Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives
Abstract
1. Introduction
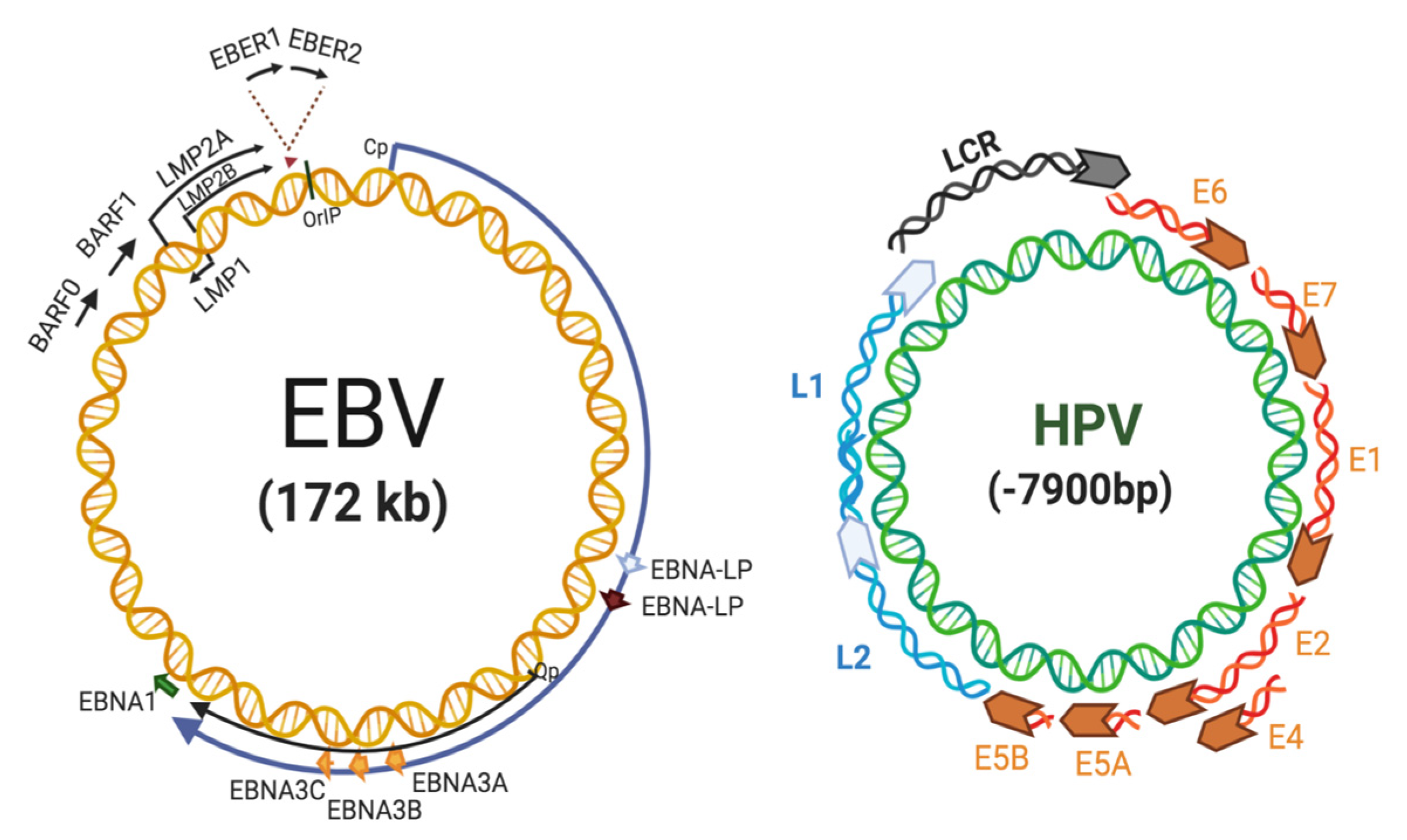
2. EBV Replication and Role in Cancer
3. HPV in Cervical Cancer
4. Frequency of HPV and EBV Coinfection in Uterine Cervix
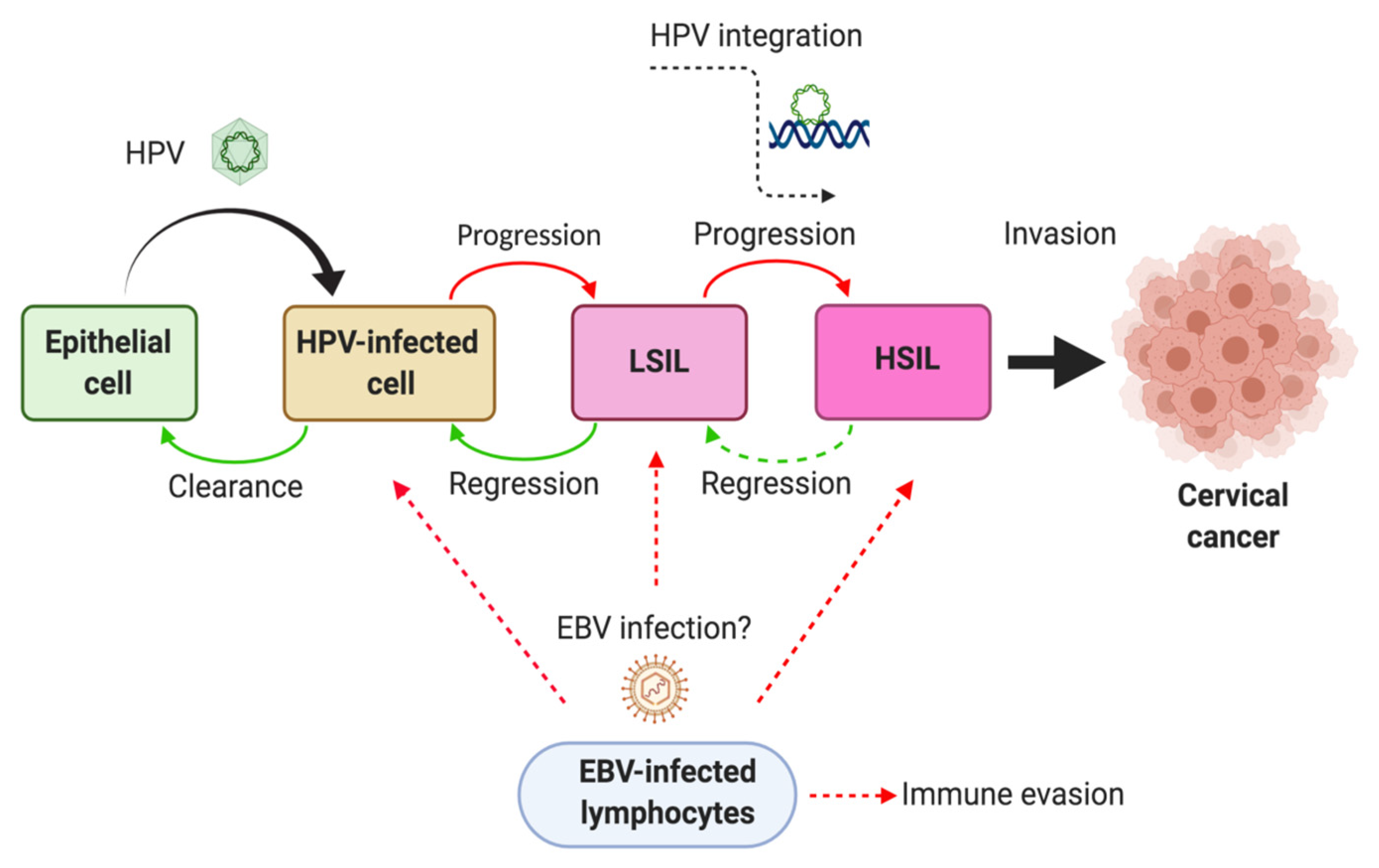
5. EBV Infection in Tumor-Infiltrating Lymphocytes from Cervical Carcinomas
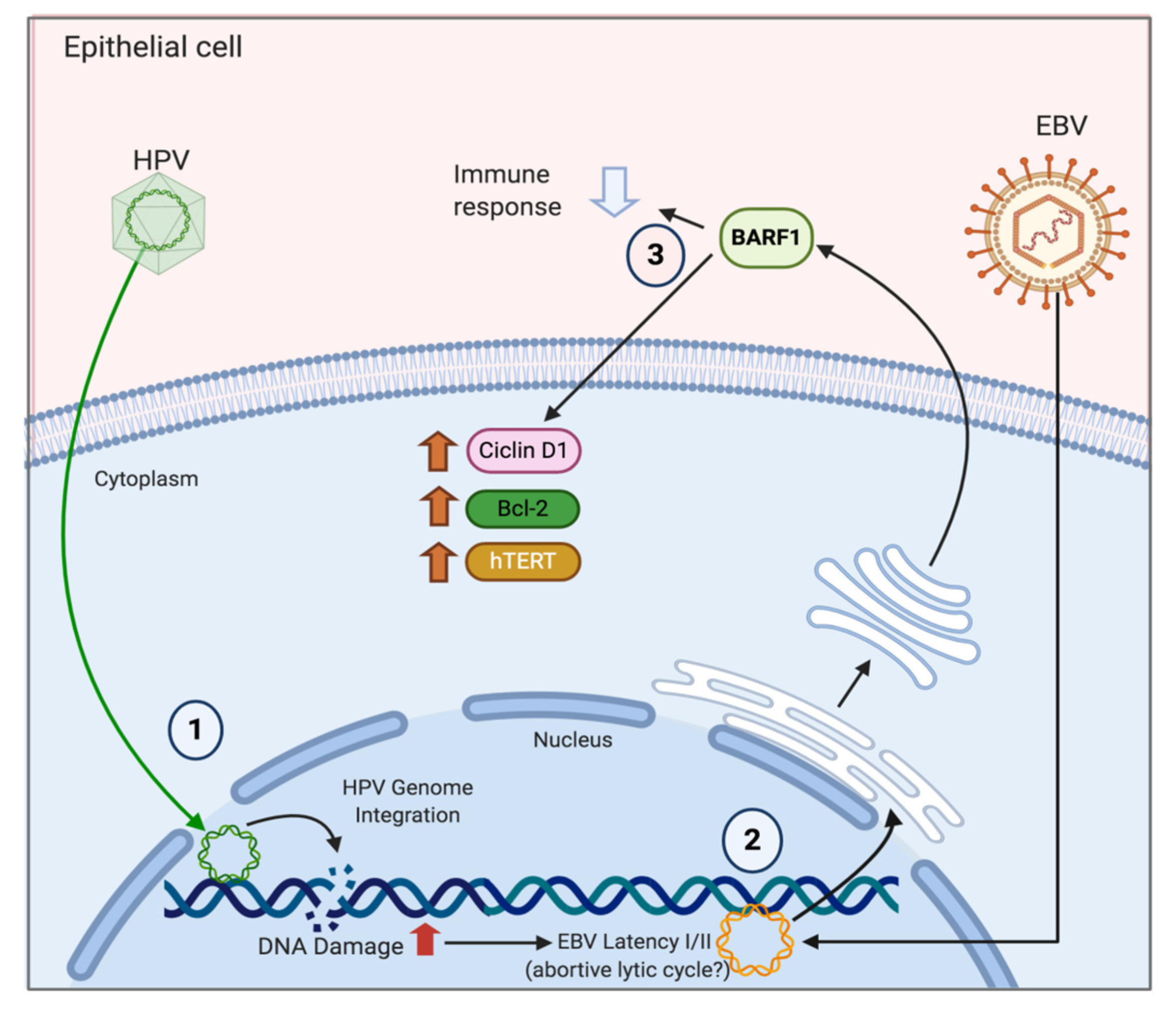
6. Mechanisms of HPV/EBV-Mediated Cervical Carcinogenesis
7. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Serjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Ciapponi, A.; Bardach, A.; Glujovsky, D.; Gibbons, L.; Piconni, M.A. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: Systematic review and meta-analysis. PLoS ONE 2011, 6, e25493. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Pons-Salort, M.; Favre, M.; Heard, I.; Delarocque-Astagneau, E.; Guillemot, D.; Thiebaut, A.C.M. Comparing human papillomavirus prevalences in women with normal cytology or invasive cervical cancer to rank genotypes according to their oncogenic potential: A meta-analysis of observational studies. BMC Infect. Dis. 2013, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Howard, N.; Gallagher, K.E.; Mounier-Jack, S.; Burchett, H.E.D.; Kabakama, S.; Lamontagne, D.S.; Watson-Jones, D. What works for human papillomavirus vaccine introduction in low and middle-income countries? Papillomavirus Res. (Amst. Neth.) 2017, 4, 22–25. [Google Scholar] [CrossRef]
- Murillo, R.; Reyes, C.O. Human papillomavirus (HPV) vaccination: From clinical studies to immunization programs. Int. J. Gynecol. Cancer 2019, 29, 1317–1326. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Platt, R.W.; Duarte-Franco, E.; Costa, M.C.; Sobrinho, J.P.; Prado, J.C.M.; Ferenczy, A.; Rohan, T.E.; Villa, L.L.; Franco, E. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 2003, 95, 1336–1343. [Google Scholar] [CrossRef]
- Silveira, F.; Almeida, G.; Furtado, Y.; Cavalcanti, S.M.B.; Silva, K.; Maldonado, P.; Carvalho, M. The association of HPV genotype with the regression, persistence or progression of low-grade squamous intraepithelial lesions. Exp. Mol. Pathol. 2015, 99, 702–706. [Google Scholar] [CrossRef]
- Prayitno, A. Cervical cancer with Human Papilloma Virus and Epstein-Barr Virus positive. J. Carcinog. 2006, 5, 13. [Google Scholar] [CrossRef]
- Szostek, S.; Zawilinska, B.; Kopec, J.; Kosz-Vnenchak, M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 2009, 56, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kahla, S.; Oueslati, S.; Achour, M.; Kochbati, L.; Chanoufi, M.B.; Maalej, M.; Oueslati, R. Correlation between ebv co-infection and HPV16 genome integrity in Tunisian cervical cancer patients. Braz. J. Microbiol. 2012, 43, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Khenchouche, A.; Sadouki, N.; Boudriche, A.; Houali, K.; Graba, A.; Ooka, T.; Bouguermouh, A. Human Papillomavirus and Epstein-Barr virus co-infection in Cervical Carcinoma in Algerian women. Virol. J. 2013, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Aromseree, S.; Pientong, C.; Swangphon, P.; Chaiwongkot, A.; Patarapadungkit, N.; Kleebkaow, P.; Tangsiriwatthana, T.; Kongyingyoes, B.; Vendrig, T.; Middeldorp, J.M.; et al. Possible contributing role of Epstein-Barr virus (EBV) as a cofactor in human papillomavirus (HPV)-associated cervical carcinogenesis. J. Clin. Virol. 2015, 73, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr virus and cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Grünewald, K.; Desai, P.; Winkler, D.C.; Heymann, J.B.; Belnap, D.M.; Baumeister, W.; Steven, A.C. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 2003, 302, 1396–1398. [Google Scholar] [CrossRef]
