Norovirus Is the Most Frequent Cause of Diarrhea in Hospitalized Patients in Monterrey, Mexico
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Microbial Detection
2.2.1. Strains Used as Controls
2.2.2. Bacterial Detection
2.2.3. Parasite Detection
2.2.4. Norovirus Detection
2.3. Statistical Analyses
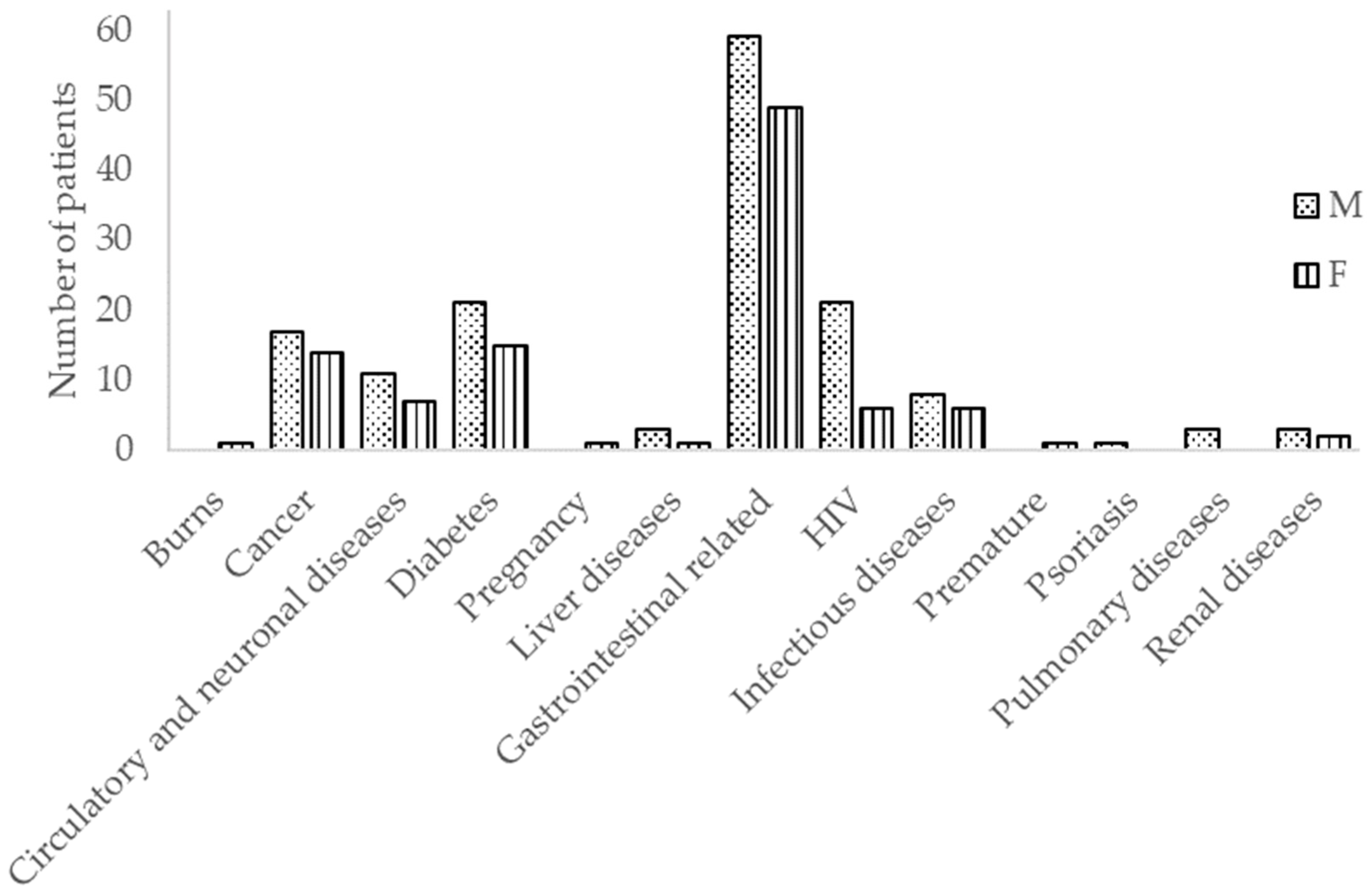
3. Results
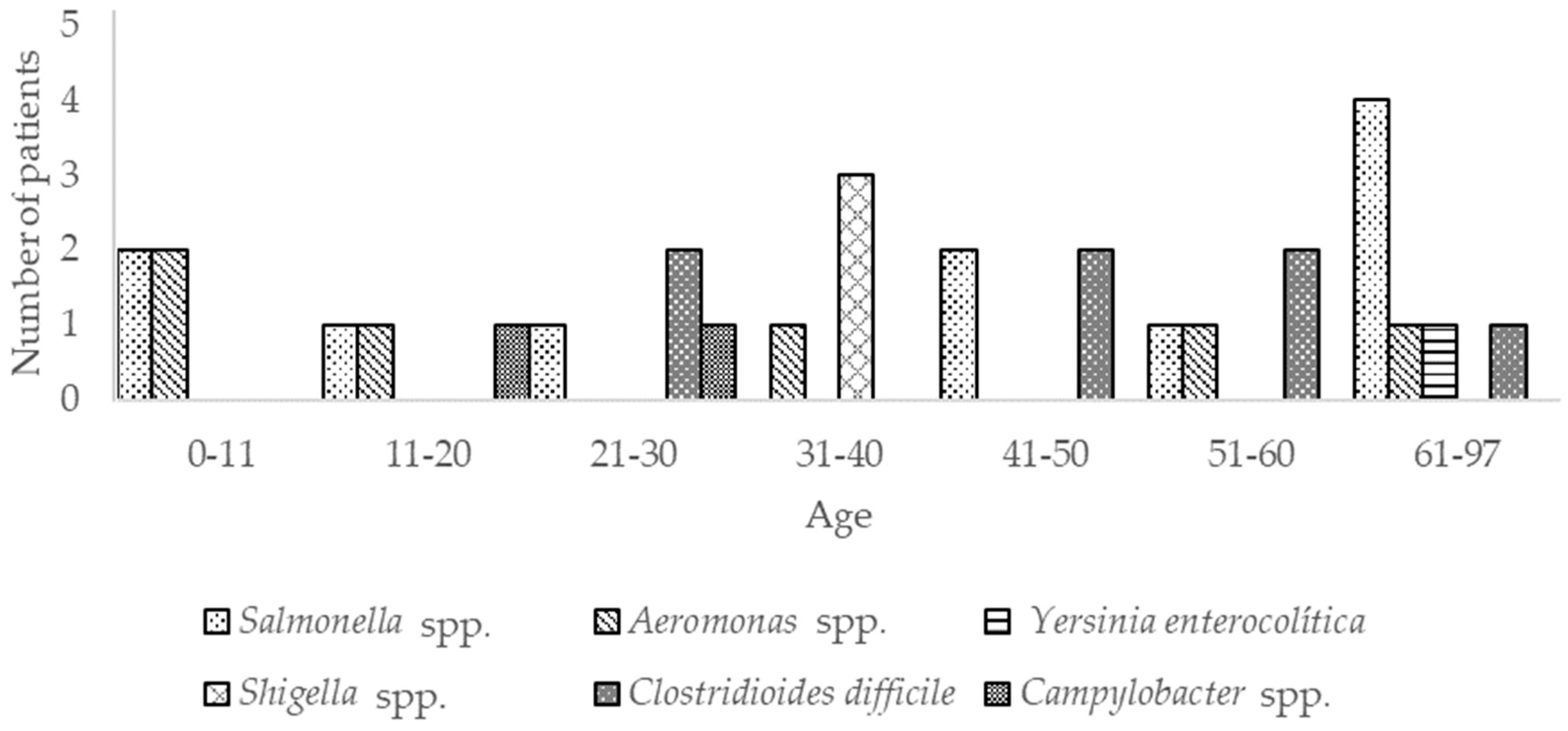
3.1. Bacteria
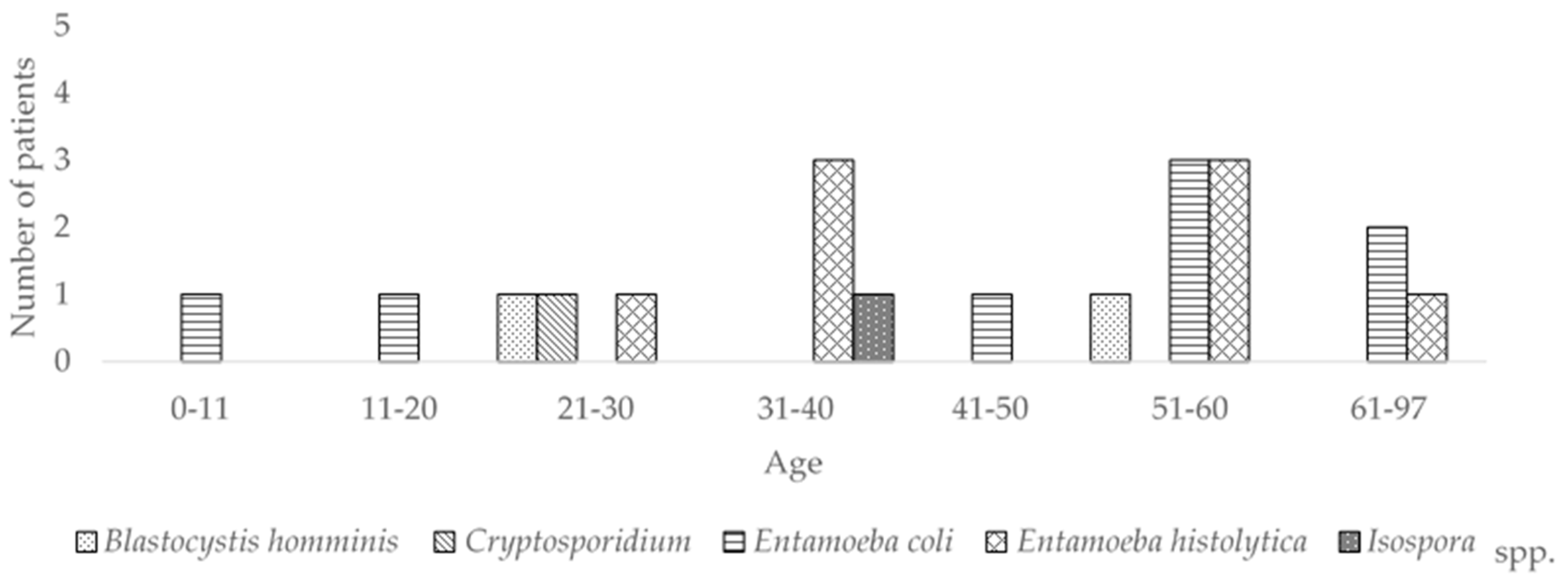
3.2. Parasites
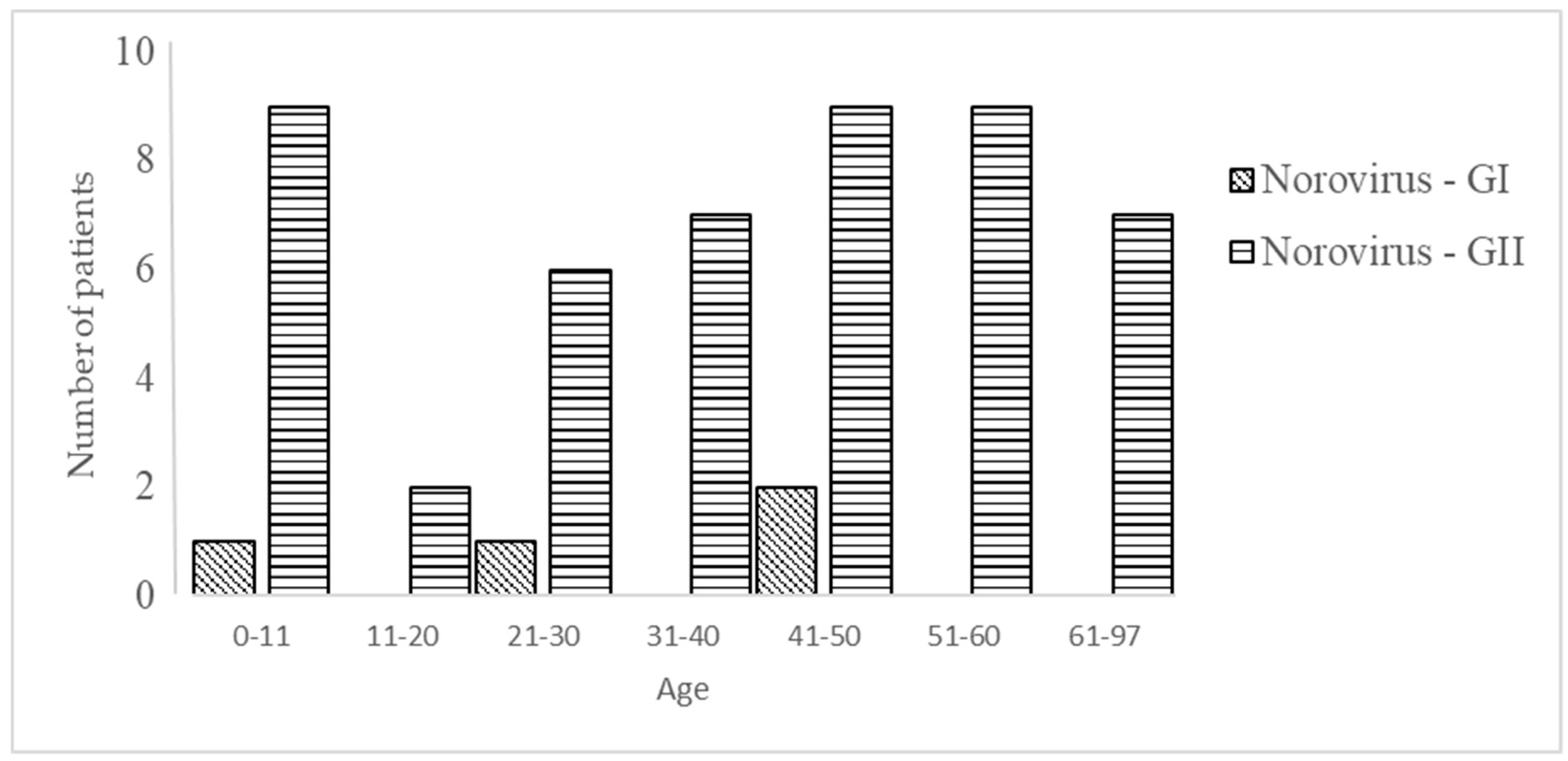
3.3. Virus: Norovirus
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Law, J.W.-F.; Letchumanan, V.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Insights into detection and identification of foodborne pathogens. In Foodborne Pathogens and Antibiotic Resistance; Singh, O.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 153–186. [Google Scholar]
- DuPont, H.L. Persistent diarrhea, a clinical review. JAMA 2016, 315, 2712–2723. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, CDC. CDC Annual Summaries of Foodborne Outbreaks; US Department of Health and Human Services CDC: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/fdoss/annual-reports/index.html (accessed on 15 July 2020).
