Abstract
Powered Lower Limb Exoskeletons (PLLE) have attracted much interest due to their potential applications. They provide assistance for persons with disabilities to accomplish activities of daily living (ADL), and more importantly, assist them in achieving their rehabilitation goals. However, there is still uncertainty regarding the quality and benefits that PLLEs can offer to patients. This is due to limited usability and performance of current PLLEs, insufficient clinical use of PLLEs for different patients with high diversity in their disability type and impairment, and also the large gap between the technological state of the art and clinical expectations. In this study, we review and analyse various factors that can improve the effectiveness of PLLEs at yielding better assistance and rehabilitation training for patients with motor impairments. First, we define a set of criteria that characterize the majority of expectations for the rehabilitation and assistance domains and we use them for evaluating PLLEs depending on the context. Then, we include the effects of control strategies and combined approaches which include auxiliary devices such as functional electrical stimulation and smart crutches applied to PLLEs with regard to the criteria we defined.
1. Introduction
The effectiveness of powered lower limb exoskeletons (PLLEs) in assisting people with locomotion difficulties is pretty new to most of people [1,2,3,4]. Indeed, their initial use dates back to the 1960s [5] and their evolution has constantly been progressing over time, even attracting market and clinical interests in recent years. PLLEs are electro-mechanical robotic devices designed to be worn by human end-users which are used to assist them to enhance their lower limb locomotion capabilities.
PLLEs as robotic devices aim to fully or partially replicate human lower limb motor functionalities. Human lower limbs exploit a higher number of degrees of freedom (DoF) and possess a kinematically and dynamically redundant actuation system through muscle-ligament interactions [6,7]. PLLEs, instead, in the vast majority of cases, are under-actuated robotic platforms that incorporate the lower limbs in a parallel arrangement, and adopt rigid mechanical parts (links) and electromechanical motors (joints) located correspondingly to the lower limb joints, for their actuation systems.
Generally, PLLEs can be classified into three main types based on their applications [5,8]. The first type is used for power augmentation of healthy people whose target users are mainly soldiers or factory workers to increase their walking distance and durability and workers to help them with carrying and lifting heavy objects in factories. The second type aims at assisting subjects with locomotion impairments such as elderly people and individuals who suffer from stroke, spinal cord injury (SCI) or similar neurological diseases and disorders. Lastly, the third type has therapeutic applications and is used for training gait patterns and recovering locomotion capabilities of individuals with lower limb disabilities. Assistive and therapeutic PLLEs, despite structural and design similarities, have different purposes and are often referred to rehabilitation lower limb exoskeletons in the literature.
In this review paper, we mainly focus on rehabilitation PLLEs and investigate the performance of such platforms. Here, by platform, we do not define just the lower limb exoskeleton itself, but we also include control algorithms and strategies adopted to drive PLLEs and, additionally, we comprise auxiliary devices and sensors that can play a complementary but crucial role in the effectiveness of such machines. Although performance assessment of rehabilitation PLLEs is not straightforward and one could evaluate them with any arbitrary metric function due to lack of a ground truth that establishes a reference point of evaluation, we define a series of criteria that we hypothesize, among other important ones, offer more reasonable evaluations of the efficacy of PLLEs from the point of view of rehabilitation expectations and outcomes. In Section 3, we will present these criteria in detail and will address how and to what extent different platform aspects can affect one or more of these criteria.
Several recent review papers have already addressed the effectiveness of PLLEs and their various characteristics. Some of these reviews, clinically evaluated the rehabilitation outcomes of PLLEs in terms of ambulatory spatiotemporal parameters and energy expenditure measurements [9,10,11,12,13,14]. However, these papers did not evaluate the effect of robotic platform design choices on the rehabilitation outcomes. As the results of robot assisted rehabilitation training suggest, the positive outcomes of PLLEs are still poorly organised and, as a consequence, despite the overall positive results, their benefits and their correlation with the type of patient or the specific technology employed for the training are still unclear.
The lack of works that could systematically and deterministically prove the superiority of PLLEs over conventional therapy methods in gait rehabilitation has raised skepticism and disappointment among researchers and clinicians. In our opinion, this can be mainly because of the following reasons. First, PLLEs, despite their recent considerable advancements, still are emerging technologies at their early development stage, and their current form suffers from many limitations as they are not able to fully provide functional solutions for rehabilitation aims. Second, since PLLEs have not reached a sufficient clinical use yet; thus, robotic rehabilitation is restricted to an insufficient number of patients and/or trials with high heterogeneity. Indeed, diversity in age, weight, gender and underlying pathology type and level, make it difficult to discover subject-specific training protocols that can optimally benefit patients [15].
Third, research and industry groups are rapidly developing new PLLEs and delivering them to the market without considering their use in rehabilitation programs. The gap between engineering designs and clinical expectation is due to the lack of expertise in the vast and distributed rehabilitation science and poor understanding of physiological concepts by engineers who struggle to evaluate the development of PLLEs aligned with rehabilitation outcomes.
Due to the aforementioned reasons, assessment of PLLEs for rehabilitation purposes remains challenging. To overcome this challenge, one desirable but unfeasible approach is to evaluate rehabilitation outcomes of existing PLLEs with respect to a number of platform-related aspects and patient-specific condition variables including a broad range of parameters. Another potential approach, i.e., the one that we propose in this paper, is to study the effects of various platform-related aspects and to exploit reports of rehabilitation trial outcomes to identify the solutions that may improve the efficacy of current existing PLLEs through the performance evaluation criteria that we define in Section 3. In other words, in this work we explore various aspects of PLLEs to discover the most effective design paradigms and how to exploit them for rehabilitation purposes.
Many other review papers investigated PLLEs [16,17,18,19,20,21,22,23,24], mainly focusing on their mechanical structure, types, design, and clinical applications. A smaller number of reviews, explored control algorithms and strategies employed to drive PLLEs [25,26,27,28]. Alternative approaches such as the CYBATHLON competition [29], could provide a benchmark to compare the capability of exoskeletons in the accomplishment of daily life problems through competitions. However, metrics employed to define efficacy, such as the successful execution of a fixed set of tasks in a given time, are not sufficient to appropriately address the performance of exoskeletons [30]. Moreover, the skill of users can substantially influence the results. A more relevant approach in assessing performance of lower limb wearable devices was proposed in the EUROBENCH project [31]. They attempt to introduce standard benchmarks and appropriate performance indicators to help developers and engineers to select an optimal solution based on their needs. However, this approach mainly focuses on performance evaluation at an abstract level where the effect of platform-related solutions are not considered in evaluation process.
The remainder of this paper is organised as follows: Section 2, describes the inclusion and exclusion methods we adopted to select papers for both analysis and review parts. In Section 3, we introduce our performance evaluation criteria and study important parameters that can affect them. In Section 4, we present platform-related aspects in detail and investigate how they can promote different criteria and overall performance of the PLLEs, and Section 5 and Section 6 discuss and conclude this paper.
2. Search Methodology and Classification Method
The main goal of this paper is to gather all relevant publications presented in the literature to form a review and analysis over vast and distributed parameters that increase the effectiveness of PLLEs for patients with mobility problems. We focus on different influential factors and evaluate their corresponding effects on the criteria that we define in Section 3. We selected a number of topics to search as follows: (i) neurological disorders and their adverse effects on patients, (ii) clinical reports on outcomes of rehabilitation programs, (iii) works focused on the evaluation and benchmarking PLLEs, (iv) design and structure of existing PLLEs, (v) control strategies, and (vi) auxiliary devices. We found the majority of the papers with repeated queries for all the indicated topics on Google scholar and Scopus websites. Following the flowchart depicted in Figure 1, we attempted to include more sources by adding highly relevant papers cited in this step, and exclude the irrelevant papers while screening them by title or abstract. We employed a two-layer inclusion and exclusion approach, where the first layer excludes publications not meeting general requirements with the second layer excluding the remaining papers when they did not meet requirement of each specific topic technically.
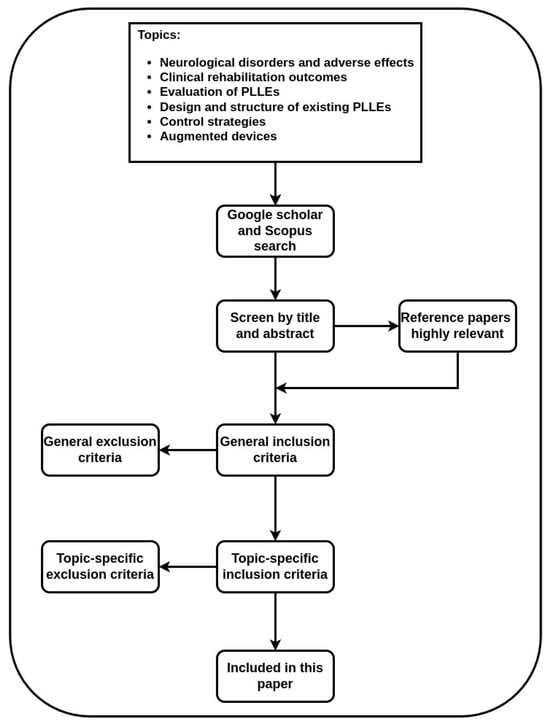
Figure 1.
Flowchart representing inclusion and exclusion criteria we adopted for paper selection.
The general inclusion approach comprises high-quality publications written in English, peer-reviewed conference or journal papers that study overground PLLEs. Works focusing on treadmill-based PLLEs, upper limb exoskeletons and prosthetic devices are immediately excluded. We imposed specific inclusion criteria for each topic such as neurological disorders that affect lower limbs. Publications on clinical reports of rehabilitation should address the indicated disorders and adopt PLLEs for rehabilitation. Papers regarding evaluation and benchmarking should focus on wearable robots and consider physical interactions with the user and the environment. The selected PLLEs in this paper are commercial products or are actively being used as research devices. Publications on control strategies apply the control algorithms on PLLEs and are published after 2016, as we observed that since that year, a huge number of publications directly target rehabilitation PLLEs. Papers with the focus on augmented devices include devices with a proper PLLE.
3. Performance Evaluation Criteria
In this section we discuss the evaluation of PLLEs, introducing the existing methods and we exploit them to support our proposed criteria that we use to evaluate PLLE platforms. We justify our approach in detail, explore the criteria we introduce, and identify underlying influential parameters for our eventual analysis. Furthermore, we define the target population of rehabilitation programs and highlight the deficits and impairments that should be addressed.
3.1. Criteria Selection
Assessing the emerging rehabilitation technologies in PLLEs is challenging due to the absence of the defined standard benchmarks for evaluation. The rapid development and delivery of PLLEs by research and industry groups necessitate the development of evaluation and benchmarking tools [32]. Consequently, the authors of [33] introduced a method for assessing and benchmarking for wearable robotic devices, which can be adopted for rehabilitation PLLEs assessment as well, since PLLEs belong to the same family and share many similarities with them in their structure, design and the interaction they have with the human body. They proposed to consider two important perspectives for evaluating these devices: (1) how these devices affect motor functions of the subjects, and (2) the interaction quality between patient and the device, as shown in Figure 2. As functional evaluation considers stability, efficiency, kinematics and dynamics of the patient and exoskeleton as a whole, the interaction evaluation includes physical and cognitive aspects between human and exoskeleton and also takes into account safety issues for the patient.
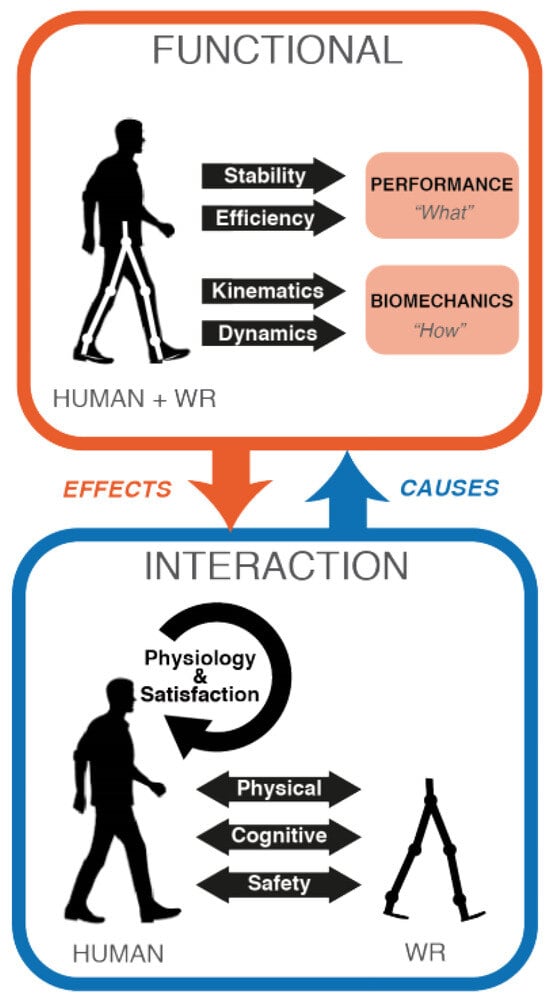
Figure 2.
Wearable robot (WR) evaluation perspectives to be considered as proposed by [33].
In functional evaluation, performance is defined as the level of accomplishment of a certain task such as walking speed, or body displacement. It can be further divided it into two sub-modules, namely, stability as an index to assess performance in the presence of disturbances and efficiency as an index to measure cost of transportation and energy consumption. The functional evaluation related to biomechanical aspects considers how PLLEs can compensate and improve walking patterns. This can be achieved through either the study of kinematic models where various aspects of the motion such as center of mass (CoM), center of pressure (CoP) or inter-limb coordination are important or focusing on dynamic parameters, where inertia, ground/contacts reaction forces and gravity are considered.
In interaction evaluation, physical human–robot interaction becomes of high importance and it measures how the interaction is exploited to assist during walking, not to limit the user movements and its capability in ensuring comfort of the user. Instead, cognitive interaction metric measures the gap between human wishes and robot actions.
Despite such a precise selection of evaluation perspectives and aspects, the presented method considers all the wearable robots and does not address rehabilitation PLLEs specifically. Consequently, functional and interaction aspects are defined as abstract concepts and require accurate and specific implementations to match to rehabilitation PLLEs and their expected outcomes. Furthermore, in many cases, evaluation of performance aspects individually does not lead to meaningful outcomes, while a combined set of aspects might give a better perspective.
To have a clear evaluation of rehabilitation PLLEs which considers the evaluation perspectives described above, we identify a series of performance evaluation criteria as follows:
- Gait rehabilitation (GAR);
- Balance maintenance (BAM);
- Decision making (DEM);
- Locomotion modes (LOM);
- Safety, ergonomy and clinical use (SEC).