- Murata, T.; Tsurumi, T. Switching of EBV cycles between latent and lytic states. Rev. Med Virol. 2013, 24, 142–153. [Google Scholar] [CrossRef]
- Barth, S.; Meister, G.; Grässer, F.A. EBV-encoded miRNAs. Biochim. Biophys. Acta 2011, 1809, 631–640. [Google Scholar] [CrossRef]
- Kang, M.S.; Kieff, E. Epstein-Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef]
- Babcock, G.J.; Hochberg, D.; Thorley-Lawson, D.A. The expression pattern of epstein-barr virus latent genes in vivo is dependent upon the differentiation stage of the infected b cell. Immunity 2000, 13, 497–506. [Google Scholar] [CrossRef]
- Klein, E.; Nagy, N.; Rasul, A.E. EBV genome carrying B lymphocytes that express the nuclear protein EBNA-2 but not LMP-1. OncoImmunology 2013, 2, e23035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Price, A.M.; Luftig, M.A. To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis. PLoS Pathog. 2015, 11, e1004656. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Koizumi, S.; Sugiura, M.; Tokunaga, M.; Uemura, Y.; Yamamoto, N.; Tanaka, S.; Sato, E.; Osato, T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9131–9135. [Google Scholar] [CrossRef] [PubMed]
- Neparidze, N.; Lacy, J. Malignancies associated with -epstein-barr virus: Pathobiology, clinical features, and evolving treatments. Clin. Adv. Hematol. Oncol. 2014, 12, 358–371. [Google Scholar] [PubMed]
- Dugan, J.P.; Coleman, B.P.; Haverkos, B. Opportunities to target the life cycle of epstein-barr virus (EBV) in EBV-associated lymphoproliferative disorders. Front. Oncol. 2019, 9, 127. [Google Scholar] [CrossRef]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef]
- Grywalska, E.; Rolinski, J. Epstein-Barr virus-associated lymphomas. Semin. Oncol. 2015, 42, 291–303. [Google Scholar] [CrossRef]
- Sivachandran, N.; Wang, X.; Frappier, L. Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. J. Virol. 2012, 86, 6146–6158. [Google Scholar] [CrossRef]
- Wang, L.; Tian, W.-D.; Xu, X.; Nie, B.; Lu, J.; Liu, X.; Zhang, B.; Dong, Q.; Sunwoo, J.B.; Li, G.; et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer 2013, 120, 363–372. [Google Scholar] [CrossRef]
- O’Neil, J.D.; Owen, T.J.; Wood, V.H.J.; Date, K.L.; Valentine, R.; Chukwuma, M.B.; Arrand, J.R.; Dawson, C.W.; Young, L.S. Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. J. Gen. Virol. 2008, 89, 2833–2842. [Google Scholar] [CrossRef]
- Yin, L.; Liao, W.; Deng, X.; Tang, M.; Gu, H.; Li, X.; Yi, W.; Cao, Y. LMP1 activates NF-kappa B via degradation of I kappa B alpha in nasopharyngeal carcinoma cells. Chin. Med. J. 2001, 114, 718–722. [Google Scholar] [PubMed]
- Yang, Y.; Shi, Y.; Yuan, Q.; Liu, X.; Yan, B.; Chen, L.; Tao, Y.; Cao, Y.; Song, X. Heterodimer formation between c-Jun and Jun B proteins mediated by Epstein-Barr virus encoded latent membrane protein 1. Cell Sign. 2004, 16, 1153–1162. [Google Scholar]
- Chen, H.; Lee, J.; Zong, Y.; Borowitz, M.; Ng, M.H.; Ambinder, R.F.; Hayward, S.D. Linkage between STAT regulation and Epstein-Barr virus gene expression in tumors. J. Virol. 2001, 75, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yang, L.; Middeldorp, J.M.; Sheen, T.-S.; Chen, J.-Y.; Fukayama, M.; Eizuru, Y.; Ooka, T.; Takada, K. Epstein-Barr virus (EBV)-encodedBARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J. Med. Virol. 2005, 76, 82–88. [Google Scholar] [CrossRef]
- Hoebe, E.K.; Le Large, T.Y.S.; Greijer, A.E.; Middeldorp, J.M. BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator. Rev. Med. Virol. 2013, 23, 367–383. [Google Scholar] [CrossRef]
- Elegheert, J.; Bracke, N.; Pouliot, P.; Gutsche, I.; Shkumatov, A.V.; Tarbouriech, N.; Verstraete, K.; Bekaert, A.; Burmeister, W.P.; Svergun, D.I.; et al. Allosteric competitive inactivation of hematopoietic CSF-1 signaling by the viral decoy receptor BARF1. Nat. Struct. Mol. Boil. 2012, 19, 938–947. [Google Scholar] [CrossRef]