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef]
- ECDPC. The European Union one health 2018 zoonoses report 2018. EFSA J. 2019, 17, 5926. [Google Scholar]
- Rajko-Nenow, P.; Keaveney, S.; Flannery, J.; O’Flaherty, V.; Dore, W. Characterization of norovirus contamination in an Irish shellfishery using real time RT-qPCR and sequencing analysis. Int. J. Food Microbiol. 2012, 160, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Parada-Fabia, J.C.; Juarez-Garcıa, P.; Natividad-Bonifacio, I.; Vazquez-Salinas, C.; Quiñones-Ramırez, E.I. Identification of enteric viruses in foods from Mexico City. Food Environ. Virol. 2016, 8, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.-A.T.; Farr, L.; Watanabe, K.; Moonah, S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.; Sisay, D. A review on major food borne bacterial illnesses. J. Trop. Dis. 2015, 3, 4. [Google Scholar]
- Yamamoto-Furusho, J.K. Diagnosis and management of inflammatory bowel disease (IBD) in Mexico and Central America. In WHO Handbook on Inflammatory Bowel Disease (IBD): Navigating Evolving Therapies in an Evolving Disease; Bernstein, C.N.N., Graber, R., Eds.; World Gastroenterology Organisation: Milwaukee, WI, USA, 2017; pp. 7–11. [Google Scholar]
- McFarland, L.V. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am. J. Infect. Control 1995, 23, 295–305. [Google Scholar] [CrossRef]
- Alteruse, S.F.; Swerdlow, D. The changing epidemiology of foodborne diseases. Am. J. Epidemiol. Foodborne Dis. 1996, 311, 23–29. [Google Scholar]
- Fincher, C.L.; Thornhill, R.; Murray, D.R.; Schaller, M. Pathogen prevalence predicts human cross-cultural variability in individualism/collectivism. Proc. R. Soc. B 2008, 275, 1279–1285. [Google Scholar] [CrossRef]
- Mota-Hernandez, F. Presentación del manual de procedimientos del servicio de hidratación oral del hospital infantil de Mexico “Federico Gomez”. Gac. Med. Mex. 1990, 126, 419–422. [Google Scholar] [PubMed]
- Lagier, J.C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, S.R.; Kim, Y.G. Detection of Escherichia coli O157: H7, Salmonella spp., Staphylococcus aureus and Listeria monocytogenes in kimchi by multiplex polymerase chain reaction (mPCR). J. Microbiol. 2006, 44, 92–97. [Google Scholar] [PubMed]
- Vidal, M.; Kruger, E.; Durán, C.; Lagos, R.; Levine, M.; Prado, V.; Toro, C.; Vidal, R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J. Clin. Microbiol. 2005, 43, 5362–5365. [Google Scholar] [CrossRef]
- Leblanc-Maridor, M.; Garénaux, A.; Beaudeau, F.; Chidaine, B.; Seegers, H.; Denis, M.; Belloc, C. Quantification of Campylobacter spp. in pig feces by direct real-time PCR with an internal control of extraction and amplification. J. Microbiol. Methods 2011, 85, 53–61. [Google Scholar] [CrossRef]
- Gómez-Duarte, O.G.; Bai, J.; Newell, E. Detection of Escherichia coli, Salmonella spp., Shigella spp., Yersinia enterocolitica, Vibrio cholerae, and Campylobacter spp. enteropathogens by 3-reaction multiplex polymerase chain reaction. Diag. Microbiol. Infect. Dis. 2009, 63, 1–9. [Google Scholar]
- Lee, C.; Cho, J.C.; Lee, S.H.; Lee, D.G.; Kim, S.J. Distribution of Aeromonas spp. as identified by 16S rDNA restriction fragment length polymorphism analysis in a trout farm. J. Appl. Microbiol. 2002, 93, 976–985. [Google Scholar] [CrossRef]
- Lemee, L.; Dhalluin, A.; Trstelin, S.; Mattrat, M.-A.; Maillard, K.; Lemaland, J.-F.; Pons, J.L. Multiplex PCR targeting tpi (trialose phosphate isomerase), tcdA (toxin A) and tcdB (toxin B) genes for toxigenic cultures of Clostridium difficile. J. Clin. Microbiol. 2004, 42, 5710–5714. [Google Scholar] [CrossRef]