Each of these criteria incorporates one or more of the mentioned functional and interaction evaluation perspectives as shown in Table 1. Note that these criteria are not mutually independent and they may overlap in some aspects, but each of them potentially considers important functionalities that PLLEs can deliver. They have been selected so that their overall contribution covers a wide and representative range of expected outcomes of both therapeutic and assistance applications. Later in this section, we will discuss in detail the importance of these criteria and potential factors that can influence them.

Table 1.
The one-to-some mappings between the evaluation perspectives presented in [33], and our performance evaluation criteria for PLLEs.
Table 1 describes the relationship and mappings between our described criteria and the ones defined in [33]. For instance, GAR criteria involves kinematics, dynamics, cognitive and physical interaction aspects. This is because different parameters, such as gait pattern, the quality of interaction between patient and the PLLE, and cognitive encouragement of patient have direct and indirect influence on improving GAR. Later in this section, we elaborate all the parameters that have heavy impacts on these mappings for all the mentioned criteria.
Robot-Assisted Rehabilitation and Target Population
Robot-assisted rehabilitation provides additional benefits compared to conventional rehabilitation methods. This is a consequence of many different factors that contribute at offering repeatable and intensive movement patterns. These are: robots are able to be highly precise, to convey a wide range of motions and forces, to sustain intensive assistance and training for a long period of time, to offer a standardized environment, to increase the dose and intensity of the therapy, and in some ways to assess the progress of recovery in terms of kinematics and kinetics.
PLLEs are expected to respond to a wide spectrum of demands to achieve efficient results within rehabilitation programs. Their primary objectives are to: (i) adaptively assist patients with various pathology types and levels in their daily living activities, (ii) provide modulated therapeutic solutions for recovery and training programs, (iii) reduce the physical burden of physiotherapists in rehabilitation activities, and (iv) continuously monitor performance and improvement of patients to adapt themselves accordingly to time-varying circumstances of patients.
Target population for PLLE-based rehabilitation are patients that have complete or severe impairment of lower limb mobility due to, e.g., neurological disorders with central nervous system (CNS) impairments, that mainly result from stroke, spinal cord injury (SCI), severe brain injury (SBI), multiple sclerosis (MS) and Parkinson’s disease.
To better understand how rehabilitation can effectively impact gait recovery, we need first to define what adverse effects different neurological disorders can cause on gait. Patients with SCI, SBI and MS have many deficits in common, as these disorders stem from CNS impairment. In many cases, findings made in rehabilitation of a CNS lesion such as stroke are valid for other lesions such as SCI and can hence be equivalent [34]. However, described neural disorders are caused due to different reasons and they manifest the impairments in various forms and levels. Despite the type and level of symptoms that patients may develop following various incidents, we need to consider that their mobility is compromised.
Stroke is the most common neural disorder with the largest patient size among all patients with CNS lesion. Road vehicle accidents [35] are common causes of SCI. SCI, also, depending on completeness and location of injury, leaves patients with different levels of impairment and it mainly manifests in the form of paraparesis and in severe cases quadriparesis.
The aforementioned target patients, despite their varying type and level of mobility impairments, share some common biological deficits. They suffer from reduced or null force and torque outputs of the muscles in their paretic limb, although reports stated that unaffected limbs also suffer to some degree from this deficit [36,37,38,39]. Furthermore, these patients have compromised motor control synergy, whereby synergy refers to the template of complex neuromechanical outputs based on a modular combination of basic activation patterns of muscle groups shifted in time and amplitude [40]. Abnormal synergies reduce the ability of patients to control certain muscle groups independently from others and lead to activation of undesired secondary torques (torques created in adjacent joints while attempting to exert torque in a certain joint) [41,42].
3.2. Selected Performance Criteria
In this part, we describe our selected performance criteria in detail. We also discuss the importance of them technically. Various parameters that can leave substantial impacts for improvement of these performance criteria are considered and we discuss how these parameters can be implemented employing PLLEs.
3.2.1. Gait Rehabilitation (GAR)
Gait rehabilitation or recovery is the primary objective of neurorehabilitation training [43] as neurological disorders leave individuals with gait abnormalities such as reduced walking speed, uncoordinated inter-limb walking pattern, asymmetric step length, muscle weakness, compromised balance and force reduction [44]. However, regaining all described skills and functionalities is typically possible and at the same time complete recovery requires extensive time and effort. Therefore, success in robot-assisted rehabilitation is defined by achieving the primary outcome, that is, regaining the ability to walk independently, and measuring secondary outcomes such as walking speed and walking capacity (metres walked in a certain time) [14,45]. The spectrum of human locomotion phases characterised by biomechanical events can be found in Figure 3.
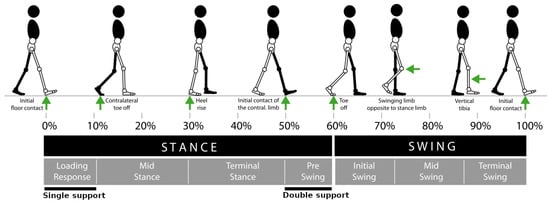
Figure 3.
Spectrum of human locomotion phases characterised by bio mechanical events. Overall gait is divided into stance and swing phases. Each phase comprises some sub-phase segments representing bio mechanical events [46].
GAR training should aim at overcoming not only primary complications of the incident but also to prevent deconditioning of patients due to effects of secondary complications. Early training plays a crucial role, specifically for stroke survivors, as the patients in the first six months after stroke have the maximum likelihood of recovery [47,48,49].
Generally, GAR is a process which involves motor learning that can be promoted through both recovery and compensation mechanisms. Recovery is referred as employment of undamaged regions of the brain to activate the same muscles used before the injury, while compensation, in contrast, is the recruitment of alternative muscles to accomplish the same task [50]. There are several factors that have proven to influence the gait rehabilitation program effectiveness. Intensity of activity is a fundamental factor for the improvement of the performances in motor learning [51]. Although intensive activity in very simple forms, i.e., repeating the same activities over and over again, might be considered the most effective approach, it can be misleading in terms of providing temporary improvements [52]; therefore, it can not be considered the sole factor contributing to optimal gait rehabilitation. Variability of activities, i.e., performing various activities in a random fashion, shifting the order of activities and changing the frequency of the rest times among them, leads to the achievement of a more effective performance in motor learning rather than performing simple repetitive activities [53,54]. This can be associated with the fact that, in random activities, each action is considered a problem to be solved and it will lead to skill learning, whereas simple activities promote the memorizing of motions and forces in time sequences and replicating them later [50]. Furthermore, therapy limited to simple tasks and targeting specific muscles may improve muscle motion and strength; however often it fails to translate into motor learning and function improvement [55,56,57]. Another important factor in recovery and motor learning is active physical and cognitive engagement of patients in training [34]. There are a number of ways to actively engage the patients during training. Adaptive assistance, for instance, cooperatively engages the patient and introduces gait variations that can lead to retraining the neural network in the spinal cord [58]. Cognitive challenges also can lead to actively engage patients to simultaneously improve motor and cognitive deficits [59]. Another approach to challenge patients while preventing cognitive stress and frustration is to adaptively change the difficulty of the training according to patients capabilities [60], as it has been shown that matching the complexity of tasks to the skill level of the patients leads to improvement in learning efficacy [61].
3.2.2. Balance Maintenance (BAM)
Balance dysfunction in patients with CNS lesions typically results in muscle weakness and uncoordinated gait patterns. Maintaining good levels of balance and stability over the rehabilitation session is one important limitation in current PLLEs and patients often struggle to keep their balance after standing up despite using crutches or while receiving physical support from a therapist. The balance mechanism revolves around the interaction of ground reaction forces (GRFs) applied to the human body and the kinematic configuration of body segments. The sum of GRF vectors acting on various parts of the human body pass through the CoP. Similarly, the configuration of body segments with different masses defines a point of application referred to as CoM. The Euclidean distance between the CoP and CoM is the determinant factor for balance and stability, thus balance assistance deals with both kinematics and kinetics of PLLE and the wearer as a whole. As shown in Figure 4 (left), whenever CoP resultant of GRF passes through the CoM, the net rate of angular momentum becomes zero and stability is guaranteed, while, in Figure 4 (right), the notable Euclidean distance between the GRF vector passing from the CoP to CoM creates a non-zero rate of angular momentum causing a fall situation. BAM can be defined as a sustainable locomotion pattern without risks of falling or injury. Balance allows for safe return to a statically stable configuration [62] and maintaining the upright posture of a human wearing a PLLE.
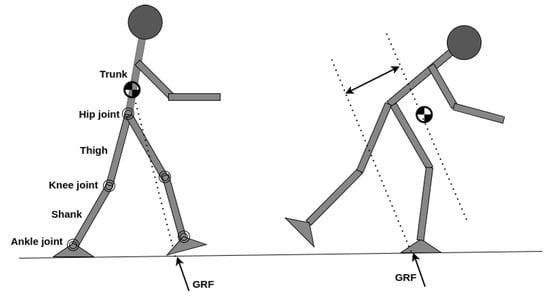
Figure 4.
(left) Vector of GRF (summation of ground normal reaction and friction forces) passes through CoM and rate of angular momentum is zero and balance is maintained. (right) GRF vector is creating a clock-wise rate of angular momentum and causing an excessive forward lean and enhancing fall danger.
Humans implicitly adopt ankle strategy, or hip strategy, or foot placement, or a modular combination of them [63], to reach a plateau state of balance while walking or standing. Ankle strategy regulates CoP positioning by means of ankle motions, and hip strategy by attempts to adjust angular momentum of body segments by means of hip flexion and extensions in order to reach a static balance. Ankle and hip strategies are more prevalent for the stance phase of locomotion, while foot placement adjustment is predominantly employed for dynamic balance while walking [64]. In static stability, projection of CoM in the transverse plane is restricted to remain inside the support polygon, that is, the contact area of the stance foot with the ground in case of single support and the polygon area that bounds both feet in the transverse plane in case of double support. Note that in the case of using a cane or crutches, the support polygon is formed by the bound area of feet and cane/crutches. Instead, in dynamic stability, CoM is able to freely move and exit the support polygon and provide faster gaits [65].
As dynamical balance of locomotion heavily relies on step length in the sagittal plane, step width in the frontal plane also plays a crucial role in maintaining balance [66]. Hip flexion and extension are required for balancing in the sagittal plane and hip abduction and adduction guarantee stability in the frontal plane. Figure 5 depicts human body planes in anatomical positions. Humans do not have direct control over GRF and CoP adjustment, and modulate it through dynamic coupling [67]; thus, it seems the optimal solution to maintain balance in PLLEs is to implement hip strategies in stance phase and adjust foot placements in swing phase.
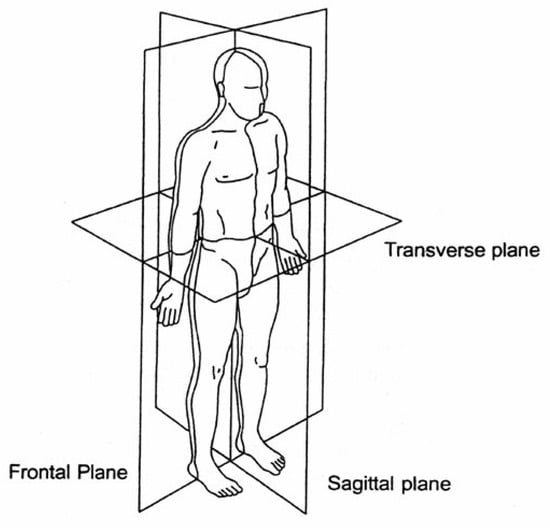
Figure 5.
The reference planes of the human body in the standard anatomical position [68].
Apart from the effective role of lower limb segments in BAM, upper limb segments such as trunk and arms are also important in both static and dynamic balance maintenance and recovery [69]. Humans exploit arm and trunk motions to adjust to or cancel angular momentum resultant from GRF in both the sagittal and frontal planes to keep a stable locomotion or to recover stability in the presence of perturbations. Note that, in PLLEs, the trunk and arms are not actuated and in many cases the trunk is spatially restricted and arms do not follow physiological motions to adjust the overall angular momentum of the body as crutches or walker are employed to guarantee stability. Nevertheless, a feedback from upper limb configurations and motions can be helpful with stability strategies.
BAM, balance recovery and fall prevention are not only meant to guarantee safety; in theory, appropriate designs of PLLEs and/or control strategies would free patients from dependence on the support of crutches or walkers, since walking the aid of crutches or walkers induces asymmetric and discrete walking patterns and uncoordinated lower and upper limb motions. This may lead to slow or inefficient rehabilitation programs, as training follows non-physiological patterns. Moreover, recovered patients may adapt to non-physiological lower and upper limb synergies with added asymmetries, leading to secondary complications as discussed in Section 3.2.1.
As discussed above, balance maintenance is a complex issue and both PLLE structure and control strategies play critical roles in determining it. However, we need to consider a number of factors once aiming to include balance and fall recovery solutions into a PLLE. Firstly, the design of PLLEs should follow human anatomy and be able to mimic human body and motions as precisely as possible to avoid introducing abnormalities. Secondly, balance maintenance solutions should not override normal human motions and do not act as opposing forces to muscles unless in severe balance challenges [70]. Third, following the definition of balance, as stated previously, balance solutions should be actively monitoring locomotion to predict falling hazard, and must be triggered only if danger of fall is detected.
3.2.3. Locomotion Modes (LOM)
Activities of daily living (ADL) are not limited to walking on level grounds and they incorporate numerous modes from ascending and descending stairs and slopes, to walking on unstructured terrain. One of the main drawbacks of current PLLEs that prevents them from being widely used both for assistive and therapeutic applications in daily activities is their inefficiency in autonomous recognition of LOMs, immediate adaptation and smooth switching among them. Current solutions employ manual switching of locomotion modes triggered by the user or an assistant. Although manual switching guarantees reliability and robustness, it poses discontinuity in locomotion and can implement only few locomotion switching schemes with prior knowledge of locomotion parameters. Moreover, manual switching to some degrees decreases the independence of the user in mobility. Switching LOM entails a number of considerations as kinematic and kinetic aspects may change drastically and need prompt actions to be taken by the PLLE.
It is noteworthy to indicate that errors in mode recognition would lead to incorrect mode changes and result in actions opposed to volitional control of the user, increasing the risk of harm [71].
The ability of switching from one locomotion mode to another, enhances balance maintenance and recovery, and ensures safety. Moreover, smooth transition among modes promotes natural and continuous gait patterns in patients.