- Wiech, T.; Nikolopoulos, E.; Lassman, S.; Heidt, T.; Schöpflin, A.; Sarbia, M.; Werner, M.; Shimizu, Y.; Sakka, E.; Ooka, T.; et al. Cyclin D1 expression is induced by viral BARF1 and is overexpressed in EBV-associated gastric cancer. Virchows Arch. 2008, 452, 621–627. [Google Scholar] [CrossRef]
- Chang, M.S.; Kim, D.H.; Roh, J.K.; Middeldorp, J.M.; Kim, Y.S.; Kim, S.; Han, S.; Kim, C.W.; Lee, B.L.; Kim, W.H.; et al. Epstein-Barr Virus-Encoded BARF1 Promotes Proliferation of Gastric Carcinoma Cells through Regulation of NF-B. J. Virol. 2013, 87, 10515–10523. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Zhang, X.; Chu, F.; Zhou, T. MAPK/c-Jun signaling pathway contributes to the upregulation of the anti-apoptotic proteins Bcl-2 and Bcl-xL induced by Epstein-Barr virus-encoded BARF1 in gastric carcinoma cells. Oncol. Lett. 2018, 15, 7537–7544. [Google Scholar] [CrossRef]
- Zhang, Y.; Ohyashiki, J.H.; Takaku, T.; Shimizu, N.; Ohyashiki, K. Transcriptional profiling of Epstein-Barr virus (EBV) genes and host cellular genes in nasal NK/T-cell lymphoma and chronic active EBV infection. Br. J. Cancer 2006, 94, 599–608. [Google Scholar] [CrossRef][Green Version]
- Cohen, J.I.; Lekstrom, K. Epstein-Barr virus BARF1 protein is dispensable for B-cell transformation and inhibits alpha interferon secretion from mononuclear cells. J. Virol. 1999, 73, 7627–7632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Cabras, G.; Sheng, W.; Zeng, Y.; Ooka, T. Synergism of BARF1 with ras induces malignant transformation in primary primate epithelial cells and human nasopharyngeal epithelial cells. Neoplasia 2009, 11, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.P.; Brink, A.A.; Vervoort, M.B.; Middeldorp, J.M.; Meijer, C.J.; van den Brule, A.J. Expression of Epstein-Barr virus (EBV) transcripts encoding homologues to important human proteins in diverse EBV associated diseases. Mol. Pathol. 1999, 52, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pinidis, P.; Tsikouras, P.; Iatrakis, G.; Zervoudis, S.; Koukouli, Z.; Bothou, A.; Galazios, G.; Vladareanu, S. Human papilloma virus’ life cycle and carcinogenesis. Maedica (Buchar.) 2016, 11, 48–54. [Google Scholar] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; Group, I.A.f.R.o.C.M.C.C.S. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Park, J.S.; Hwang, E.S.; Park, S.N.; Ahn, H.K.; Um, S.J.; Kim, C.J.; Kim, S.J.; Namkoong, S.E. Physical status and expression of hpv genes in cervical cancers. Gynecol. Oncol. 1997, 65, 121–129. [Google Scholar] [CrossRef]
- Li, K.; Jin, X.; Fang, Y.; Wang, C.; Gong, M.; Chen, P.; Liu, J.; Deng, D.; Ai, J. Correlation between physical status of human papilloma virus and cervical carcinogenesis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 97–102. [Google Scholar] [CrossRef]
- Klaes, R.; Woerner, S.M.; Ridder, R.; Wentzensen, N.; Duerst, M.; Schneider, A.; Lotz, B.; Melsheimer, P.; von Knebel Doeberitz, M. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999, 59, 6132–6136. [Google Scholar]
- Hopman, A.H.; Smedts, F.; Dignef, W.; Ummelen, M.; Sonke, G.; Mravunac, M.; Vooijs, G.P.; Speel, E.J.; Ramaekers, F.C. Transition of high-grade cervical intraepithelial neoplasia to micro-invasive carcinoma is characterized by integration of HPV 16/18 and numerical chromosome abnormalities. J. Pathol. 2004, 202, 23–33. [Google Scholar] [CrossRef]
