Macromol. 2017, 103, 226–233. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Wang, Y.L.; Luo, H.L.; Cheng, G.X.; Yao, K.D. Carbon Fiber-Reinforced Gelatin Composites. I. Preparation and Mechanical Properties. J. Appl. Polym. Sci. 2000, 75, 987–993. [Google Scholar] [CrossRef]
- Ravishankar, P.; Ozkizilcik, A.; Husain, A.; Balachandran, K. Anisotropic Fiber-Reinforced Gly-cosaminoglycan Hydrogels for Heart Valve Tissue Engineering. Tissue Eng. Part A 2021, 27, 513–525. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.A.; Sarker, B.; Saha, S.; Das, A.K.; Noor, N.; Huq, T.; Khan, A.; Dey, K.; Saha, M. Fabrication and Characterization of Gelatin Fiber-based Linear Low-density Polyethylene Foamed Composite. J. Reinf. Plast. Compos. 2010, 29, 2438–2449. [Google Scholar] [CrossRef]
- Torrejon, V.M.; Song, J.; Yu, Z.; Hang, S. Gelatin-Based Cellular Solids: Fabrication, Structure and Properties. J. Cell. Plast. 2022, 58, 797–858. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Andrieux, S.; Quell, A.; Stubenrauch, C.; Drenckhan, W. Liquid foam templating—A route to tailor-made polymer foams. Adv. Colloid Interface Sci. 2018, 256, 276–290. [Google Scholar] [CrossRef]
- Frazier, S.D.; Aday, A.N.; Srubar, W. On-Demand Microwave-Assisted Fabrication of Gelatin Foams. Molecules 2018, 23, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Sun, Y.; Wang, J.; Ma, C.; Peng, S.; Cao, X.; Yang, L.; Ma, C.; Duan, G.; Liu, Z.; et al. A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption. E-Polymers 2022, 22, 468–477. [Google Scholar] [CrossRef]
- Murakami, K.; Yamakawa, Y.; Zhao, J.; Johnsen, E.; Ando, K. Ultrasound-Induced Nonlinear Oscillations of a Spherical Bubble in a Gelatin Gel. J. Fluid Mech. 2021, 924, A38. [Google Scholar] [CrossRef]
- Pezeshki Modaress, M.; Mirzadeh, H.; Zandi, M. Fabrication of a Porous Wall and Higher Inter-connectivity Scaffold Comprising Gelatin/Chitosan via Combination of Salt-Leaching and Lyophilization Methods. Iran. Polym. J. 2012, 21, 191–200. [Google Scholar] [CrossRef]
- Lu, B.; Wang, T.; Li, Z.; Dai, F.; Lv, L.; Tang, F.; Yu, K.; Liu, J.; Lan, G. Healing of skin wounds with a chitosan–gelatin sponge loaded with tannins and platelet-rich plasma. Int. J. Biol. Macromol. 2016, 82, 884–891. [Google Scholar] [CrossRef]
- La Gatta, A.; Tirino, V.; Cammarota, M.; La Noce, M.; Stellavato, A.; Pirozzi, A.V.A.; Portaccio, M.; Diano, N.; Laino, L.; Papaccio, G.; et al. Gelatin-Biofermentative Unsulfated Glycosaminoglycans Semi-Interpenetrating Hydrogels via Microbial-Transglutaminase Crosslinking Enhance Osteogenic Poten-tial of Dental Pulp Stem Cells. Regen. Biomater. 2021, 8, rbaa052. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, J.; Zhao, H.; Zhao, G.; Wan, Z.; Guo, R. A muscle-like magnetorheological actuator based on bidisperse magnetic particles enhanced flexible alginate-gelatin sponges. Smart Mater. Struct. 2020, 29, 015019. [Google Scholar] [CrossRef]
- Ohl, S.-W.; Klaseboer, E.; Khoo, B.C. Bubbles with shock waves and ultrasound: A review. Interface Focus 2015, 5, 20150019. [Google Scholar] [CrossRef]
- Ohl, C.D.; Ikink, R. Shock-Wave-Induced Jetting of Micron-Size Bubbles. Phys. Rev. Lett. 2003, 90, 214502. [Google Scholar] [CrossRef] [Green Version]
- Ter Haar, G. Ultrasonic imaging: Safety considerations. Interface Focus 2011, 1, 686–697. [Google Scholar] [CrossRef]
- Liang, H.-D.; Noble, J.A.; Wells, P.N.T. Recent advances in biomedical ultrasonic imaging techniques. Interface Focus 2011, 1, 475–476. [Google Scholar] [CrossRef]
- Wong, Z.Z.; Kripfgans, O.D.; Qamar, A.; Fowlkes, J.B.; Bull, J.L. Bubble evolution in acoustic droplet vaporization at physiological temperature via ultra-high speed imaging. Soft Matter 2011, 7, 4009–4016. [Google Scholar] [CrossRef]
- Dear, J.P.; Field, J.E. A study of the collapse of arrays of cavities. J. Fluid Mech. 1988, 190, 409–425. [Google Scholar] [CrossRef]