3.2.4. Decision Making (DEM)
DEM is an important criteria for the effectiveness of the PLLEs. By DEM, we refer to the capability of the PLLEs in managing between the decisions and intentions coming from the patient and the actuation control of the PLLE. In other words, DEM evaluates how well the priority between human decisions and the exoskeleton actuation, and ultimately, the safety in the double-agent paradigm context, is addressed. We emphasize that the decisions are not related only to low-level gait pattern parameters; they also include high-level LOM functionalities.
PLLEs must ensure an effective physical and engaging cognitive interaction with the patients, as it is essential for both assistive and therapeutic applications. Solutions which focus on realising effective DEM promote patient engagement. They also cognitively encourage and motivate patients to take the lead in their activities and prevent them from slacking. Moreover, including decisions of patients in locomotion considers them as active human counterpart subjects rather than passive objects.
PLLEs can adapt to wearer decisions both proactively and reactively. Proactive adaptation refers to the recruitment of signals acquired from CNS or other musculoskeletal segments and identification of intents prior to muscle motions, and accordingly actuate the PLLE to fulfill human intentions, whereas reactive adaptation is functionally triggered by measuring signals such as force and position that arise from physical interaction between human and PLLE. In contrast to reactive adaptation that is formed on the basis of physical interaction, proactive adaptation is resultant from cognitive interactions.
The inclusion of decisions and intentions of patients can be obtained within volitional-based rehabilitation strategies such as assistance-as-needed [72,73], human-in-the-loop [74,75] and user-centered concepts [76], where the level of assistance is regulated with regard to detected intentions and the evolution of the therapy [77].
The aforementioned assistive strategies require precise intent recognition and analysis that takes into account the specific impairment of the patient, since different pathologies require specific interventions in assisting them.
Biological signals received from injured limbs are different from those of healthy limbs and in some cases are absent. Hence, in many cases they may provide misleading information of patient intents. Therefore, a PLLE might need to implement alternative approaches exploiting unaffected limbs [78,79,80,81], walking supports such as a cane, crutches or a walker, since they take part in the coordination of the gait and their movements represent upper limb motions and contribute to the whole synergy of locomotion [82].
The main factors that would help patients to gain control over the locomotion activities are gait initiation, gait termination, walking at a preferred speed, ability to switch between gait activities such as sitting down, standing up, ascending and descending stairs and slopes. To this aim, recognition of human decisions in locomotion can be divided into two types: gait phase recognition and gait activity recognition [83]. Human gait is formed by two main phases of stance and swing as shown in Figure 3. Generally, gait can be divided into seven discrete and ordered inter-state transitions, namely, loading response, mid stance, terminal stance and pre swing in the stance phase, and initial swing, mid swing and terminal swing in the swing phase. Since recognition of numerous state transitions is very complex and prone to error, in some cases, a more simple division, where only single support, double support and swing phases are detected, might lead to satisfactory results.
Among all the gait-related DEM activities, gait initiation and termination are of higher importance, as they potentially promote cognitive capability and provide a positive feeling for patients on being independent in controlling their locomotion. Gait initiation is the transition from the upright standing position to a toe-off event and is characterized by anticipatory postural adjustments where CoP is shifted [84], whereas gait termination is the transition from a steady-state gait to the upright standing; the person in the final step increases braking forces of the lead foot and reduces push-off of trailing foot [85], preparing for a complete stop. Recognition of gait initiation and termination, due to their complex biomechanical transition and the independent volition of the patient that can occur at any time, is a challenging task, whereas detecting gate phases between initiation and termination, due to cyclic nature of the gait, remains less challenging.
Similarly, gait activity recognition can be defined as a smooth transition from one locomotion mode to another where the termination of the former and the initiation of the latter are neglected to provide continuous and monotonic gait motion. Gait activity change detection, for analogous reasons of gait initiation and termination detection, is a challenging task and demands accurate detection and processing.
3.2.5. Safety, Ergonomics and Clinical Use (SEC)
Safety is a crucial aspect in the evaluation of PLLEs. Despite their indubious advantage on supporting patients, PLLEs can potentially cause severe adverse events. Safety requirements should be considered from the early development phase of design and throughout its entire lifetime. Safety can be defined as an abstract representation of the ability of PLLEs in reducing a specific risk or dealing with a specific hazard [86]. Adverse effects often vary from discomfort, cardiovascular events, pain and injuries to bone fractures [87,88]. Adverse events do not only compromise the health of patients, but they also prevent patients from receiving continuous training and assistance from PLLEs. Reports in [14] indicate that a notable number of dropouts in clinical trials for gait training were due to adverse events related to pain and skin breakdown [88]. The main sources of concern regarding safety arise from the PLLE itself, the prolonged interaction of vulnerable subjects with PLLEs and their interaction as a whole with the environment. Those risks might be relevant even in controlled environments such as clinics. PLLEs are required to be compliant to the human body to avoid injuries and, at the same time, they should be stiff enough to convey forces to the human musculoskeletal system through contact points such as straps and foot plates to achieve the desired effects [44,86].
Although all PLLEs are required to acquire safety and conformity approvals from representative authorities before being delivered to the market, several adverse events and safety issues have been reported in [89]. This study, in particular, reported adverse events and identified risks associated with a series of PLLEs which had received necessary approvals as commercial medical devices. Non-ergonomic design and device malfunctioning seem to be the major reasons for the incidents.
Fully ergonomic design of PLLEs is in theory difficult and in practice impossible, since PLLEs are designed to provide the minimal functional representation of a human lower body. A complete and human-like design of PLLEs with existing technologies adds high complexity in design, compromises technical performance and deviates PLLEs from their final objectives. However, monitoring and controlling ergonomy-related issues such as misalignment of PLLEs to lower body and non-physiological joint ranges can substantially reduce the associated risks.
Misalignment of PLLEs with the human lower body is related to non concentric alignment of PLLE actuators with human joint axes that can be the result of either wrong dimension of PLLE segments, or poor adherence of straps or cuffs with the patient during locomotion. This misalignment negatively impacts the physical interaction with the patient and introduces hazards to the human body [90]. To alleviate this concern, some works proposed polycentric knee joints [91] for improving kinematic compatibility with human knee, especially at a high degree of knee flexion. Another likely hazard is exceeding human joint limits such as moving the limb beyond anatomical joint range of motion (RoM), moving at high velocity and acceleration and introducing jerky motions, especially at the beginning of the gait. These limits are subjective and specific considerations need to be taken into account for each patient at different recovery levels.
Another important factor for rehabilitation in PLLEs is their applicability in clinical use. This can be achieved by considering several factors while designing PLLEs. Clinicians prefer to operate with easy-to-handle devices, where they can easily take control of the device and configure it for their clinical aims. Patients with different physical conditions demand a modular PLLE, easily adjustable to their physical characteristics. They also differ in their neurological disorder, and this requires configurable levels of assistance and modes of use, which should be readily accessible for clinicians. Another possible approach for improving SEC is to include human-centred design methods from the initial phases of design and development of PLLEs. However, these kind of methods are usually complicated and entail ethical, methodological and financial challenges [92].
Durability of batteries and possibility for easy and fast battery recharging and replacement are also important to ensure clinical use. Lower weight of the PLLE also increases portability and overall performance, since it is easier for clinicians to guide patients with severe balance problems. Other design choices, such as compact design of PLLEs, employing compact motors, avoiding exposed cables and proper placement of batteries and computer systems [93], are also fundamental for clinical use.
4. Platform-Related Apparatus
In this section, we study the main components which form the platform, namely, exoskeletons, control strategies and augmented devices. For exoskeletons, we investigate different types of PLLEs, their structure and components. We review current PLLE technologies and compare them with the best solutions using our proposed performance evaluation criteria. Similarly, for control strategies, we present a review of control strategies adopted for rehabilitation PLLEs and analyse how these approaches can best provide rehabilitative and assistive solutions considering our criteria. Finally, we explore possible accessory devices that can increase the performance, compensate for the limits of PLLEs, or provide other functionalities for the control of the devices. We investigate to what extent they are used and analyse their contribution in extending the performance of PLLEs.
4.1. Lower limb Exoskeletons
Rehabilitation PLLEs are designed and manufactured to partially or completely compensate for lower limbs’ loss of motor function, as shown in Figure 6. Those which partially compensate the patient strength provide support only for one or few joints or a single limb. In contrast, complete ones cover both legs and provide at least support for locomotion in the sagittal and/or frontal plane. Some examples of complete PLLEs are shown in Figure 6f–g. In this review paper we mainly focus on complete PLLEs.
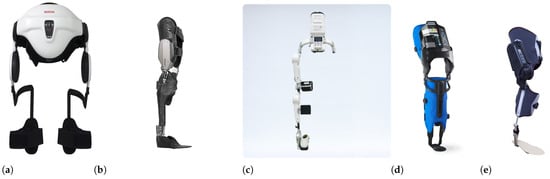
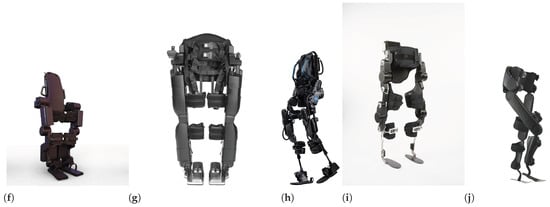
Figure 6.
Some examples of partial PLLEs: (a) Honda Walking Assist, with two active hip joints [94], (b) C-Brace orthotic device [95], (c) Hal single-leg exoskeleton with actuated hip and knee joints [79], (d) AlterG Bionic Leg with actuated knee joint [96] and (e) MAK, actuated knee [97]. Some examples of complete PLLEs: (f) Atalante of Wandercraft with 12 DoF [94], (g) REX PLLE with ten actuated joints [98], (h) Ekso GT exoskeleton with four actuated joints [99], (i) TWIN PLLE with four actuated joints [100] and (j) Indego PLLE with four actuated joints [101].
To achieve safe and efficient physical interaction between a PLLE and the wearer, PLLEs are required to give assistance following the anthropomorphic structure of the human body and to mimic biological lower limb functionalities. Each lower limb is composed of three main joints: One hip joint with three DoFs provides flexion and extension, abduction and adduction, and internal and external rotations, respectively, in sagittal, frontal and transverse anatomical planes. One single DoF joint actuates knee for both flexion and extension in sagittal plane. Similar to the hip, one joint with three DoFs applies rotations of the ankle in all three planes. Therefore, to achieve fully anthropomorphic kinematics, PLLEs should possess seven DoFs for each leg. Anthropomorphic design has a direct impact on BAM and SEC as humans maintain their static and dynamic balance by using all the joints and the corresponding muscles. We can hypothesize that anthropomorphic design can also affect the quality of GAR, since it enables the training of all the major and interconnected muscles. However, among developed PLLEs, a small number of them possess seven or more DoFs for each leg. Berkeley lower extremity exoskeleton (BLEEX) [102] was the first PLLE to aim for fully anthropomorphic design of PLLEs. Atalante and REX to date, are the only rehabilitation PLLEs that follow an anthropomorphic design with complete DoFs for each leg, although not all of their DoFs are actuated. The majority of rehabilitation PLLEs are underactuated, typically providing support only in the sagittal plane with active hip and knee without ankle actuators. Obviously enough, technological barriers, excessive weight, cost and size, geometrical and design limitations are the main factors preventing optimal anthropomorphic design of current PLLEs. A list of commercially available or common PLLEs used in research are shown in Figure 7a. As it is shown, all of the listed PLLEs support hip and knee flexion/extension (FE) actuation. Only few of them support ankle actuation and in the majority of designs, ankles are passive joints. Considerations about the weight and complexities of the system, design limitations and small range of motion (RoM) of some biological DoFs are the main reasons to alternate active actuators with passive joints. This implies that research groups and industries over the years have reached a consensus on commonalities of the design of PLLEs. However, the ankle joint, despite having a low range of motion, has a predominant role in the locomotion and its active presence provides immediate changes to gait kinematics [103]. It is the first joint to meet GRFs applied to the human foot and therefore its active presence becomes necessary in different phases of the gait. In the toe-off phase, it can act as a thrust force in pushing the human to move forward. In the swing phase, it applies enough force to damp the released energy of the toe-off phase to guarantee a stable and level foot positioning, and in the heel-strike phase, it absorbs the energy resulting from the impact with the ground and prevents the disturbance to knee and hip joints [104]. Information of RoM of the joints of PLLEs in Figure 7a demonstrate that there is considerable deviation between the RoM of joints and those of human walking [18]. This incompatibility is mainly corrected at the software level and by means of control strategies. However, exceeding the normal RoM of human joints, especially for patients with stiff muscles and fragile joints, imposes a high risk of danger and has negative influence on SEC.
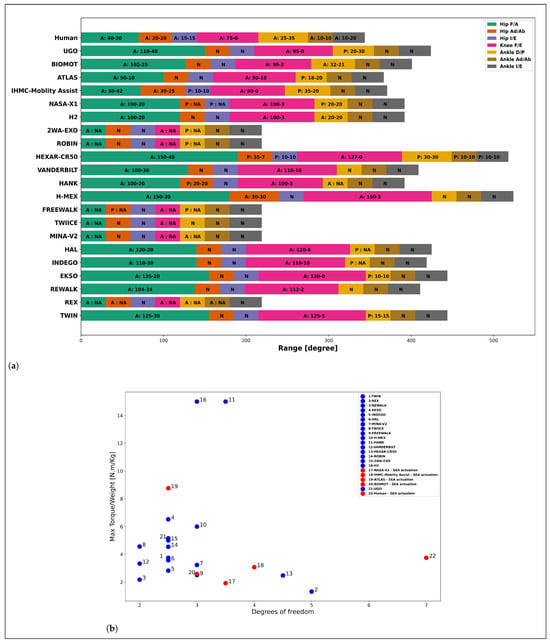
Figure 7.
(a) RoM of actuators for various PLLEs. A—Active joint, P—Passive joint, N—No joint, NA—information is not available. The x-axis represents the range in degrees. (b) Ratio of PLLE maximum torque of motors to overall weight of the exoskeleton as an indicator for deliverable power, w.r.t DoF of each leg. Passive joints are considered to have 0.5 DoF. Blue circles depict stiff joints and red circles joints with SEA actuation. Human biological lower limb is considered to weigh 40 kg and provide 150 Nm torque. We considered 50 Nm torque for PLLEs with unknown max torque value.