- Münger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Laura, R.; Hepner, K.; Guccione, E.; Sawyers, C.; Lasky, L.; Banks, L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene 2002, 21, 5088–5096. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, J.; Schlosser, S.; Müller, M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014, 4, 285. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, O.; Veeraraghavalu, K.; Tergaonkar, V.; Liu, Y.; Androphy, E.J.; Stanley, M.A.; Krishna, S. Human papillomavirus type 16 E6 amino acid 83 variants enhance E6-mediated MAPK signaling and differentially regulate tumorigenesis by notch signaling and oncogenic Ras. J. Virol. 2004, 78, 5934–5945. [Google Scholar] [CrossRef] [PubMed]
- Spangle, J.M.; Münger, K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J. Virol. 2010, 84, 9398–9407. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.O.; Duerksen-Hughes, P.J. Modulation of apoptosis by human papillomavirus (HPV) oncoproteins. Arch Virol. 2006, 151, 2321–2335. [Google Scholar] [CrossRef]
- Yuan, C.H.; Filippova, M.; Tungteakkhun, S.S.; Duerksen-Hughes, P.J.; Krstenansky, J.L. Small molecule inhibitors of the HPV16-E6 interaction with caspase 8. Bioorg. Med. Chem. Lett. 2012, 22, 2125–2129. [Google Scholar] [CrossRef]
- Ohlenschläger, O.; Seiboth, T.; Zengerling, H.; Briese, L.; Marchanka, A.; Ramachandran, R.; Baum, M.; Korbas, M.; Meyer-Klaucke, W.; Dürst, M.; et al. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene 2006, 25, 5953–5959. [Google Scholar] [CrossRef]
- Boyer, S.N.; Wazer, D.E.; Band, V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res 1996, 56, 4620–4624. [Google Scholar]
- Veldman, T.; Liu, X.; Yuan, H.; Schlegel, R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 8211–8216. [Google Scholar] [CrossRef]
- Wang, Y.W.; Chang, H.S.; Lin, C.H.; Yu, W.C. HPV-18 E7 conjugates to c-Myc and mediates its transcriptional activity. Int. J. Biochem. Cell Boil. 2007, 39, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Hellner, K.; Mar, J.; Fang, F.; Quackenbush, J.; Münger, K. HPV16 E7 oncogene expression in normal human epithelial cells causes molecular changes indicative of an epithelial to mesenchymal transition. Virology 2009, 391, 57–63. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Kramer, R.E.; Tan, M.J.; Hayes, S.D.; Harper, J.W.; Howley, P.M. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J. Virol. 2012, 86, 13174–13186. [Google Scholar] [CrossRef] [PubMed]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV integration and its progression to cervical cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA integration and carcinogenesis: Putative roles for inflammation and oxidative stress. Future Virol. 2011, 6, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Deacon, J.M.; Evans, C.D.; Yule, R.; Desai, M.; Binns, W.; Taylor, C.; Peto, J. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: A case–control study nested within the Manchester cohort. Br. J. Cancer 2000, 83, 1565–1572. [Google Scholar] [CrossRef]
- Peña, N.; Carrillo, D.; Muñoz, J.P.; Chnaiderman, J.; Urzúa, U.; León, O.; Tornesello, M.L.; Corvalán, A.H.; Soto-Rifo, R.; Aguayo, F. Tobacco smoke activates human papillomavirus 16 p97 promoter and cooperates with high-risk E6/E7 for oxidative DNA damage in lung cells. PLoS ONE 2015, 10, e0123029. [Google Scholar] [CrossRef]
- Muñoz, J.P.; González, C.; Parra, B.; Corvalán, A.H.; Tornesello, M.L.; Eizuru, Y.; Aguayo, F. Functional interaction between human papillomavirus type 16 E6 and E7 oncoproteins and cigarette smoke components in lung epithelial cells. PLoS ONE 2012, 7, e38178. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Carrillo-Beltrán, D.; Aedo-Aguilera, V.; Calaf, G.M.; León, O.; Maldonado, E.; Tapia, J.C.; Boccardo, E.; Ozbun, M.A.; Aguayo, F. Tobacco exposure enhances human papillomavirus 16 oncogene expression via EGFR/PI3K/Akt/c-Jun signaling pathway in cervical cancer cells. Front. Microbiol. 2018, 9, 3022. [Google Scholar] [CrossRef]