- Dear, J.P.; Field, J.E.; Walton, A.J. Gas compression and jet formation in cavities collapsed by a shock wave. Nature 1988, 332, 505–508. [Google Scholar] [CrossRef]
- Bourne, N.K.; Field, J.E. Shock-induced collapse of single cavities in liquids. J. Fluid Mech. 1992, 244, 225–240. [Google Scholar] [CrossRef]
- Kodama, T.; Takayama, K. Dynamic behavior of bubbles during extracorporeal shock-wave lithotripsy. Ultrasound Med. Biol. 1998, 24, 723–738. [Google Scholar] [CrossRef]
- Hopfes, T.; Wang, Z.; Giglmaier, M.; Adams, N.A. Collapse Dynamics of Bubble Pairs in Gelati-nous Fluids. Exp. Therm. Fluid Sci. 2019, 108, 104–114. [Google Scholar] [CrossRef]
- Gillitzer, R.; Neisius, A.; Wöllner, J.; Hampel, C.; Brenner, W.; Bonilla, A.A.; Thüroff, J. Low-Frequency Extracorporeal Shock Wave Lithotripsy Improves Renal Pelvic Stone Disintegration in a Pig Model. BJU Int. 2009, 103, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Saliu, O.D.; Mamo, M.; Ndungu, P.; Ramontja, J. The Making of a High Performance Supercapac-itor Active at Negative Potential Using Sulphonic Acid Activated Starch-Gelatin-TiO2 Nano-Hybrids. Arab. J. Chem. 2021, 14, 103242. [Google Scholar] [CrossRef]
- Fan, H.; Shen, W. Gelatin-Based Microporous Carbon Nanosheets as High Performance Superca-pacitor Electrodes. ACS Sustain. Chem. Eng. 2016, 4, 1328–1337. [Google Scholar] [CrossRef]
- Wang, S.; Han, L.; Liu, H.; Dong, Y.; Wang, X. Ionic Gelatin-Based Flexible Thermoelectric Gener-ator with Scalability for Human Body Heat Harvesting. Energies 2022, 15, 3441. [Google Scholar] [CrossRef]
- Wei, L.; Huang, J.; Yan, Y.; Cui, J.; Zhao, Y.; Bai, F.; Liu, J.; Wu, X.; Zhang, X.; Du, M. Substrate-Independent, Mechanically Tunable, and Scalable Gelatin Methacryloyl Hydrogel Coating with Drag-Reducing and Anti-Freezing Properties. ACS Appl. Polym. Mater. 2022, 4, 4876–4885. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Florez-Rojas, J.S.; Pradilla, D.; Valderrama-Rincón, J.D.; Cruz, J.C.; Reyes, L.H. Formulation and Characterization of Gelatin-Based Hydrogels for the Encapsulation of Kluyveromyces lactis—Applications in Packed-Bed Reactors and Probiotics Delivery in Humans. Polymers 2020, 12, 1287. [Google Scholar] [CrossRef]
- Zeng, L.; Tao, W.; Zhao, J.; Li, Y.; Li, R. Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement. Nanotechnol. Rev. 2022, 11, 625–636. [Google Scholar] [CrossRef]
- Simsek, G.M.; Barthes, J.; Muller, C.; McGuinness, G.B.; Vrana, N.E.; Yapici, G.G. PVA/gelatin-based hydrogel coating of nickel-titanium alloy for improved tissue-implant interface. Appl. Phys. A 2021, 127, 387. [Google Scholar] [CrossRef]
- Tung, A.T.; Park, B.-H.; Liang, D.H.; Niemeyer, G. Laser-machined shape memory alloy sensors for position feedback in active catheters. Sens. Actuators A Phys. 2008, 147, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Whitehead, D.; Boor, A.; Oppenlander, W.; Liu, Z.; Li, L. Picosecond Laser Micromachining of Nitinol and Platinum–Iridium Alloy for Coronary Stent Applications. Appl. Phys. A 2012, 106, 607–617. [Google Scholar] [CrossRef]
- Tan, K.M.; Tay, C.M.; Tjin, S.C.; Chan, C.C.; Rahardjo, H. High Relative Humidity Measurements Using Gelatin Coated Long-Period Grating Sensors. Sens. Actuators B Chem. 2005, 110, 335–341. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. In-situ polymerized cellulose nanocrystals (CNC)—Poly(l-lactide) (PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr. Polym. 2016, 153, 549–558. [Google Scholar] [CrossRef]
- Lee, C.; Kim, M.; Kim, Y.J.; Hong, N.; Ryu, S.; Kim, H.J.; Kim, S. Soft Robot Review. Int. J. Control Autom. Syst. 2017, 15, 3–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H. A Scientometric Review of Soft Robotics: Intellectual Structures and Emerging Trends Analysis (2010–2021). Front. Robot. AI 2022, 9, 868682. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Huang, W.; Shang, W.; Huang, Y.; Long, H.; Wu, X. Insect-Scale SMAW-Based Soft Robot With Crawling, Jumping, and Loading Locomotion. IEEE Robot. Autom. Lett. 2022, 7, 9287–9293. [Google Scholar] [CrossRef]