Another fundamental requirement for PLLE design is their mechanical compliance. PLLEs, due to their tight physical interaction with the lower limbs, are required to mechanically conform to lower limbs by compliantly adapting to the configuration of biological legs to avoid risks and ensure SEC. Moreover, compliant legs are essential to achieve basic walking mechanics [105], since stiff legs are not able to reproduce human-like motions. In humans, actuation and compliance mechanisms are possible by means of an integrated complex of muscles and tendons. Compliance is the result of the visco-elastic properties of muscles and series-elastic behavior of tendons. The activation and contraction velocity of muscles also play a role in the stiffness of the joints [46]. However, to implement human-like actuation and compliance mechanisms, recent works have inclined toward using elastic elements embedded within actuators. Although elastic elements in robotic systems in recent years have become prevalent and are widely used even in prosthetic systems, they are rarely adopted in the actuation of complete PLLEs as shown in Figure 7b.
Elastic elements can mainly be categorized into elements with fixed or variable stiffness, or in their arrangement form with the actuators, in series or in parallel. Each form of these arrangements has some advantages for PLLEs. Variable stiffness actuation (VSA) has been developed to replicate the ability of mammals in regulating joint stiffness. However, practical implementation of VSAs within PLLEs is difficult, given the typically cumbersome and complex nature of these devices. Alternatively, series-elastic actuation (SEA) or parallel elastic actuation (PEA), with fixed stiffness, provide an acceptable level of compliance for the actuation of PLLEs. A complete review on the use of compliant actuation in lower limb exoskeletons is addressed in [19]. Obviously enough, compliant behaviour of the mechanical structure of PLLEs and the corresponding actuation system, as it promotes human-like motion and interaction with the environment, has substantial effects on BAM and SEC.
4.2. Control Algorithms and Strategies
Control strategies play a fundamental role in the mobilization of the PLLE and the patient as a whole. To date, many different control strategies have been proposed in the field of PLLEs. In this section, we group these control strategies and evaluate their advantages w.r.t the criteria we defined before. We categorize control strategies based on their relevance in addressing rehabilitation aims and also in accomplishing ADL.
We differentiate them into following four categories: (I) assistive, (II) challenge-based, (III) volitional and (IV) autonomous controllers. In Table 2, we list a number of recent and promising works that represent interesting assistive, volitional and autonomous controllers. Please note that we could not find recent works compliant with our topic-specific criteria according to Figure 1 for challenge-based controllers.
4.2.1. Assistive Controllers
Assistive controllers aim for partial or complete assistance of the patients that comes in different frameworks as depicted in Figure 8, and are mainly in the following forms:
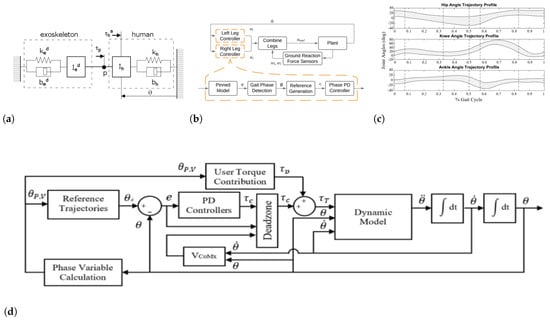
Figure 8.
Assistive controllers: (a) Linear model of the coupled 1-DOF human–exoskeleton system [106]. (b) Hybrid zero dynamic-based controller supplemented with foot detection-based switching condition [107]. (c) Performance of the proposed controller acting on paretic leg in forcing the trajectories back into the deadzone throughout gait [108]. (d) AAN controller with the user torque contribution for different gait pathologies [108].
- Trajectory-based (position, velocity);
- Force/flow field;
- Impedance and admittance;
- Virtual constraints.
Please note that the aforementioned assistive controllers are low-level controllers that may be adopted in other categories with an appropriate mid/high-level control framework. However, they are the common types of assistive controllers.
Trajectory-based controllers are widely adopted, especially in commercial devices, to ensure a stable walking pattern [109,110,111,112,113,114] and mainly promote SEC criteria. In these controllers, a predefined trajectory or pattern is regulated to fit patient-specific walking pattern requirements. Trajectories are usually based on biological walking patterns of humans in either the task or joint space. A low-level controller, usually a PID controller, is responsible for tracking the motor position/velocity trajectory with respect to time or the phase of the gait. These controllers provide full assistance to the patients for all walking modalities from sit-to-stand to walking. Trajectories are predefined for the whole walking time and patients are able only in some cases to start or stop a step through adjusting trunk tilts or sending manual commands.
The force/flow field control modality partially assists the patient in tracking the predefined trajectory in the context of assistance-as-needed [115,116,117,118,119,120]. These controllers allow patients to contribute to walking by inserting forces at any phase. Therefore, in this control modality, patients are allowed to deviate from the main trajectory, which can be predefined or designed online, to an admissible extent that is bound within a limited zone.

Table 2.
Description of the methods that some selected works implemented for assistive, volitional, or autonomous control strategies on PLLEs.
Table 2.
Description of the methods that some selected works implemented for assistive, volitional, or autonomous control strategies on PLLEs.
| Strategy | Ref. | Method | Description |
|---|---|---|---|
| Assistive | [121] | Impedance | Implemented an adaptive impedance controller by introducing an adaptive control law that considers interaction torque of human–exoskeleton. A radial basis function neural network was adopted to approximate the uncertain dynamic. |
| [114] | Tracking | Developed a parameter identification system for both human and exoskeleton and adopted linear quadratic programming method for trajectory tracking. | |
| [118] | AAN | A velocity field in task space was constructed to determine motion velocity limits at any configuration, and implemented a force field controller in joint space, embedding a tunnel for position tracking and control. | |
| [120] | Torque field | Proposed a torque field controller to guarantee coordination between limb joints and also allows the user to vary step length and time. | |
| [108] | Virtual constraints | Proposed a virtual constraint model to generate trajectories autonomously and to ensure stability of the user. This work adopted also AAN method to allow the user to deviate from desired trajectories within a deadzone that is defined according to velocity of CoM. | |
| Volitional | [122] | BMI | Implemented a control framework to feed human detected intentions to the PLLE considering safety issues. This work successfully detects six different intentions. |
| [123] | HMI | This work combined EEG and EMG signals to detect three movement classes of human intentions. | |
| Autonomous | [124] | Synergy | An adaptive synergy-based control is developed to realize impedance adjustment on affected leg following the kinesiological information of healthy leg. |
| [125] | Balancing | A safety index based on extrapolated CoM of human–exoskeleton model together with dynamic movement primitives is presented to promote BAM and control of exoskeleton. | |
| [126] | CoG transfer | It presents a control scheme capable of minimizing the reliance on pilot for transferring centre of gravity. It also provides an online trajectory planning considering CoG transfer and safety. |
Virtual potential fields, corresponding to deviations from the main trajectory, are created to guide the limbs toward the reference pattern. The force potential field [127,128] acts as a virtual wall for the motion of the legs, allowing the legs to move within a bounded area and not exceed that area. It is mainly formed of tangential, normal and damping terms. Tangential and normal forces are regulated w.r.t tangential and normal distances between the current and desired trajectories in the configuration space, while the damping force limits the velocity of the leg. Normal forces push the leg toward the desired trajectory, whereas the tangential forces help the leg to track the desired trajectory. Flow potential fields instead create a viscous fluid field and the force control is calculated relatively to the Euclidean distance of the actual velocity from the desired velocity and limit the velocity of the motion of the legs corresponding to the gait phase. Force/flow field controllers promote physical engagement of the patients in gait and also provide a sort of DEM ability for patients. However, superiority of this approach over conventional methods (assistive controllers) for GAR is not confirmed yet [72].
Controllers based on impedance/admittance are highly beneficial for PLLEs [121,129,130,131], since the soft physical contact of PLLEs with the user and also the hard GRF acting on user soles, specifically while walking on uneven terrains or crossing obstacles [106,129], demands impedance/admittance controllers to track the predefined trajectory well. The main objective of the impedance controller is to minimise the influence of external forces on the PLLE and ensure motion trajectory tracking. The impedance controller simulates the interaction forces as mechanical impedance. The controller, following the impedance model, generates forces and applies them to the perturbed PLLE to both guarantee a proper position control and also dissipate the effect of external forces [132]. In admittance control, on the other hand, the controller has the role of mechanical admittance and the PLLE is considered as mechanical impedance. Thus, the admittance controller, by following the impedance model and observing external forces, generates desired position motions and sends them to the PLLE for position control. Impedance-based controllers are essential for SEC, as they consider physical interactions between the user and the environment. They also have shown positive effects on GAR in SCI patients [133].
Virtual constraints control methods make it possible to virtually add limits to some parameters or shape the output trajectories to meet specific constraints [108,134,135,136,137]. This method provides inputs considering the actual state of the PLLE and also satisfying predefined virtual constraints. Therefore, in contrast to trajectory tracking, these methods can deliver online and stable trajectory solutions that take inter-limb coordination problem into consideration. Defined constraints are usually kinematic functions such as desired forward velocity, or dynamic functions such as desired angular momentum to follow. These constraints applied with the physical constraints, that is, the dynamic model of PLLE together with the wearer, yield dynamic and stable solutions in the presence of uncertainties. Moreover, the controller is not time-dependent and it mainly depends on the states of the system. An overview of the lower limb exoskeleton shared control system can be found in Figure 9.
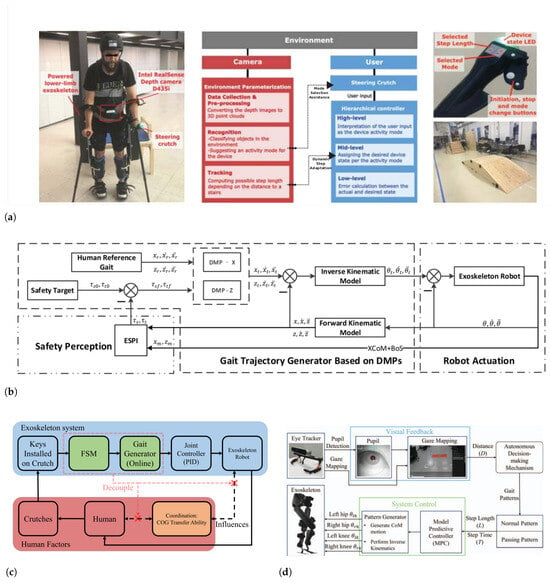
Figure 9.
(a) Overview of the lower limb exoskeleton shared control system [138]. (b) The architecture of the balance control strategy presented in [125]. (c) Control structure of the human–exoskeleton system [126], (d) Autonomous gait pattern planning framework presented in [139].
4.2.2. Challenge-Based Controllers
Challenge-based controllers aim to increase the physical engagement of the patients rather than partially or fully assist them. They can increase the difficulty of the training task, or oppose the movements of the limbs. This can be achieved through various strategies such as resistive training, error augmentation, inducing constraints and applying perturbations [140]. Resistive training adds resistance to the movement of the paretic limb of the patient as a mean to increase muscle strength [141,142,143]; however, this method does not lead to improvements of functional skills.
The error augmentation method was proposed to promote implicit learning and motor adaptation capacity, that is, the capability of modifying motor commands to archive desired motions. Error augmentation is accomplished by applying forces to the limbs in order to push them away from approaching to the desired trajectory. Usually a force/viscous potential field is adopted to generate the disruptive force opposite to assistive force/viscous field forces. This method has proved to be efficient in motor adaptation [144,145,146,147,148,149]. Another approach to challenge and engage the patients is to induce constraints on intact limbs so that the patient relies more on affected limb. Induced constraints promote and enforce symmetric use of limbs in walking [150,151].
It is noteworthy to mention that challenge-based controllers have rarely been studied and adopted despite the diffusion of PLLEs in robot-assisted therapy in recent years. However, these methods in traditional therapies have been widely used and have proved to be efficient in achieving positive GAR outcomes in the studies mentioned above.
4.2.3. Volitional Controllers
A possible alternative to keep patients cognitively engaged to promote GAR, and allow them to participate in DEM for accomplishing tasks, is to introduce a higher level of controllers that include the intention of patients in controlling PLLEs. Volitional controller frameworks embed at least two hierarchical levels of control: one to identify the intentions of the patient and the other to integrate and implement the intentions that can be related to any of the assistive or challenge-based methods we introduced previously. Intentions can range from walking pattern adjustments such as step length and velocity, to switching between LOMs. Intentions are mainly detected using sensory data or patient manual inputs. These sensors can be categorised into physiological sensors such as electromyographic (EMG) and electroencephalographic (EEG) that detect intentions prior to patient movements, proprioceptive/exteroceptive sensors such as IMU, GRF, and camera sensors that provide intention information after the movements. Various algorithms have been proposed to exploit the acquired data and transform them to meaningful actions. Various control schemes also have been developed to integrate human intentions to the low-level controllers to ensure stable and safe locomotion.
Methods based on EEG signals [122,123,152] attempt to recognise human intentions in the context of brain–machine interaction (BMI), adopting different classifiers such as linear discriminant analysis (LDA) and Gaussian mixture model (GMM). However, the problem with these methods is the reliability of signal acquisition and precision of the classifiers. EMG-based intent recognition also has its own problems, since reliability of the signals and robustness of the classification methods have excessive reliance on the placement location of EMG electrodes and user-specific adjustments. Therefore, EMG-based models are frequently employed in conjunction with other sensors such as GRF and IMU [153,154,155,156,157].
Human-recognised intentions at their best efficiency are not enough to independently drive PLLEs. Therefore, results of the recognition are usually limited to follow specific state transitions of control frameworks such as finite state machines (FSM).
4.2.4. Autonomous Controllers
Autonomous controllers are more adaptive and consider the environment, kinematics and dynamics of interactions and human posture effects for gait pattern design and control, compared to what discussed previously, [124,158,159,160,161,162,163,164,165,166,167,168]. These controllers have more complex schemes and are composed of different layers, including perception, high-level for decision making and low-level control for gait generation and actuation. These controllers aim at assisting patients, either fully autonomously or by means of human-in-loop strategies, with ADL rather than rehabilitation goals. These controllers aim at integrating different control modalities to promote flexibility in HMI and ensure safety and SEC.
The work presented in [138] provides a shared level of autonomy and control with the patient. In that work, a vision perception system is actively monitoring the environment for possible variations in LOM and shares the adaptation strategies with the patient for their further decisions as shown Figure 9a. It also provides adaptive step length adjustments for the user based on environmental conditions.
A recent work [125] introduced a safety index to generate adaptive solutions for gait patterns considering human balance and also exploiting reference trajectories. They used a framework, as shown in Figure 9b, to observe the integrated model of PLLE together with the user and by considering a safety and balance index, generate safe targets. They proposed a novel theory to trace the overall CoM of user and exoskeleton online and evaluate the safety and take further actions. A different approach for online gait pattern design was implemented in [126]. That work addressed the limitations of CoM transfer from one foot to another to start a step for paraplegic patients. The authors of that work introduced a control scheme as shown in Figure 9c, where the motion of patient and exoskeleton are separated and using a finite state machine, CoM transfer is accomplished in specific states of a step to relieve the patient of relying on CoM transfer for the initiation of a step.