- Se Thoe, S.Y.; Wong, K.K.; Pathmanathan, R.; Sam, C.K.; Cheng, H.M.; Prasad, U. Elevated secretory IgA antibodies to Epstein-Barr virus (EBV) and presence of EBV DNA and EBV receptors in patients with cervical carcinoma. Gynecol. Oncol. 1993, 50, 168–172. [Google Scholar] [CrossRef]
- Landers, R.J.; O’Leary, J.J.; Crowley, M.; Healy, I.; Annis, P.; Burke, L.; O’Brien, D.; Hogan, J.; Kealy, W.F.; Lewis, F.A. Epstein-Barr virus in normal, pre-malignant, and malignant lesions of the uterine cervix. J. Clin. Pathol. 1993, 46, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Shimakage, M.; Nakamura, M.; Sakaike, J.; Ishikawa, H.; Inoue, M. Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: A comparative study with human papillomavirus (HPV) infection. Hum. Pathol. 2000, 31, 318–326. [Google Scholar] [CrossRef]
- Shimakage, M.; Sasagawa, T. Detection of Epstein-Barr virus-determined nuclear antigen-2 mRNA by in situ hybridization. J. Virol. Methods 2001, 93, 23–32. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Wal, M.; Kuch, A.; Pieta, P. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) cervical infections in women with normal and abnormal cytology. Pol. J. Microbiol. 2004, 53, 95–99. [Google Scholar] [PubMed]
- Hilton, D.A.; Brown, L.J.; Pringle, J.H.; Nandha, H. Absence of Epstein-Barr virus in carcinoma of the cervix. Cancer 1993, 72, 1946–1948. [Google Scholar] [CrossRef]
- Shoji, Y.; Saegusa, M.; Takano, Y.; Hashimura, M.; Okayasu, I. Detection of the Epstein-Barr virus genome in cervical neoplasia is closely related to the degree of infiltrating lymphoid cells: A polymerase chain reaction and in situ hybridization approach. Pathol. Int. 1997, 47, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Elgui de Oliveira, D.; Furtado Monteiro, T.A.; Alencar de Melo, W.; Amaral Rebouças Moreira, M.; Alvarenga, M.; Bacchi, C.E. Lack of Epstein-Barr virus infection in cervical carcinomas. Arch. Pathol. Lab. Med. 1999, 123, 1098–1100. [Google Scholar]
- Yang, Y.Y.; Koh, L.W.; Tsai, J.H.; Tsai, C.H.; Wong, E.F.; Lin, S.J.; Yang, C.C. Correlation of viral factors with cervical cancer in Taiwan. J. Microbiol. Immunol. Infect. 2004, 37, 282–287. [Google Scholar]
- Seo, S.S.; Kim, W.H.; Song, Y.S.; Kim, S.H.; Kim, J.W.; Park, N.H.; Kang, S.B.; Lee, H.P. Epstein-Barr virus plays little role in cervical carcinogenesis in Korean women. Int. J. Gynecol. Cancer 2005, 15, 312–318. [Google Scholar]
- Wei, Z.; Shunqian, J.; Junyao, L.; Xiao, L.; Lihua, M.; Xiaohong, W.; Ming, S.; Airu, W.; Jianheng, S.; Xixia, W.; et al. The infection of EBV for cervical epithelium, a new causative agent in the development of cervical carcinomas. Chin. J. Cancer Res. 1992, 4, 23–29. [Google Scholar]
- McCormick, T.M.; Canedo, N.H.; Furtado, Y.L.; Silveira, F.A.; de Lima, R.J.; Rosman, A.D.; Almeida Filho, G.L.; da C Carvalho, M.D.G. Association between human papillomavirus and Epstein-Barr virus DNA and gene promoter methylation of RB1 and CDH1 in the cervical lesions: A transversal study. Diagn. Pathol. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lattario, F.; Furtado, Y.L.; Fonseca, R.; Silveira, F.A.; do Val, I.C.; Almeida, G.; Carvalho, M.G. Analysis of human papillomavirus and Epstein-Barr virus infection and aberrant death-associated protein kinase methylation in high-grade squamous intraepithelial lesions. Int. J. Gynecol. Cancer 2008, 18, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.E.; Rositch, A.F.; Vielot, N.A.; Mugo, N.R.; Kwatampora, J.K.L.; Waweru, W.; Gilliland, A.E.; Hagensee, M.E.; Smith, J.S. Epstein-Barr virus, high-risk human papillomavirus and abnormal cervical cytology in a prospective cohort of african female sex workers. Sex. Transm. Dis. 2018, 45, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.E.; Dennis, D.C.; Herrel, N.R.; Chapple, A.G.; Hagensee, M.E. Risk of abnormal cervical cytology in HIV-infected women testing positive for both human papillomavirus and Epstein-Barr virus in genital tract specimens. Cancer Causes Control. 2020, 31, 365–375. [Google Scholar] [CrossRef]