- Jothikumar, N.; Lowther, J.A.; Henshilwood, K.; Lees, D.N.; Hill, V.R.; Vinjé, J. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl. Environ. Microbiol. 2005, 71, 1870–1875. [Google Scholar] [CrossRef]
- Garcia, L.S.; Arrowood, M.; Kokoskin, E.; Paltridge, G.P.; Pillai, D.R.; Procop, G.W.; Ryan, N.; Shimizu, R.Y.; Visvesvara, G. Laboratory diagnosis of parasites from the gastrointestinal tract. Clin. Microbiol. Rev. 2018, 31, e00025-17. [Google Scholar] [CrossRef]
- Heidary, M.; Pahlevan-Kakhki, M. TRIzol-based RNA Extraction: A Reliable Method for Gene Expression Studies. J. Sci. Islamic Repub. Iran 2014, 25, 13–17. [Google Scholar]
- Breurec, S.; Vanel, N.; Bata, P.; Chartier, L.; Farra, A.; Favennec, L.; Franck, T.; Giles-Vernick, T.; Gody, J.-C.; Luang Nguyen, L.B. Etiology and epidemiology of diarrhea in hospitalized children from low income country: A matched case-control study in Central African Republic. PLoS Negl. Trop. Dis. 2016, 10, e0004283. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Murray, R.; Flockhart, L.; Pintar, K.; Fazil, A.; Nesbitt, A.; Marshall, B.; Tataryn, J.; Pollari, F. Estimates of foodborne illness–related hospitalizations and deaths in Canada for 30 specified pathogens and unspecified agents. Foodborne Pathog. Dis. 2015, 12, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Cauteren, D.V.; Strat, Y.L.; Sommen, C.; Bruyand, C.; Tourdjman, M.; Jourdan-Da Silva, N.; Coulurier, E.; Fournet, N.; de Valk, H.; Desenclos, J.-C. Estimated annual numbers of foodborne pathogen–associated illnesses, hospitalizations, and deaths, France, 2008–2013. Emerg. Infect. Dis. 2017, 23, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Vazquez-Polanco, A.M.; Argueta-Donohue, J.; Stephens, C.R.; Jimenez-Trejo, F.; Ceballos-Liceaga, S.E.; Mantilla-Beniers, N. Incidence of intestinal infectious diseases due to protozoa and bacteria in Mexico: Analysis of National surveillance records from 2003 to 2012. BioMed Res. Int. 2018, 2018, 2893012. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cortez, C.; Aguilera Arreola, M.G.; Castro-Escapulli, G. Situacion de las enfermedades gastrointestinales en Mexico. Enferm. Infecc. Microbiol. 2011, 31, 137–151. [Google Scholar]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Umesh, D.; Koopman, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- EFSA-ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 13, 3991. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Buzby, J.C.; Roberts, T. ERS Updates U.S. foodborne disease costs for seven pathogens. Food Rev. 1996, 19, 20–25. [Google Scholar]

| Microorganism | Target Gene | Primers Sequence | Amplicon Size (bp) | Run Conditions | Ref. |
|---|---|---|---|---|---|
| Salmonella spp. | ITS | 5′-TATAGCCCCATCGTGTAGTCAGAAC-3′ F | 312 | 5 min at 94 °C, 35 cycles of 30 s at 94 °C, 35 s at 61 °C, and 35 s at 72 °C, and final extension at 72 °C for 5 min. | [16] |
| 5′-TGCGGCTGGATCACCTCCTT-3′ R | |||||
| Shigella spp. | VirF | 5′-AGCTCAGGCAATGAAACTTTGAC-3′ F | 628 | 5 min at 95 °C. 30 cycles of 30 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C. and final extension at 72 °C for 5 min. | [17] |
| 5′-TGGGCTTGATATTCCGATAAGTC-3′ R | |||||
| Campylobacter spp. | 16S rRNA | 5′-CACGTGTCACAATGGCATAT-3′ F | 115 | 6 min at 95 °C, 30 cycles of 30 s at 95 °C, 30 s at 59 °C, and 30 s at 72 °C, and final extension at 72 °C for 7 min. | [18] |