Different approaches such as vision-assisted gait pattern generation [139], within more sophisticated control schemes, Figure 9d, have been proposed and implemented. Although many of these works proved to provide stable solutions for assisting patients, still their safety concerns and their reliance on extra instruments, uncertain reliability and robustness, and more importantly, the cognitive load and stress of the patients, make them less popular.
4.3. Augmented Devices and Sensors
Augmented devices, to date, are rarely adopted for assistive applications of PLLEs, and mainly are used for research purposes. However, their role can potentially increase the efficiency of PLLEs and provide additional functionalities for human locomotion. In the following part we list some of them that are widely used.
4.3.1. Functional Electrical Stimulation (FES)
The use of functional electrical stimulation (FES), either with PLLE or alone, for both gait rehabilitation aims [169] and assistance of ADL, has proved positive outcomes as shown in a review paper that gathered together all the rehabilitation benefits of FES [170]. Studies in [171] showed slight improvements in gait performance (speed, stride length and cadence) of patients with SCI lesions. Another study [172] presented more interesting results of using FES in reducing spastic muscle tone. FES can also be employed to assist the exoskeletons in the propulsion of patients. Stimulation of muscles generate metabolic energy and can be harvested to reduce the energy consumption of the PLLE during different LOM and gait phases [173,174,175,176,177,178].
However, recruitment of FES with PLLEs includes many challenges and limitations as follows: (i) stimulated muscles generate forces with random values that vary in time and for each patient, (ii) muscles go through physical fatigue after a certain time and dosage and (iii) difficulty in electrode placement for specific muscle groups. However, the use of FES with PLLEs has grown recently and different control frameworks have been proposed to overcome these limitations [179].
4.3.2. Sensors
Various sensors such as inertial measurement systems (IMU), GRF sensors, force and pressure sensors and distance measuring sensors have been adopted to measure different aspects of kinematics or dynamics of the interaction between patient and PLLE. IMU sensors are mainly used to detect the position of body segments, phase and event detection and also for LOM detection [180,181,182,183,184].
GRF and pressure sensors are usually placed on the soles of the shoes and provide information about the vector of interaction forces with the ground. These data are used to find CoP for BAM and proper control of the PLLE [185]. They also provide information on foot configuration relative to the ground [186], or directly being used for force control methods [187]. Mechanical sensors play complementary roles within different control schemes to promote BAM and DEM criteria.
4.3.3. EEG/EMG Sensors
EEG signals are widely used to measure brain activities in BMI systems with respect to their counterpart methods. This is due to their non-invasive monitoring and portability, and that they can be easily placed on the scalp to monitor brain activities. Different methods have been developed to detect neural features relevant to movement and walking [188]. EEG signals are classified based on range of frequencies, where each range represents a certain type of activity. For instance, EEG signals with range of 13–30 Hz, known as beta rhythm, represent activities regarding to movements, preparation for a movement, planning and imagining a movement [189]. Movement-related cortical potential and event-related desynchronization features are two major techniques used for movement activities detection [158,190,191,192]. The problem with EEG signals are the artifacts related to eye motion, cardiac activity and scalp muscle contraction [193]. They also suffer from high complexity that renders the feature extraction process error prone and time consuming. Another limitation of EEG signals for driving PLLEs is the separation of patterns regarding intention and motion of different legs since the motor cortex area of legs are quite close to each other [123]. Furthermore, long-term prior training is required for non real-time feature extractions that are composed of mainly 2–4 classes, including move, stop and turn left/right commands [190,191,194,195]. Reliability and real-time control of EEG signals to drive PLLEs, to date, remains challenging. Therefore, in many studies, EMG signals are integrated to better address BMI issues. However, BMI methods are extremely useful for DEM and have proved to be efficient in improving motor recovery and GAR [192].
EMG signals detect voluntary muscle contractions prior to muscle movements and provide information on muscle forces [196,197]. EMG signals, compared to EEG signals, have higher signal to noise ratio and have less time delay [198]. However, finding mapping models for EMG signal data and estimated active joint torques is challenging [199,200]. Additionally, electrode displacements, the time-varying property of EMG signals, and the problem of recalibrating the EMG-torque models after doffing and donning, limit their applications [201].
4.3.4. Smart Crutches (SMC)
As discussed in Section 4.1, the majority of complete PLLEs lack the actuation in the frontal plane and miss the ankle actuation in the sagittal plane. Therefore, users of PLLEs need to use crutches or walker to transfer their body weight and maintain balance. Moreover, they can be equipped with additional sensors and controls to provide more information on human motion. However, a limited number of PLLEs and researches employed smart crutches in their platforms. Smart crutches can potentially benefit PLLEs for different control schemes and establish a human machine interaction mean. Smart crutches employed in [202], exploit force sensors attached to the bottom of the structure to estimate GRF and IMU sensor to gain information on the placement distance for further step length control. A similar work [203] adopted strain gauges to measure GRF to feed the inverse dynamic model. The study presented in [204] attempted an online gait planning based on calculation of CoP exploiting GRF coming from smart crutches and also GRF from human-machine complex. Moreover, smart crutches are important for providing additional information on human–exoskeleton posture, therefore promoting DEM and SEC by adopting different control schemes.
5. Discussion
Evaluating the performance of PLLEs considering only parameters related to exoskeletons may result in incorrect conclusions. Moreover, the evaluation requires a large search space including all one-to-one or many-to-one relationships among all parameters and the performance index. Furthermore, physical characteristics and skill of the users may heavily influence the efficacy of the results. Our proposed method, besides the effects of exoskeletons, considers the effect of control algorithms and accessories on the performance evaluation process. Moreover, our method attempts to narrow down the large-space of the parameters into a small set of functional criteria that directly evaluate the rehabilitation and assistive performance of the PLLEs isolating the wearer-related impacts. Our proposed criteria, includes all the rehabilitation and assistive aspects as ultimate goals of PLLEs. However, the application of our method is limited to qualitative performance evaluation and for benchmarks, competitions or comparative evaluations, further quantifying mappings are needed to be integrated to this method.
Additionally, we conducted a review to identify the major factors that were reported to have high impacts on our selected criteria. We also introduced different parameters of PLLEs, i.e., aspects of exoskeletons such as kinematics, actuation and design, different types of controllers and various augmented devices that can be optionally utilized. To address the performance evaluation issue, based on accomplished studies and reviews in the literature, we performed an analysis on how those parameters of PLLEs can promote the major factors of our proposed criteria, and ultimately how performance of PLLEs is improved.
6. Conclusions
In this paper, we reviewed and analysed PLLE platforms including different design choices from PLLEs, control strategies and auxiliary devices. To better address assistance and rehabilitation challenges, we studied neurological disorders and the adverse effects that they have on patients. We also defined a set of criteria that address assistance and rehabilitation purposes for patients with neurological disorders. For all aspects of platform, we thoroughly investigated how they can affect the outcome of performance evaluation criteria we defined. We also reviewed recent works that adopted these aspects and included their advantages in terms of assistance and rehabilitation benefits to support the factors that promote our defined criteria. Our study aims at helping researchers and engineers, who design PLLEs or develop control strategies for rehabilitation goals, to highlight the gap between current technologies and rehabilitation expectations and also to indicate possible directions for improvement. Rehabilitation using PLLEs requires deep and vast knowledge over design, engineering and neuro-rehabilitation that are not readily accessible for all researchers. Moreover, the observed convergence in the design and structure of PLLEs in recent years to similar solutions due to many different reasons such as technological barriers, certification problems and lack of investments in the area of rehabilitation robotics, hinder outstanding technological breakthroughs towards effective rehabilitation.
Author Contributions
Conceptualization, All authors; writing–original draft preparation, H.K.; writing—review and editing, S.M.; visualization, C.V., M.L. and L.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Federici, S.; Meloni, F.; Bracalenti, M.; De Filippis, M.L. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: A systematic review. NeuroRehabilitation 2015, 37, 321–340. [Google Scholar] [PubMed]
- Lajeunesse, V.; Vincent, C.; Routhier, F.; Careau, E.; Michaud, F. Exoskeletons’ design and usefulness evidence according to a systematic review of lower limb exoskeletons used for functional mobility by people with spinal cord injury. Disabil. Rehabil. Assist. Technol. 2016, 11, 535–547. [Google Scholar] [PubMed]
- Lerner, Z.F.; Damiano, D.L.; Bulea, T.C. A lower-extremity exoskeleton improves knee extension in children with crouch gait from cerebral palsy. Sci. Transl. Med. 2017, 9, eaam9145. [Google Scholar]
- Benson, I.; Hart, K.; Tussler, D.; van Middendorp, J.J. Lower-limb exoskeletons for individuals with chronic spinal cord injury: Findings from a feasibility study. Clin. Rehabil. 2016, 30, 73–84. [Google Scholar] [PubMed]
- Dollar, A.M.; Herr, H. Lower Extremity Exoskeletons and Active Orthoses: Challenges and State-of-the-Art. IEEE Trans. Robot. 2008, 24, 144–158. [Google Scholar] [CrossRef]
- Pandy, M.G.; Andriacchi, T.P. Muscle and joint function in human locomotion. Annu. Rev. Biomed. Eng. 2010, 12, 401–433. [Google Scholar]
- Stanev, D.; Moustakas, K. Modeling musculoskeletal kinematic and dynamic redundancy using null space projection. PLoS ONE 2019, 14, e0209171. [Google Scholar]
- Babič, J.; Laffranchi, M.; Tessari, F.; Verstraten, T.; Novak, D.; Šarabon, N.; Ugurlu, B.; Peternel, L.; Torricelli, D.; Veneman, J.F. Challenges and solutions for application and wider adoption of wearable robots. Wearable Technol. 2021, 2, e14. [Google Scholar]
- Bunge, L.R.; Davidson, A.J.; Helmore, B.R.; Mavrandonis, A.D.; Page, T.D.; Schuster-Bayly, T.R.; Kumar, S. Effectiveness of powered exoskeleton use on gait in individuals with cerebral palsy: A systematic review. PLoS ONE 2021, 16, e0252193. [Google Scholar]
- Nedergård, H.; Arumugam, A.; Sandlund, M.; Bråndal, A.; Häger, C.K. Effect of robotic-assisted gait training on objective biomechanical measures of gait in persons post-stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2021, 18, 64. [Google Scholar]
- Shackleton, C.; Evans, R.; Shamley, D.; West, S.; Albertus, Y. Effectiveness of over-ground robotic locomotor training in improving walking performance, cardiovascular demands, secondary complications and user-satisfaction in individuals with spinal cord injuries: A systematic review. J. Rehabil. Med. 2019, 51, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Harvey, L.; Thomas, S.; Elsner, B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord 2017, 55, 722–729. [Google Scholar] [PubMed]
- Yeung, L.F.; Lau, C.C.; Lai, C.W.; Soo, Y.O.; Chan, M.L.; Tong, R.K. Effects of wearable ankle robotics for stair and over-ground training on sub-acute stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 19. [Google Scholar] [PubMed]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 5, CD006185. [Google Scholar]
- Esquenazi, A.; Talaty, M.; Jayaraman, A. Powered exoskeletons for walking assistance in persons with central nervous system injuries: A narrative review. PM&R 2017, 9, 46–62. [Google Scholar]
- Wang, T.; Zhang, B.; Liu, C.; Liu, T.; Han, Y.; Wang, S.; Ferreira, J.P.; Dong, W.; Zhang, X. A Review on the Rehabilitation Exoskeletons for the Lower Limbs of the Elderly and the Disabled. Electronics 2022, 11, 388. [Google Scholar]
- Kalita, B.; Narayan, J.; Dwivedy, S.K. Development of active lower limb robotic-based orthosis and exoskeleton devices: A systematic review. Int. J. Soc. Robot. 2021, 13, 775–793. [Google Scholar]
- Shi, D.; Zhang, W.; Zhang, W.; Ding, X. A review on lower limb rehabilitation exoskeleton robots. Chin. J. Mech. Eng. 2019, 32, 74. [Google Scholar]
- del Carmen Sanchez-Villamañan, M.; Gonzalez-Vargas, J.; Torricelli, D.; Moreno, J.C.; Pons, J.L. Compliant lower limb exoskeletons: A comprehensive review on mechanical design principles. J. Neuroeng. Rehabil. 2019, 16, 55. [Google Scholar]
- Huo, W.; Mohammed, S.; Moreno, J.C.; Amirat, Y. Lower limb wearable robots for assistance and rehabilitation: A state of the art. IEEE Syst. J. 2014, 10, 1068–1081. [Google Scholar] [CrossRef]
- Pamungkas, D.S.; Caesarendra, W.; Soebakti, H.; Analia, R.; Susanto, S. Overview: Types of lower limb exoskeletons. Electronics 2019, 8, 1283. [Google Scholar] [CrossRef]
- Contreras-Vidal, J.L.; Bhagat, N.A.; Brantley, J.; Cruz-Garza, J.G.; He, Y.; Manley, Q.; Nakagome, S.; Nathan, K.; Tan, S.H.; Zhu, F.; et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J. Neural Eng. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Ma, X.F.; Huo, B.; Zhang, Y.D.; Yu, X.Y. Recent advances on lower limb exoskeleton rehabilitation robot. Recent Patents Eng. 2017, 11, 194–207. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Cempini, M.; Oddo, C.M.; Vitiello, N. Review of assistive strategies in powered lower-limb orthoses and exoskeletons. Robot. Auton. Syst. 2015, 64, 120–136. [Google Scholar] [CrossRef]