- Ammatuna, P.; Giovannelli, L.; Giambelluca, D.; Mancuso, S.; Rubino, E.; Colletti, P.; Mazzola, G.; Belfiore, P.; Lima, R. Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J. Med. Virol. 2000, 62, 410–415. [Google Scholar] [CrossRef]
- Smith, J.R.; Kitchen, V.S.; Botcherby, M.; Hepburn, M.; Wells, C.; Gor, D.; Forster, S.M.; Harris, J.R.; Steer, P.; Mason, P. Is HIV infection associated with an increase in the prevalence of cervical neoplasia? Br. J. Obstet. Gynaecol. 1993, 100, 149–153. [Google Scholar] [CrossRef]
- Abudoukadeer, A.; Niyazi, M.; Aikula, A.; Kamilijian, M.; Sulaiman, X.; Mutailipu, A.; Abudula, A. Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur. J. Gynaecol. Oncol. 2015, 36, 546–550. [Google Scholar]
- Al-Thawadi, H.; Ghabreau, L.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.E. Co-Incidence of Epstein-Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian W omen. Front. Oncol. 2018, 8, 250. [Google Scholar] [CrossRef]
- Schindl, M.; Oberhuber, G.; Obermair, A.; Schoppmann, S.F.; Karner, B.; Birner, P. Overexpression Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res. 2001, 61, 5703–5706. [Google Scholar]
- Darnel, A.D.; Wang, D.; Ghabreau, L.; Yasmeen, A.; Sami, S.; Akil, N.; Al Moustafa, A.E. Correlation between the presence of high-risk human papillomaviruses and Id gene expression in Syrian women with cervical cancer. Clin. Microbiol. Infect. 2010, 16, 262–266. [Google Scholar] [CrossRef]
- Resende Rodrigues, F.; Lourenco MIranda, N.; Carvalho da Fonseca, E.; Rodrigues Cordovil Pires, A.; Pedra Dias, E. Investigação da LMP1 do EBV e a coinfeçcão do HPV em lesões genitais de pacientes infectados ou não pelo HIV. Bras. Patol. Med. Lab. 2010, 46, 6. [Google Scholar] [CrossRef][Green Version]
- Albanese, M.; Tagawa, T.; Bouvet, M.; Maliqi, L.; Lutter, D.; Hoser, J.; Hastreiter, M.; Hayes, M.; Sugden, B.; Martin, L.; et al. Epstein-Barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6467–E6475. [Google Scholar] [CrossRef] [PubMed]
- Jochum, S.; Moosmann, A.; Lang, S.; Hammerschmidt, W.; Zeidler, R. The EBV Immunoevasins vIL-10 and BNLF2a Protect Newly Infected B Cells from Immune Recognition and Elimination. PLoS Pathog. 2012, 8, e1002704. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Fujisaki, S.; Nakamura, N.; Miyazaki, K.; Katsuki, T.; Okamura, H. Immunological Disorder against the Epstein-Barr Virus Infection and Prognosis in Patients with Cervical Carcinoma. Gynecol. Oncol. 1995, 57, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Shimabuku, T.; Tamanaha, A.; Kitamura, B.; Tanabe, Y.; Tawata, N.; Ikehara, F.; Arakaki, K.; Kinjo, T. Dual expression of Epstein-Barr virus, latent membrane protein-1 and human papillomavirus-16 E6 transform primary mouse embryonic fibroblasts through NF-κB signaling. Int. J. Clin. Exp. Pathol. 2014, 7, 1920–1934. [Google Scholar] [PubMed]
- Wang, L.W.; Jiang, S.; Gewurz, B.E. Epstein-Barr Virus LMP1-Mediated Oncogenicity. J. Virol. 2017, 91, 21. [Google Scholar] [CrossRef]
- Tsai, C.L.; Li, H.P.; Lu, Y.J.; Hsueh, C.; Liang, Y.; Chen, C.L.; Tsao, S.W.; Tse, K.P.; Yu, J.S.; Chang, Y.S. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH2-terminal kinase signaling. Cancer Res. 2006, 66, 11668–11676. [Google Scholar] [CrossRef]