- Yang, X.; Shang, W.; Lu, H.; Liu, Y.; Yang, L.; Tan, R.; Wu, X.; Shen, Y. An agglutinate magnetic spray transforms inanimate objects into millirobots for biomedical applications. Sci. Robot. 2020, 5, eabc8191. [Google Scholar] [CrossRef]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.-F.; Tao, H.-Y.; Lin, C.-H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Fernandez, P.; Bausch, A.R. The compaction of gels by cells: A case of collective mechanical activity. Integr. Biol. 2009, 1, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Shi, X.; Wang, Y. Injectable Alendronate-Functionalized GelMA Hydrogels for Mineralization and Osteogenesis. RSC Adv. 2018, 8, 22764–22776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Young, J.L.; Holle, A.W.; Jeong, K.; Major, L.G.; Jeong, J.H.; Aman, Z.; Han, D.-W.; Hwang, Y.; Spatz, J.P.; et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann. Biomed. Eng. 2020, 48, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wachendörfer, M.; Schräder, P.; Buhl, E.M.; Palkowitz, A.L.; Ben Messaoud, G.; Richtering, W.; Fischer, H. A Defined Heat Pretreatment of Gelatin Enables Control of Hydrolytic Stability, Stiffness, and Microstructural Architecture of Fibrin–Gelatin Hydrogel Blends. Biomater. Sci. 2022, 10, 5552–5565. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, R.; Ren, X.; Zhang, K.; Li, J. 2D Gelatin Methacrylate Hydrogels with Tunable Stiffness for Investigating Cell Behaviors. ACS Appl. Bio Mater. 2019, 2, 570–576. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell Loaded GelMA: HEMA IPN Hydrogels for Corneal Stroma Engineering. J. Mater. Sci. Mater. Med. 2020, 31, 2. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M. Material Properties in Unconfined Compression of Gelatin Hydro-gel for Skin Tissue Engineering Applications. Biomed. Eng. Biomed. Tech. 2014, 59, 6. [Google Scholar]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D Bioprinting Using Cross-Linker-Free Silk–Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef]
- Serafim, A.; Tucureanu, C.; Petre, D.-G.; Dragusin, D.-M.; Salageanu, A.; Van Vlierberghe, S.; Dubruel, P.; Stancu, I.-C. One-Pot Synthesis of Superabsorbent Hybrid Hydrogels Based on Methacrylamide Gela-tin and Polyacrylamide. Effortless Control of Hydrogel Properties through Composition Design. New J. Chem. 2014, 38, 3112–3126. [Google Scholar] [CrossRef]
- Moghanian, A.; Portillo-Lara, R.; Sani, E.S.; Konisky, H.; Bassir, S.H.; Annabi, N. Synthesis and characterization of osteoinductive visible light-activated adhesive composites with antimicrobial properties. J. Tissue Eng. Regen. Med. 2020, 14, 66–81. [Google Scholar] [CrossRef]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and Characterization of Methacrylated Gelatin/Bacterial Cellulose Composite Hydrogels for Cartilage Tissue En-gineering. Regen. Biomater. 2020, 7, 195–202. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs: Gelatin-Methacrylamide Hydrogels as Potential Bio-materials for Fabrication. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edward, S.; Golecki, H.M. Gelatin Soft Actuators: Benefits and Opportunities. Actuators 2023, 12, 63. https://doi.org/10.3390/act12020063
Edward S, Golecki HM. Gelatin Soft Actuators: Benefits and Opportunities. Actuators. 2023; 12(2):63. https://doi.org/10.3390/act12020063
Chicago/Turabian StyleEdward, Sandra, and Holly M. Golecki. 2023. "Gelatin Soft Actuators: Benefits and Opportunities" Actuators 12, no. 2: 63. https://doi.org/10.3390/act12020063
APA StyleEdward, S., & Golecki, H. M. (2023). Gelatin Soft Actuators: Benefits and Opportunities. Actuators, 12(2), 63. https://doi.org/10.3390/act12020063