- Baud, R.; Manzoori, A.R.; Ijspeert, A.; Bouri, M. Review of control strategies for lower-limb exoskeletons to assist gait. J. Neuroeng. Rehabil. 2021, 18, 119. [Google Scholar] [CrossRef]
- Li, W.Z.; Cao, G.Z.; Zhu, A.B. Review on Control Strategies for Lower Limb Rehabilitation Exoskeletons. IEEE Access 2021, 9, 123040–123060. [Google Scholar] [CrossRef]
- Tucker, M.R.; Olivier, J.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; Millán, J.d.R.; Riener, R.; Vallery, H.; Gassert, R. Control strategies for active lower extremity prosthetics and orthotics: A review. J. Neuroeng. Rehabil. 2015, 12, 1. [Google Scholar] [CrossRef]
- Riener, R. The Cybathlon promotes the development of assistive technology for people with physical disabilities. J. Neuroeng. Rehabil. 2016, 13, 49. [Google Scholar] [CrossRef]
- Pinto-Fernandez, D.; Torricelli, D.; del Carmen Sanchez-Villamanan, M.; Aller, F.; Mombaur, K.; Conti, R.; Vitiello, N.; Moreno, J.C.; Pons, J.L. Performance evaluation of lower limb exoskeletons: A systematic review. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1573–1583. [Google Scholar] [CrossRef]
- Torricelli, D.; Pons, J.L. Eurobench: Preparing robots for the real world. In Proceedings of the International Symposium on Wearable Robotics; Springer: Berlin/Heidelberg, Germany, 2018; pp. 375–378. [Google Scholar]
- Torricelli, D.; Rodriguez-Guerrero, C.; Veneman, J.F.; Crea, S.; Briem, K.; Lenggenhager, B.; Beckerle, P. Benchmarking wearable robots: Challenges and recommendations from functional, user experience, and methodological perspectives. Front. Robot. AI 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, D.; Del Ama, A.J.; Gonzalez, J.; Moreno, J.; Gil, A.; Pons, J.L. Benchmarking lower limb wearable robots: Emerging approaches and technologies. In Proceedings of the 8th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Corfu, Greece, 1–3 July 2015; pp. 1–4. [Google Scholar]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [PubMed]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995. [Google Scholar] [PubMed]
- Adams, R.; Gandevia, S.; Skuse, N. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain 1990, 113, 1459–1476. [Google Scholar] [CrossRef]
- Da Vies, J.; Mayston, M.; Newham, D. Electrical and mechanical output of the knee muscles during isometric and isokinetic activity in stroke and healthy adults. Disabil. Rehabil. 1996, 18, 83–90. [Google Scholar] [CrossRef]
- Harris, M.; Polkey, M.; Bath, P.M.; Moxham, J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin. Rehabil. 2001, 15, 274–281. [Google Scholar] [CrossRef]
- McComas, A.; Sica, R.; Upton, A.; Aguilera, N. Functional changes in motoneurones of hemiparetic patients. J. Neurol. Neurosurg. Psychiatry 1973, 36, 183–193. [Google Scholar]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Ting, L.H.; McKay, J.L. Neuromechanics of muscle synergies for posture and movement. Curr. Opin. Neurobiol. 2007, 17, 622–628. [Google Scholar]
- Hidler, J.; Carroll, M.; Federovich, E.; Lacsamana, C. Loss of differential muscle control leads to weakness and discoordination in individuals with acute hemiparetic stroke. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; IEEE: New York, NY, USA, 2003; Volume 2, pp. 1468–1470. [Google Scholar]
- Dobkin, B.H. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Berté, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [PubMed]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [PubMed]
- Torricelli, D.; Gonzalez, J.; Weckx, M.; Jiménez-Fabián, R.; Vanderborght, B.; Sartori, M.; Dosen, S.; Farina, D.; Lefeber, D.; Pons, J.L. Human-like compliant locomotion: State of the art of robotic implementations. Bioinspiration Biomimetics 2016, 11, 051002. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.; Brocklehurst, J.; Richards, B.; Laycock, P. The rate of recovery from stroke-and its measurement. Int. Rehabil. Med. 1981, 3, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Skilbeck, C.E.; Wade, D.T.; Hewer, R.L.; Wood, V.A. Recovery after stroke. J. Neurol. Neurosurg. Psychiatry 1983, 46, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Cifu, D.X.; Stewart, D.G. Factors affecting functional outcome after stroke: A critical review of rehabilitation interventions. Arch. Phys. Med. Rehabil. 1999, 80, S35–S39. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Schmidt, R.A.; Lee, T.D.; Winstein, C.; Wulf, G.; Zelaznik, H.N. Motor Control and Learning: A Behavioral Emphasis; Human Kinetics: Windsor, ON, Canada, 2018. [Google Scholar]
- Winstein, C.; Wing, A.; Whitall, J. Motor control and learning principles for rehabilitation of upper limb movements after brain injury. Handb. Neuropsychol. 2003, 9, 79–138. [Google Scholar]
- Shea, J.B.; Morgan, R.L. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J. Exp. Psychol. Hum. Learn. Mem. 1979, 5, 179. [Google Scholar] [CrossRef]
- Hanlon, R.E. Motor learning following unilateral stroke. Arch. Phys. Med. Rehabil. 1996, 77, 811–815. [Google Scholar] [CrossRef]
- Van Peppen, R.P.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.; Van der Wees, P.J.; Dekker, J. The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clin. Rehabil. 2004, 18, 833–862. [Google Scholar] [PubMed]
- Langhammer, B.; Stanghelle, J.K. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: A randomized controlled study. Clin. Rehabil. 2000, 14, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Pennycott, A.; Wyss, D.; Vallery, H.; Klamroth-Marganska, V.; Riener, R. Towards more effective robotic gait training for stroke rehabilitation: A review. J. Neuroeng. Rehabil. 2012, 9, 65. [Google Scholar] [CrossRef]
- Riener, R.; Lünenburger, L.; Colombo, G. Human-centered robotics applied to gait training and assessment. J. Rehabil. Res. Dev. 2006, 43, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Heins, S.; Dehem, S.; Montedoro, V.; Dehez, B.; Edwards, M.; Stoquart, G.; Rocca, F.; De Deken, P.H.; Mancas, M.; Lejeune, T. Robotic-assisted serious game for motor and cognitive post-stroke rehabilitation. In Proceedings of the 2017 IEEE 5th International Conference on Serious Games and Applications for Health (SeGAH), Perth, WA, Australia, 2–4 April 2017; IEEE: New York, NY, USA, 2017; pp. 1–8. [Google Scholar]
- Metzger, J.C.; Lambercy, O.; Califfi, A.; Dinacci, D.; Petrillo, C.; Rossi, P.; Conti, F.M.; Gassert, R. Assessment-driven selection and adaptation of exercise difficulty in robot-assisted therapy: A pilot study with a hand rehabilitation robot. J. Neuroeng. Rehabil. 2014, 11, 154. [Google Scholar]
- Choi, Y.; Qi, F.; Gordon, J.; Schweighofer, N. Performance-based adaptive schedules enhance motor learning. J. Mot. Behav. 2008, 40, 273–280. [Google Scholar] [CrossRef]
- Goswami, A.; Kallem, V. Rate of change of angular momentum and balance maintenance of biped robots. In Proceedings of the IEEE International Conference on Robotics and Automation, ICRA’04, New Orleans, LA, USA, 26 April–1 May 2004; IEEE: New York, NY, USA, 2004; Volume 4, pp. 3785–3790. [Google Scholar]
- Kuo, A.D.; Zajac, F.E. Human standing posture: Multi-joint movement strategies based on biomechanical constraints. Prog. Brain Res. 1993, 97, 349–358. [Google Scholar]
- Townsend, M.A. Biped gait stabilization via foot placement. J. Biomech. 1985, 18, 21–38. [Google Scholar] [CrossRef]
- Hamza, M.F.; Ghazilla, R.A.R.; Muhammad, B.B.; Yap, H.J. Balance and stability issues in lower extremity exoskeletons: A systematic review. Biocybern. Biomed. Eng. 2020, 40, 1666–1679. [Google Scholar]
- MacKinnon, C.D.; Winter, D.A. Control of whole body balance in the frontal plane during human walking. J. Biomech. 1993, 26, 633–644. [Google Scholar] [CrossRef]
- Vukobratovic, M.; Borovac, B.; Surla, D.; Stokic, D. Biped Locomotion: Dynamics, Stability, Control and Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 7. [Google Scholar]
- Vaughan, C.L.K. The Biomechanics of Human Locomotion. 2009. Available online: https://open.uct.ac.za/handle/11427/3491 (accessed on 26 July 2023).
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieen, J.H. The effects of arm swing on human gait stability. J. Exp. Biol. 2010, 213, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Vallery, H.; Bögel, A.; O’ Brien, C.; Riener, R. Cooperative control design for robot-assisted balance during gait. De Gruyter (O) 2012, 60, 715–720. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Huang, H. Effects of locomotion mode recognition errors on volitional control of powered above-knee prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Alingh, J.; Fleerkotte, B.; Groen, B.; Rietman, J.; Weerdesteyn, V.; van Asseldonk, E.; Geurts, A.; Buurke, J. Effect of assist-as-needed robotic gait training on the gait pattern post stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 26. [Google Scholar] [CrossRef]
- Cai, L.L.; Fong, A.J.; Liang, Y.; Burdick, J.; Edgerton, V.R. Assist-as-needed training paradigms for robotic rehabilitation of spinal cord injuries. In Proceedings of the 2006 IEEE International Conference on Robotics and Automation, ICRA 2006, Orlando, FL, USA, 15–19 May 2006; IEEE: New York, NY, USA, 2006; pp. 3504–3511. [Google Scholar]
- Li, Z.; Zhao, K.; Zhang, L.; Wu, X.; Zhang, T.; Li, Q.; Li, X.; Su, C.Y. Human-in-the-loop control of a wearable lower limb exoskeleton for stable dynamic walking. IEEE/ASME Trans. Mechatronics 2020, 26, 2700–2711. [Google Scholar] [CrossRef]
- Zhang, J.; Fiers, P.; Witte, K.A.; Jackson, R.W.; Poggensee, K.L.; Atkeson, C.G.; Collins, S.H. Human-in-the-loop optimization of exoskeleton assistance during walking. Science 2017, 356, 1280–1284. [Google Scholar] [CrossRef]
- Meyer, J.T.; Schrade, S.O.; Lambercy, O.; Gassert, R. User-centered design and evaluation of physical interfaces for an exoskeleton for paraplegic users. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; IEEE: New York, NY, USA, 2019; pp. 1159–1166. [Google Scholar]
- Rajasekaran, V.; Aranda, J.; Casals, A. Compliant gait assistance triggered by user intention. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: New York, NY, USA, 2015; pp. 3885–3888. [Google Scholar]
- Suzuki, K.; Kawamura, Y.; Hayashi, T.; Sakurai, T.; Hasegawa, Y.; Sankai, Y. Intention-based walking support for paraplegia patient. In Proceedings of the 2005 IEEE International Conference on Systems, Man and Cybernetics, Waikoloa, HI, USA, 12 October 2005; IEEE: New York, NY, USA, 2005; Volume 3, pp. 2707–2713. [Google Scholar]
- Kawamoto, H.; Hayashi, T.; Sakurai, T.; Eguchi, K.; Sankai, Y. Development of single leg version of HAL for hemiplegia. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; IEEE: New York, NY, USA, 2009; pp. 5038–5043. [Google Scholar]
- Ferris, D.P.; Huang, H.J.; Kao, P.C. Moving the arms to activate the legs. Exerc. Sport Sci. Rev. 2006, 34, 113–120. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Lamontagne, A.; De Serres, S.J. The coordination of upper and lower limb movements during gait in healthy and stroke individuals. Gait Posture 2009, 29, 11–16. [Google Scholar] [CrossRef]
- Hassan, M.; Kadone, H.; Suzuki, K.; Sankai, Y. Exoskeleton robot control based on cane and body joint synergies. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012; pp. 1609–1614. [Google Scholar]
- Li, Y.; Sun, H.; Wang, C.; Yang, Y.; Fan, C.; Zhang, Z.; Rui, Y. Key Technologies of Lower Limb Power-Assisted Exoskeleton Robots: A Review. In Proceedings of the 2021 6th International Conference on Control, Robotics and Cybernetics (CRC), Shanghai, China, 9–11 October 2021; IEEE: New York, NY, USA, 2021; pp. 25–31. [Google Scholar]
- Mickelborough, J.; Van Der Linden, M.; Tallis, R.; Ennos, A. Muscle activity during gait initiation in normal elderly people. Gait Posture 2004, 19, 50–57. [Google Scholar] [CrossRef]
- Novak, D.; Reberšek, P.; De Rossi, S.M.M.; Donati, M.; Podobnik, J.; Beravs, T.; Lenzi, T.; Vitiello, N.; Carrozza, M.C.; Munih, M. Automated detection of gait initiation and termination using wearable sensors. Med. Eng. Phys. 2013, 35, 1713–1720. [Google Scholar] [CrossRef]
- Bessler, J.; Prange-Lasonder, G.B.; Schaake, L.; Saenz, J.F.; Bidard, C.; Fassi, I.; Valori, M.; Bach Lassen, A.; Buurke, J.H. Safety assessment of rehabilitation robots: A review identifying safety skills and current knowledge gaps. Front. Robot. AI 2021, 8, 33. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef]
- Bessler, J.; Prange-Lasonder, G.B.; Schulte, R.V.; Schaake, L.; Prinsen, E.C.; Buurke, J.H. Occurrence and type of adverse events during the use of stationary gait robots—A systematic literature review. FRontiers Robot. AI 2020, 7, 557606. [Google Scholar] [CrossRef]
- He, Y.; Eguren, D.; Luu, T.P.; Contreras-Vidal, J.L. Risk management and regulations for lower limb medical exoskeletons: A review. Med. Devices 2017, 10, 89. [Google Scholar] [CrossRef]
- Rocon, E.; Ruiz, A.; Raya, R.; Schiele, A.; Pons, J.L.; Belda-Lois, J.; Poveda, R.; Vivas, M.; Moreno, J. Human-robot physical interaction. Wearable Robot. Biomechatron. Exoskelet. 2008, 127–163. [Google Scholar]
- Chaichaowarat, R.; Macha, V.; Wannasuphoprasit, W. Passive knee exoskeleton using brake torque to assist stair ascent. In Proceedings of the 2020 IEEE REGION 10 CONFERENCE (TENCON), Osaka, Japan, 16–19 November 2020; IEEE: New York, NY, USA, 2020; pp. 1165–1170. [Google Scholar]
- Ármannsdóttir, A.L.; Beckerle, P.; Moreno, J.C.; van Asseldonk, E.H.; Manrique-Sancho, M.T.; Del-Ama, A.J.; Veneman, J.F.; Briem, K. Assessing the involvement of users during development of lower limb wearable robotic exoskeletons: A survey study. Hum. Factors 2020, 62, 351–364. [Google Scholar] [CrossRef]
- Harman, E.; Hoon, K.; Frykman, P.; Pandorf, C. The Effects of Backpack Weight on the Biomechanics of Load Carriage; Technical Report; Army Research Inst of Environmental Medicine Natick Ma Military Performancediv: Natick, MA, USA, 2000. [Google Scholar]
- Kitatani, R.; Ohata, K.; Takahashi, H.; Shibuta, S.; Hashiguchi, Y.; Yamakami, N. Reduction in energy expenditure during walking using an automated stride assistance device in healthy young adults. Arch. Phys. Med. Rehabil. 2014, 95, 2128–2133. [Google Scholar] [CrossRef]
- Pröbsting, E.; Kannenberg, A.; Zacharias, B. Safety and walking ability of KAFO users with the C-Brace® Orthotronic Mobility System, a new microprocessor stance and swing control orthosis. Prosthetics Orthot. Int. 2017, 41, 65–77. [Google Scholar] [CrossRef]
- AlterG, Inc. Bionic Leg User Manual. 2015. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiZ4Or6l5-CAxW9slYBHZeXDp0QFnoECBUQAQ&url=https%3A%2F%2Falterg.com%2Fwp-content%2Fuploads%2F2022%2F08%2FAlterG-M-F320-User-Man-114422-00-Rev-C.pdf&usg=AOvVaw1pLx3T8QMD1QjeJyEBjt2k&opi=89978449 (accessed on 25 July 2023).