- Horikawa, T.; Yang, J.; Kondo, S.; Yoshizaki, T.; Joab, I.; Furukawa, M.; Pagano, J.S. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are Associated with metastatic nasopharyngeal carcinoma. Cancer Res. 2007, 67, 1970–1978. [Google Scholar] [CrossRef]
- Horikawa, T.; Yoshizaki, T.; Kondo, S.; Furukawa, M.; Kaizaki, Y.; Pagano, J.S. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial–mesenchymal transition in metastatic nasopharyngeal carcinoma. Br. J. Cancer 2011, 104, 1160–1167. [Google Scholar] [CrossRef]
- Al Moustafa, A.E.; Al-Antary, N.; Aboulkassim, T.; Akil, N.; Batist, G.; Yasmeen, A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum. Vaccines Immunother. 2016, 12, 1936–1939. [Google Scholar] [CrossRef]
- Moon, J.W.; Kong, S.K.; Kim, B.S.; Kim, H.J.; Lim, H.; Noh, K.; Kim, Y.; Choi, J.W.; Lee, J.H.; Kim, Y.S. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci. Rep. 2017, 7, 17810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dakic, A.; Chen, R.; Dai, Y.; Schlegel, R.; Liu, X. Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation. Oncotarget 2017, 8, 96323–96339. [Google Scholar] [CrossRef] [PubMed]
- McMurray, H.R.; McCance, D.J. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J. Virol. 2003, 77, 9852–9861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Månér, S.; Betz, R.; Angström, T.; Stendahl, U.; Bergman, F.; Zetterberg, A.; Wallin, K.L. Genetic alterations in cervical carcinomas: Frequent low-level amplifications of oncogenes are associated with human papillomavirus infection. Int. J. Cancer 2002, 101, 427–433. [Google Scholar] [CrossRef]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef]
- Guidry, J.T.; Myers, J.E.; Bienkowska-Haba, M.; Songock, W.K.; Ma, X.; Shi, M.; Nathan, C.O.; Bodily, J.M.; Sapp, M.J.; Scott, R.S. Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes. J. Virol. 2018, 93, 2. [Google Scholar] [CrossRef]
- Aromseree, S.; Middeldorp, J.M.; Pientong, C.; van Eijndhoven, M.; Ramayanti, O.; Lougheed, S.M.; Pegtel, D.M.; Steenbergen, R.D.; Ekalaksananan, T. High Levels of EBV-Encoded RNA 1 (EBER1) Trigger Interferon and Inflammation-Related Genes in Keratinocytes Expressing HPV16 E6/E7. PLoS ONE 2017, 12, e0169290. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Jin, M.; Zhang, J.; Li, S.; Tong, F.; Zhou, Y. Differential expression of EBV proteins LMP1 and BHFR1 in EBV-associated gastric and nasopharyngeal cancer tissues. Mol. Med. Rep. 2016, 13, 4151–4158. [Google Scholar] [CrossRef]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2014, 44, 28–36. [Google Scholar] [CrossRef]
- Hoebe, E.; Wille, C.; Hagemeier, S.; Kenney, S.; Greijer, A.; Middeldorp, J. Epstein-Barr Virus Gene BARF1 expression is regulated by the epithelial differentiation factor ΔNp63α in undifferentiated nasopharyngeal carcinoma. Cancers (Basel) 2018, 10, 76. [Google Scholar] [CrossRef]
- Temple, R.M.; Meyers, C.; Sample, C.E. Generation and infection of organotypic cultures with Epstein-Barr virus. Methods Mol. Biol. 2017, 1532, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Temple, R.M.; Zhu, J.; Budgeon, L.; Christensen, N.D.; Meyers, C.; Sample, C.E. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16544–16549. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Deng, W.; Yip, Y.L.; Zeng, M.S.; Lo, K.W.; Tsao, S.W. Epstein-Barr virus infection and persistence in nasopharyngeal epithelial cells. Chin. J. Cancer 2014, 33, 549–555. [Google Scholar] [PubMed]
- De Lima, M.A.P.; Neto, P.J.N.; Lima, L.P.M.; Gonçalves Júnior, J.; Teixeira Junior, A.G.; Teodoro, I.P.P.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr virus (EBV) and cervical carcinoma: A meta-analysis. Gynecol. Oncol. 2018, 148, 317–328. [Google Scholar] [CrossRef]
| Ref. | EBV | HPV | EBV/HPV Coinfection | ||
|---|---|---|---|---|---|
| Methods | Results | Methods | Results | ||
| [70] | ISH of BamHI O/K | Normal cervix = 0/15 (0%) | - | - | - |
| CIN I = 1/1 (100%) | |||||
| Cervical cancer = 5/8 (62.5%) | |||||
| [71] | ISH of BamHI W | Normal cervix = 0/25 (0%) | - | - | - |
| CIN I = 0/25 (0%) | |||||
| CIN II = 2/25 (8.0%) | |||||
| CIN III = 2/25 (8.0%) | |||||
| SCC = 5/18 (27.8%) | |||||
| [72] | ISH of EBER1 | Normal cervix = 0/5 (0%) | PCR for E6/E7 | Normal cervix = 0/5 (0%) | Normal cervix = 0/5 (0%) |
| CIN II = 1/5 (20.0%) | CIN II = 1/3 (33.3%) | CIN II = 0/3 (0%) | |||
| CIN III = 5/12 (41.7%) | CIN III = 6/8 (75.0%) | CIN III = 2/8 (25.0%) | |||
| Cervical cancer = 7/14 (50.0%) | Cervical cancer = 10/13 (76.9%) | Cervical cancer = 4/13 (30.8%) | |||
| ISH of BamHI W | Normal cervix = 0/5 (%) | Normal cervix = 0/5 (0%) | |||
| CIN II = 4/5 (80.0%) | CIN II = 0/3 (0%) | ||||
| CIN III = 8/12 (66.7%) | CIN III = 3/8 (37.5%) | ||||
| Cervical cancer = 12/14 (85.7%) | Cervical cancer = 9/13 (69.2%) | ||||
| IFI for EBNA2 | Normal cervix = 0/3 (0%) | Normal cervix = 0/3 (0%) | |||
| CIN III = 6/8 (75.0%) | CIN III = 3/6 (50.0%) | ||||
| Cervical cancer = 8/9 (88.9%) | Cervical cancer = 5/8 (62.5%) | ||||
| IFI for LMP1 | Normal cervix = 0/3 (0%) | Normal cervix = 0/3 (0%) | |||
| CIN III = 4/8 (50.0%) | CIN III = 2/6 (33.3%) | ||||
| Cervical cancer = 6/9 (66.7%) | Cervical cancer = 4/8 (50.0%) | ||||
| [73] | ISH of BamHI W | Normal cervix = 0/2 (0%) | - | - | - |
| CIN I = 2/2 (100%) | |||||
| CIN II-III = 2/2 (100%) | |||||
| Cervical cancer = 10/10 (100%) | |||||
| ISH of EBNA2 | Normal cervix = 0/3 (0%) | ||||
| CIN I = 2/2 (100%) | |||||
| CIN II-III = 2/3 (66.7%) | |||||
| Cervical cancer = 14/16 (87.5%) | |||||
| IFI for EBNA2 | Normal cervix = 0/3 (0%) | ||||
| CIN I = 0/2 (0%) | |||||
| CIN II-III = 1/3 (33.3%) | |||||
| Cervical cancer = 11/16 (68.7%) | |||||
| [74] | ISH of EBERs | CIN I-II = 4/12 (33.3%) | PCR-ELISA for MY09/MY11 | CIN-negative = 2/26 (7.7%) | CIN I-II = 3/12 (25.0%) |
| CIN III = 7/10 (70.0%) | CIN I-II = 5/12 (41.7%) | CIN III = 4/10 (40.0%) | |||
| CIN III = 7/10 (70.0%) | |||||
| [91] | IHC for LMP1 | CIN I = 1/10 (10.0%) | IHC for HPV | CIN I = 3/10 (30.0%) | CIN I = 3/10 (30.0%) |
| CIN III = 3/3 (100%) | CIN II = 2/6 (33.3%) | CIN II = 4/6 (66.7%) | |||
| [13] | IHC for EBNA1 | SCC = 8/23 (34.8%) | PCR/Hybrid Capture 2 (HC2) | Normal cervix = 2/14 (14.3%) | - |
| IHC for LMP1 | SCC = 6/23 (26.1%) | CIN I = 12/16 (75.0%) | |||
| CIN II-III = 20/21 (95.2%) | |||||
| SCC = 51/58 (87.9%) | |||||
| [88] | IHC for LMP1 | SCC = 15/44 (34.1%) | PCR for E6/E7 | SCC = 42/44 (95.5%) | SCC = 15/44 (34.1%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, R.; Carrillo-Beltrán, D.; Osorio, J.C.; Calaf, G.M.; Aguayo, F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives. Pathogens 2020, 9, 685. https://doi.org/10.3390/pathogens9090685
Blanco R, Carrillo-Beltrán D, Osorio JC, Calaf GM, Aguayo F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives. Pathogens. 2020; 9(9):685. https://doi.org/10.3390/pathogens9090685
Chicago/Turabian StyleBlanco, Rancés, Diego Carrillo-Beltrán, Julio C. Osorio, Gloria M Calaf, and Francisco Aguayo. 2020. "Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives" Pathogens 9, no. 9: 685. https://doi.org/10.3390/pathogens9090685
APA StyleBlanco, R., Carrillo-Beltrán, D., Osorio, J. C., Calaf, G. M., & Aguayo, F. (2020). Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives. Pathogens, 9(9), 685. https://doi.org/10.3390/pathogens9090685