- Puyuelo-Quintana, G.; Cano-De-La-Cuerda, R.; Plaza-Flores, A.; Garces-Castellote, E.; Sanz-Merodio, D.; Goñi-Arana, A.; Marín-Ojea, J.; García-Armada, E. A new lower limb portable exoskeleton for gait assistance in neurological patients: A proof of concept study. J. Neuroeng. Rehabil. 2020, 17, 60. [Google Scholar] [CrossRef]
- Birch, N.; Graham, J.; Priestley, T.; Heywood, C.; Sakel, M.; Gall, A.; Nunn, A.; Signal, N. Results of the first interim analysis of the RAPPER II trial in patients with spinal cord injury: Ambulation and functional exercise programs in the REX powered walking aid. J. Neuroeng. Rehabil. 2017, 14, 60. [Google Scholar] [CrossRef]
- Bionics, E. Ekso GT Lower Limb Exoskeleton. 2022. Available online: https://www.eksobionics.com/ (accessed on 28 July 2023).
- Laffranchi, M.; D’Angella, S.; Vassallo, C.; Piezzo, C.; Canepa, M.; De Giuseppe, S.; Di Salvo, M.; Succi, A.; Cappa, S.; Cerruti, G.; et al. User-Centered design and development of the modular TWIN lower limb exoskeleton. Front. Neurorobot. 2021, 15, 709731. [Google Scholar] [CrossRef]
- Parker Hannifin Corporation Indego Lower Limb Exoskeleton. 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiMv6CjmJ-CAxWzlFYBHYLnAIQQFnoECBYQAQ&url=https%3A%2F%2Fwww.medtechdive.com%2Fnews%2Fekso-bionics-parker-hannifin-sells-indego-exoskeleton%2F638167%2F&usg=AOvVaw3KABjpFrVvWrvzOAn6CyQg&opi=89978449 (accessed on 28 July 2023).
- Zoss, A.B.; Kazerooni, H.; Chu, A. Biomechanical design of the Berkeley lower extremity exoskeleton (BLEEX). IEEE/ASME Trans. Mechatronics 2006, 11, 128–138. [Google Scholar]
- Crabtree, C.A.; Higginson, J.S. Modeling neuromuscular effects of ankle foot orthoses (AFOs) in computer simulations of gait. Gait Posture 2009, 29, 65–70. [Google Scholar]
- Miyazaki, S.; Yamamoto, S.; Kubota, T. Effect of ankle-foot orthosis on active ankle moment in patients with hemiparesis. Med. Biol. Eng. Comput. 1997, 35, 381–385. [Google Scholar] [CrossRef]
- Geyer, H.; Seyfarth, A.; Blickhan, R. Compliant leg behaviour explains basic dynamics of walking and running. Proc. R. Soc. B Biol. Sci. 2006, 273, 2861–2867. [Google Scholar]
- Aguirre-Ollinger, G.; Colgate, J.E.; Peshkin, M.A.; Goswami, A. Active-impedance control of a lower-limb assistive exoskeleton. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; IEEE: New York, NY, USA, 2007; pp. 188–195. [Google Scholar]
- Goo, A.; Laubscher, C.A.; Sawicki, J.T. Hybrid Zero Dynamics Control of an Underactuated Lower-Limb Exoskeleton for Gait Guidance. J. Dyn. Syst. Meas. Control. 2022, 144, 061008. [Google Scholar]
- Campbell, S.M.; Diduch, C.P.; Sensinger, J.W. Autonomous assistance-as-needed control of a lower limb exoskeleton with guaranteed stability. IEEE Access 2020, 8, 51168–51178. [Google Scholar] [CrossRef]
- Huang, R.; Cheng, H.; Chen, Y.; Chen, Q.; Lin, X.; Qiu, J. Optimisation of reference gait trajectory of a lower limb exoskeleton. Int. J. Soc. Robot. 2016, 8, 223–235. [Google Scholar]
- Tian, J.; Yuan, L.; Xiao, W.; Ran, T.; He, L. Trajectory following control of lower limb exoskeleton robot based on Udwadia–Kalaba theory. J. Vib. Control 2021, 28, 3383–3396. [Google Scholar]
- Amiri, M.S.; Ramli, R.; Ibrahim, M.F. Hybrid design of PID controller for four DoF lower limb exoskeleton. Appl. Math. Model. 2019, 72, 17–27. [Google Scholar]
- Narayan, J.; Kalani, A.; Dwivedy, S.K. Reference trajectory based Jacobian transpose control of a novel lower limb exoskeleton system for children. In Proceedings of the 2019 5th International Conference on Signal Processing, Computing and Control (ISPCC), Solan, India, 10–12 October 2019; IEEE: New York, NY, USA, 2019; pp. 102–107. [Google Scholar]
- Wang, F.; Shi, P.; Li, S.; Zhao, S.; Liu, W. Trajectory control of lower limb exoskeleton robot with variable forgetting factor. In Proceedings of the 2016 12th World Congress on Intelligent Control and Automation (WCICA), Guilin, China, 12–15 June 2016; IEEE: New York, NY, USA, 2016; pp. 1502–1507. [Google Scholar]
- Castro, D.L.; Zhong, C.H.; Braghin, F.; Liao, W.H. Lower limb exoskeleton control via linear quadratic regulator and disturbance observer. In Proceedings of the 2018 IEEE International Conference on Robotics and Biomimetics (ROBIO), Kuala Lumpur, Malaysia, 12–15 December 2018; IEEE: New York, NY, USA, 2018; pp. 1743–1748. [Google Scholar]
- Wang, Y.; Wang, H.; Tian, Y. Adaptive interaction torque-based AAN control for lower limb rehabilitation exoskeleton. ISA Trans. 2021, 128, 184–197. [Google Scholar]
- Maggioni, S.; Reinert, N.; Lünenburger, L.; Melendez-Calderon, A. An adaptive and hybrid end-point/joint impedance controller for lower limb exoskeletons. Front. Robot. AI 2018, 5, 104. [Google Scholar]
- Shi, D.; Zhang, W.; Zhang, W.; Ding, X. Force field control for the three-dimensional gait adaptation using a lower limb rehabilitation robot. In Advances in Mechanism and Machine Science: Proceedings of the 15th IFToMM World Congress on Mechanism and Machine Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1919–1928. [Google Scholar]
- Asl, H.J.; Narikiyo, T. An assistive control strategy for rehabilitation robots using velocity field and force field. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; IEEE: New York, NY, USA, 2019; pp. 790–795. [Google Scholar]
- Hidayah, R.; Bishop, L.; Jin, X.; Chamarthy, S.; Stein, J.; Agrawal, S.K. Gait adaptation using a cable-driven active leg exoskeleton (C-ALEX) with post-stroke participants. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1984–1993. [Google Scholar] [CrossRef]
- Martinez, A.; Lawson, B.; Goldfarb, M. A controller for guiding leg movement during overground walking with a lower limb exoskeleton. IEEE Trans. Robot. 2017, 34, 183–193. [Google Scholar]
- Xia, L.; Feng, Y.; Chen, F.; Wu, X. A bio-signal enhanced adaptive impedance controller for lower limb exoskeleton. 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020; IEEE: New York, NY, USA, 2020; pp. 4739–4744. [Google Scholar]
- Vinoj, P.; Jacob, S.; Menon, V.G.; Rajesh, S.; Khosravi, M.R. Brain-controlled adaptive lower limb exoskeleton for rehabilitation of post-stroke paralyzed. IEEE Access 2019, 7, 132628–132648. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Lobov, S.A.; Grigorev, N.A.; Savosenkov, A.O.; Shamshin, M.O.; Lukoyanov, M.V.; Khoruzhko, M.A.; Kazantsev, V.B. Real-time EEG–EMG human–machine interface-based control system for a lower-limb exoskeleton. IEEE Access 2020, 8, 84070–84081. [Google Scholar]
- Wei, Q.; Li, Z.; Zhao, K.; Kang, Y.; Su, C.Y. Synergy-based control of assistive lower-limb exoskeletons by skill transfer. IEEE/ASME Trans. Mechatronics 2019, 25, 705–715. [Google Scholar]
- Xu, F.; Qiu, J.; Yuan, W.; Cheng, H. A Novel Balance Control Strategy Based on Enhanced Stability Pyramid Index and Dynamic Movement Primitives for a Lower Limb Human-Exoskeleton System. Front. Neurorobotics 2021, 15, 751642. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Yang, S.X.; Dang, C.; Liu, D.X.; Wang, C.; Wang, C.; Chen, C. Online gait planning of lower-limb exoskeleton robot for paraplegic rehabilitation considering weight transfer process. IEEE Trans. Autom. Sci. Eng. 2020, 18, 414–425. [Google Scholar] [CrossRef]
- Banala, S.K.; Agrawal, S.K.; Scholz, J.P. Active Leg Exoskeleton (ALEX) for gait rehabilitation of motor-impaired patients. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; IEEE: New York, NY, USA, 2007; pp. 401–407. [Google Scholar]
- Agarwal, P.; Deshpande, A.D. Impedance and force-field control of the index finger module of a hand exoskeleton for rehabilitation. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; IEEE: New York, NY, USA, 2015; pp. 85–90. [Google Scholar]
- Chen, L.; Wang, C.; Song, X.; Wang, J.; Zhang, T.; Li, X. Dynamic trajectory adjustment of lower limb exoskeleton in swing phase based on impedance control strategy. Proc. Inst. Mech. Eng. Part J. Syst. Control. Eng. 2020, 234, 1120–1132. [Google Scholar] [CrossRef]
- Gui, K.; Liu, H.; Zhang, D. A generalized framework to achieve coordinated admittance control for multi-joint lower limb robotic exoskeleton. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July2017; IEEE: New York, NY, USA, 2017; pp. 228–233. [Google Scholar]
- Chen, C.F.; Du, Z.J.; He, L.; Shi, Y.J.; Wang, J.Q.; Xu, G.Q.; Zhang, Y.; Wu, D.M.; Dong, W. Development and hybrid control of an electrically actuated lower limb exoskeleton for motion assistance. IEEE Access 2019, 7, 169107–169122. [Google Scholar] [CrossRef]
- Ott, C.; Mukherjee, R.; Nakamura, Y. A hybrid system framework for unified impedance and admittance control. J. Intell. Robot. Syst. 2015, 78, 359–375. [Google Scholar] [CrossRef]
- Fleerkotte, B.M.; Koopman, B.; Buurke, J.H.; van Asseldonk, E.H.; van der Kooij, H.; Rietman, J.S. The effect of impedance-controlled robotic gait training on walking ability and quality in individuals with chronic incomplete spinal cord injury: An explorative study. J. Neuroeng. Rehabil. 2014, 11, 26. [Google Scholar] [CrossRef]
- Paredes, V.; Hereid, A. Dynamic Locomotion of a Lower-Limb Exoskeleton Through Virtual Constraints Based ZMP Regulation. In Dynamic Systems and Control Conference; American Society of Mechanical Engineers: New York, NY, USA, 2020; Volume 84270, p. V001T14A001. [Google Scholar]
- Harib, O.; Hereid, A.; Agrawal, A.; Gurriet, T.; Finet, S.; Boeris, G.; Duburcq, A.; Mungai, M.E.; Masselin, M.; Ames, A.D.; et al. Feedback control of an exoskeleton for paraplegics: Toward robustly stable, hands-free dynamic walking. IEEE Control. Syst. Mag. 2018, 38, 61–87. [Google Scholar] [CrossRef]
- Gurriet, T.; Finet, S.; Boeris, G.; Duburcq, A.; Hereid, A.; Harib, O.; Masselin, M.; Grizzle, J.; Ames, A.D. Towards restoring locomotion for paraplegics: Realizing dynamically stable walking on exoskeletons. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, QLD, Australia, 21–25 May 2018; IEEE: New York, NY, USA, 2018; pp. 2804–2811. [Google Scholar]
- Agrawal, A.; Harib, O.; Hereid, A.; Finet, S.; Masselin, M.; Praly, L.; Ames, A.D.; Sreenath, K.; Grizzle, J.W. First steps towards translating HZD control of bipedal robots to decentralized control of exoskeletons. IEEE Access 2017, 5, 9919–9934. [Google Scholar] [CrossRef]
- Karacan, K.; Meyer, J.T.; Bozma, H.I.; Gassert, R.; Samur, E. An environment recognition and parameterization system for shared-control of a powered lower-limb exoskeleton. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; IEEE: New York, NY, USA, 2020; pp. 623–628. [Google Scholar]
- Bao, W.; Villarreal, D.; Chiao, J.C. Vision-based autonomous walking in a lower-limb powered exoskeleton. In Proceedings of the 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE), Cincinnati, OH, USA, 26–28 October 2020; IEEE: New York, NY, USA, 2020; pp. 830–834. [Google Scholar]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar]
- Duschau-Wicke, A.; Caprez, A.; Riener, R. Patient-cooperative control increases active participation of individuals with SCI during robot-aided gait training. J. Neuroeng. Rehabil. 2010, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Holleran, C.L.; Straube, D.D.; Kinnaird, C.R.; Leddy, A.L.; Hornby, T.G. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilit. Neural Repair 2014, 28, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Hornby, T.G.; Holleran, C.L.; Hennessy, P.W.; Leddy, A.L.; Connolly, M.; Camardo, J.; Woodward, J.; Mahtani, G.; Lovell, L.; Roth, E.J. Variable intensive early walking poststroke (views) a randomized controlled trial. Neurorehabilit. Neural Repair 2016, 30, 440–450. [Google Scholar] [CrossRef]
- Patton, J.L.; Stoykov, M.E.; Kovic, M.; Mussa-Ivaldi, F.A. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp. Brain Res. 2006, 168, 368–383. [Google Scholar]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 2007, 130, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Bastian, A.J. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. J. Neurophysiol. 2010, 103, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, S. Effects of haptic guidance and disturbance on motor learning: Potential advantage of haptic disturbance. In Proceedings of the 2010 IEEE Haptics Symposium, Waltham, MA, USA, 25–26 March 2010; IEEE: New York, NY, USA, 2010; pp. 335–342. [Google Scholar]
- Kao, P.C.; Srivastava, S.; Agrawal, S.K.; Scholz, J.P. Effect of robotic performance-based error-augmentation versus error-reduction training on the gait of healthy individuals. Gait Posture 2013, 37, 113–120. [Google Scholar] [CrossRef]
- Simon, A.M.; Gillespie, R.B.; Ferris, D.P. Symmetry-based resistance as a novel means of lower limb rehabilitation. J. Biomech. 2007, 40, 1286–1292. [Google Scholar] [CrossRef]
- Simon, A.M.; Ferris, D.P. Lower limb force production and bilateral force asymmetries are based on sense of effort. Exp. Brain Res. 2008, 187, 129–138. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Prasad, S.; Grossman, R.G.; Contreras-Vidal, J.L. High accuracy decoding of user intentions using EEG to control a lower-body exoskeleton. In Proceedings of the 2013 35th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 5606–5609. [Google Scholar]
- Fleischer, C.; Hommel, G. Calibration of an EMG-Based Body Model with six Muscles to control a Leg Exoskeleton. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Rome, Italy, 10–14 April 2007; IEEE: New York, NY, USA, 2007; pp. 2514–2519. [Google Scholar]
- Liu, M.; Zhang, F.; Huang, H.H. An adaptive classification strategy for reliable locomotion mode recognition. Sensors 2017, 17, 2020. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, F.; Hargrove, L.J.; Dou, Z.; Rogers, D.R.; Englehart, K.B. Continuous locomotion-mode identification for prosthetic legs based on neuromuscular–mechanical fusion. IEEE Trans. Biomed. Eng. 2011, 58, 2867–2875. [Google Scholar] [CrossRef]
- Kyeong, S.; Shin, W.; Yang, M.; Heo, U.; Feng, J.r.; Kim, J. Recognition of walking environments and gait period by surface electromyography. Front. Inf. Technol. Electron. Eng. 2019, 20, 342–352. [Google Scholar] [CrossRef]
- Kundu, A.S.; Mazumder, O.; Lenka, P.K.; Bhaumik, S. Hand gesture recognition based omnidirectional wheelchair control using IMU and EMG sensors. J. Intell. Robot. Syst. 2018, 91, 529–541. [Google Scholar] [CrossRef]
- Liu, D.; Chen, W.; Pei, Z.; Wang, J. A brain-controlled lower-limb exoskeleton for human gait training. Rev. Sci. Instruments 2017, 88, 104302. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.T.; Jeong, J.H.; Kim, L.; Lee, S.J.; Kim, H. Developing a motor imagery-based real-time asynchronous hybrid BCI controller for a lower-limb exoskeleton. Sensors 2020, 20, 7309. [Google Scholar] [CrossRef] [PubMed]
- Schrade, S.O.; Nager, Y.; Wu, A.R.; Gassert, R.; Ijspeert, A. Bio-inspired control of joint torque and knee stiffness in a robotic lower limb exoskeleton using a central pattern generator. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; IEEE: New York, NY, USA, 2017; pp. 1387–1394. [Google Scholar]
- Liu, J.; Zhang, Y.; Wang, J.; Chen, W. Adaptive sliding mode control for a lower-limb exoskeleton rehabilitation robot. In Proceedings of the 2018 13th IEEE Conference on Industrial Electronics and Applications (ICIEA), Wuhan, China, 31 May–2 June 2018; IEEE: New York, NY, USA, 2018; pp. 1481–1486. [Google Scholar]
- Shushtari, M.; Nasiri, R.; Arami, A. Online reference trajectory adaptation: A personalized control strategy for lower limb exoskeletons. IEEE Robot. Autom. Lett. 2021, 7, 128–134. [Google Scholar] [CrossRef]
- Jatsun, S.; Savin, S.; Yatsun, A. Motion control algorithm for a lower limb exoskeleton based on iterative LQR and ZMP method for trajectory generation. In New Trends in Medical and Service Robots: Design, Analysis and Control; Springer: Berlin/Heidelberg, Germany, 2016; pp. 305–317. [Google Scholar]
- Aole, S.; Elamvazuthi, I.; Waghmare, L.; Patre, B.; Meriaudeau, F. Improved active disturbance rejection control for trajectory tracking control of lower limb robotic rehabilitation exoskeleton. Sensors 2020, 20, 3681. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Y.; Han, S.; Wang, X. ZMP theory-based gait planning and model-free trajectory tracking control of lower limb carrying exoskeleton system. Stud. Inform. Control. 2017, 26, 161–170. [Google Scholar] [CrossRef]
- Sun, W.; Lin, J.W.; Su, S.F.; Wang, N.; Er, M.J. Reduced adaptive fuzzy decoupling control for lower limb exoskeleton. IEEE Trans. Cybern. 2020, 51, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Crespo, R.; Torricelli, D.; Huegel, J.C.; Gordillo, J.L.; Pons, J.L.; Soto, R. An adaptable human-like gait pattern generator derived from a lower limb exoskeleton. Front. Robot. AI 2019, 6, 36. [Google Scholar] [CrossRef]
- Seo, K.; Kim, K.; Park, Y.J.; Cho, J.K.; Lee, J.; Choi, B.; Lim, B.; Lee, Y.; Shim, Y. Adaptive oscillator-based control for active lower-limb exoskeleton and its metabolic impact. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, QLD, Australia, 21–25 May 2018; IEEE: New York, NY, USA, 2018; pp. 6752–6758. [Google Scholar]
- Chung, Y.; Kim, J.H.; Cha, Y.; Hwang, S. Therapeutic effect of functional electrical stimulation-triggered gait training corresponding gait cycle for stroke. Gait Posture 2014, 40, 471–475. [Google Scholar] [CrossRef]
- Nightingale, E.; Raymond, J.; Middleton, J.; Crosbie, J.; Davis, G. Benefits of FES gait in a spinal cord injured population. Spinal Cord 2007, 45, 646–657. [Google Scholar] [CrossRef]
- Granat, M.; Ferguson, A.; Andrews, B.; Delargy, M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury-observed benefits during gait studies. Spinal Cord 1993, 31, 207–215. [Google Scholar] [CrossRef]
- Krause, P.; Szecsi, J.; Straube, A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin. Rehabil. 2008, 22, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Del-Ama, A.J.; Gil-Agudo, Á.; Pons, J.L.; Moreno, J.C. Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J. Neuroeng. Rehabil. 2014, 11, 27. [Google Scholar] [CrossRef]
- Ha, K.H.; Quintero, H.A.; Farris, R.J.; Goldfarb, M. Enhancing stance phase propulsion during level walking by combining FES with a powered exoskeleton for persons with paraplegia. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: New York, NY, USA, 2012; pp. 344–347. [Google Scholar]
- Stewart, A.; Pretty, C.; Chen, X. A portable assist-as-need upper-extremity hybrid exoskeleton for FES-induced muscle fatigue reduction in stroke rehabilitation. BMC Biomed. Eng. 2019, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.H.; Murray, S.A.; Goldfarb, M. An approach for the cooperative control of FES with a powered exoskeleton during level walking for persons with paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Alouane, M.A.; Huo, W.; Rifai, H.; Amirat, Y.; Mohammed, S. Hybrid fes-exoskeleton controller to assist sit-to-stand movement. IFAC-Pap. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Quintero, H.A.; Farris, R.J.; Ha, K.; Goldfarb, M. Preliminary assessment of the efficacy of supplementing knee extension capability in a lower limb exoskeleton with FES. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: New York, NY, USA, 2012; pp. 3360–3363. [Google Scholar]
- Alibeji, N.A.; Molazadeh, V.; Moore-Clingenpeel, F.; Sharma, N. A muscle synergy-inspired control design to coordinate functional electrical stimulation and a powered exoskeleton: Artificial generation of synergies to reduce input dimensionality. IEEE Control. Syst. Mag. 2018, 38, 35–60. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.; Hwang, S. Locomotion Mode Recognition Algorithm Based on Gaussian Mixture Model Using IMU Sensors. Sensors 2021, 21, 2785. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, R.; Mai, J.; Wang, N.; Wang, Q. IMU-Based Gait Phase Recognition for Stroke Survivors. Robotica 2019, 37, 2195–2208. [Google Scholar] [CrossRef]
- Ding, S.; Ouyang, X.; Liu, T.; Li, Z.; Yang, H. Gait event detection of a lower extremity exoskeleton robot by an intelligent IMU. IEEE Sensors J. 2018, 18, 9728–9735. [Google Scholar] [CrossRef]
- Sánchez Manchola, M.D.; Bernal, M.J.P.; Munera, M.; Cifuentes, C.A. Gait phase detection for lower-limb exoskeletons using foot motion data from a single inertial measurement unit in hemiparetic individuals. Sensors 2019, 19, 2988. [Google Scholar] [CrossRef]
- Li, M.; Deng, J.; Zha, F.; Qiu, S.; Wang, X.; Chen, F. Towards online estimation of human joint muscular torque with a lower limb exoskeleton robot. Appl. Sci. 2018, 8, 1610. [Google Scholar] [CrossRef]
- Hsiao, T.; Yip, K.; Chiu, Y.J. Estimation of Ground Reaction Forces Based on Knee Joint Acceleration of Lower-Limb Exoskeletons. In Proceedings of the 2020 International Automatic Control Conference (CACS), Hsinchu, Taiwan, 4–7 November 2020; IEEE: New York, NY, USA, 2020; pp. 1–6. [Google Scholar]
- Kazerooni, H.; Racine, J.L.; Huang, L.; Steger, R. On the control of the berkeley lower extremity exoskeleton (BLEEX). In Proceedings of the 2005 IEEE International Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005; IEEE: New York, NY, USA, 2005; pp. 4353–4360. [Google Scholar]
- Şahin, Y.; Botsalı, F.M.; Kalyoncu, M.; Tinkir, M.; Önen, Ü.; Yılmaz, N.; Baykan, Ö.K.; Çakan, A. Force feedback control of lower extremity exoskeleton assisting of load carrying human. Appl. Mech. Mater. 2014, 598, 546–550. [Google Scholar]
- He, Y.; Eguren, D.; Azorín, J.M.; Grossman, R.G.; Luu, T.P.; Contreras-Vidal, J.L. Brain–machine interfaces for controlling lower-limb powered robotic systems. J. Neural Eng. 2018, 15, 021004. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishi, M.S.; Elamvazuthi, I.; Daud, S.A.; Parasuraman, S.; Borboni, A. EEG-based control for upper and lower limb exoskeletons and prostheses: A systematic review. Sensors 2018, 18, 3342. [Google Scholar] [CrossRef]
- Donati, A.R.; Shokur, S.; Morya, E.; Campos, D.S.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef]
- Lee, K.; Liu, D.; Perroud, L.; Chavarriaga, R.; Millán, J.d.R. A brain-controlled exoskeleton with cascaded event-related desynchronization classifiers. Robot. Auton. Syst. 2017, 90, 15–23. [Google Scholar] [CrossRef]
- López-Larraz, E.; Trincado-Alonso, F.; Rajasekaran, V.; Pérez-Nombela, S.; Del-Ama, A.J.; Aranda, J.; Minguez, J.; Gil-Agudo, A.; Montesano, L. Control of an ambulatory exoskeleton with a brain–machine interface for spinal cord injury gait rehabilitation. Front. Neurosci. 2016, 10, 359. [Google Scholar] [CrossRef]
- Formaggio, E.; Storti, S.F.; Boscolo Galazzo, I.; Gandolfi, M.; Geroin, C.; Smania, N.; Spezia, L.; Waldner, A.; Fiaschi, A.; Manganotti, P. Modulation of event-related desynchronization in robot-assisted hand performance: Brain oscillatory changes in active, passive and imagined movements. J. Neuroeng. Rehabil. 2013, 10, 24. [Google Scholar] [CrossRef]
- Kwak, N.S.; Müller, K.R.; Lee, S.W. A lower limb exoskeleton control system based on steady state visual evoked potentials. J. Neural Eng. 2015, 12, 056009. [Google Scholar] [CrossRef]
- Zhang, Y.; Prasad, S.; Kilicarslan, A.; Contreras-Vidal, J.L. Multiple kernel based region importance learning for neural classification of gait states from EEG signals. Front. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef]
- Selinger, J.C.; Donelan, J.M. Myoelectric control for adaptable biomechanical energy harvesting. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 364–373. [Google Scholar] [CrossRef]
- Kiguchi, K.; Hayashi, Y. An EMG-based control for an upper-limb power-assist exoskeleton robot. IEEE Trans. Syst. Man Cybern. Part B (Cybern.) 2012, 42, 1064–1071. [Google Scholar] [CrossRef]
- Rosen, J.; Brand, M.; Fuchs, M.B.; Arcan, M. A myosignal-based powered exoskeleton system. IEEE Trans. Syst. Man-Cybern.-Part A Syst. Humans 2001, 31, 210–222. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Besier, T.F. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J. Biomech. 2003, 36, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Veronica, J.; Parasuraman, S.; Khan, M.A.; DSingh, K.J. Emg-torque correction on human upper extremity using evolutionary computation. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 19–20 March 2016; IOP Publishing: Bristol, UK, 2016; Volume 149, p. 012152. [Google Scholar]
- Gui, K.; Tan, U.X.; Liu, H.; Zhang, D. Electromyography-driven progressive assist-as-needed control for lower limb exoskeleton. IEEE Trans. Med. Robot. Bionics 2020, 2, 50–58. [Google Scholar] [CrossRef]
- Chen, B.; Ma, H.; Qin, L.Y.; Guan, X.; Chan, K.M.; Law, S.W.; Qin, L.; Liao, W.H. Design of a lower extremity exoskeleton for motion assistance in paralyzed individuals. In Proceedings of the 2015 IEEE International Conference on Robotics and Biomimetics (ROBIO), Zhuhai, China, 6–9 December 2015; IEEE: New York, NY, USA, 2015; pp. 144–149. [Google Scholar]
- Lancini, M.; Serpelloni, M.; Pasinetti, S.; Guanziroli, E. Healthcare sensor system exploiting instrumented crutches for force measurement during assisted gait of exoskeleton users. IEEE Sens. J. 2016, 16, 8228–8237. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Zhang, S.; Yang, C. Lower Limb Exoskeleton Gait Planning Based on Crutch and Human-Machine Foot Combined Center of Pressure. Sensors 2020, 20, 7216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).