| 5′-GGCTTCATGCTCTCGAGTT-3′ R | |||||
| Y. enterocolitica | YST | 5′-GTTAATGCTGTCTTCATTTGGAGC-3′ F | 145 | 2 min at 94 °C, 40 cycles of 30 s at 92 °C, 30 s at 59 °C, and 30 s at 72 °C, and final extension at 72 °C for 5 min. | [19] |
| 5′-GACATCCCAATCACTACTGACTTC-3′ R | |||||
| Aeromonas spp. | 16S rRNA | 5′-CTACTTTTGCCGGCGAGCGG-3′ F | 935 | 5 min at 95 °C; then 35 cycles of 1 min at 94 °C, 30 s at 68 °C, and 45 s at 72 °C, and final extension at 72 °C for 5 min. | [20] |
| 5′-TGATTCCCGAAGGCACTCCC-3′ R | |||||
| C. difficiletoxin B | tcdB | 5′-GGAAAAGAGAATGGTTTTATTAA-3′ F | 135 | 3 min at 95 °C, 10 cycles of 30 s at 95 °C, 30 s at 65 °C, and 30 s at 72 °C, and final extension at 95 °C for 30 s. | [21] |
| 5′-ATCTTTAGTTATAACTTTGACATCTTT-3′ R | |||||
| norovirus GI | 5′-GCCATGTTCCGITGGATG-3′ F | 96 | 10 min at 95 ºC, 40 cycles of 15 s at 95 ºC and 1 min at 60 ºC. | [22] | |
| 5′-TCCTTAGACGCCATCATCAT-3′ R | |||||
| FAM-5′-TGTGGACAGGAGATCGCAATCC-3′ | |||||
| Norovirus GII | 5′-CAAGAGTCAATGTTTAGGTGGATGAG-3′ F | 89 | 10 min at 95 ºC, 40 cycles of 15 s at 95 ºC and 1 min at 60 ºC. | [22] | |
| 5′-TCGACGCCATCTTCATTCACA-3′ R | |||||
| Cy5-5′-TGGGAGGGCGATCGCAATCT-3′ |
| Pathogen | Number of Patients (n = 240) | ||||||
|---|---|---|---|---|---|---|---|
| Age Group | |||||||
| 0–10 (n = 15) | 11–20 (n = 20) | 21–30 (n = 35) | 31–40 (n = 29) | 41–50 (n = 45) | 51–60 (n = 46) | 61–97 (n = 50) | |
| Bacteria | |||||||
| Campylobacter spp. | ND | 1 | 1 | ND | ND | ND | ND |
| Clostridioidesdifficile | ND | ND | 2 | ND | 2 | 2 | 1 |
| Shigella spp. | ND | ND | ND | 3 | ND | ND | ND |
| Yersinia enterocolitica | ND | ND | ND | ND | ND | ND | 1 |
| Aeromonas spp. | 2 | 1 | ND | 1 | ND | 1 | 1 |
| Salmonella spp | 2 | 1 | 1 | ND | 2 | 1 | 4 |
| Parasites | |||||||
| Entamoeba histolytica | ND | ND | 1 | 3 | ND | 3 | 1 |
| Entamoeba coli | 1 | 1 | ND | ND | 1 | 3 | 2 |
| Blastocysts hominis | ND | ND | 1 | ND | ND | 1 | ND |
| Cryptosporidium spp. | ND | ND | ND | 1 | ND | ND | ND |
| Isospora spp. | ND | ND | ND | 1 | ND | ND | ND |
| Virus | |||||||
| norovirus GI | 1 | ND | 1 | ND | 2 | ND | ND |
| norovirus GII | 9 | 2 | 6 | 7 | 9 | 9 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casillas-Vega, N.; Flores-Rodríguez, F.; Sotelo-Coronado, I.; Vera-García, M.E.; García-Heredia, A.; Rivas-Estilla, A.M.; Lozano-Sepúlveda, S.A.; García, S.; Flores-Arechiga, A.; Heredia, N. Norovirus Is the Most Frequent Cause of Diarrhea in Hospitalized Patients in Monterrey, Mexico. Pathogens 2020, 9, 672. https://doi.org/10.3390/pathogens9090672
Casillas-Vega N, Flores-Rodríguez F, Sotelo-Coronado I, Vera-García ME, García-Heredia A, Rivas-Estilla AM, Lozano-Sepúlveda SA, García S, Flores-Arechiga A, Heredia N. Norovirus Is the Most Frequent Cause of Diarrhea in Hospitalized Patients in Monterrey, Mexico. Pathogens. 2020; 9(9):672. https://doi.org/10.3390/pathogens9090672
Chicago/Turabian StyleCasillas-Vega, Néstor, Fernanda Flores-Rodríguez, Israel Sotelo-Coronado, Magda Elizabeth Vera-García, Aldo García-Heredia, Ana Ma. Rivas-Estilla, Sonia A. Lozano-Sepúlveda, Santos García, Amador Flores-Arechiga, and Norma Heredia. 2020. "Norovirus Is the Most Frequent Cause of Diarrhea in Hospitalized Patients in Monterrey, Mexico" Pathogens 9, no. 9: 672. https://doi.org/10.3390/pathogens9090672
APA StyleCasillas-Vega, N., Flores-Rodríguez, F., Sotelo-Coronado, I., Vera-García, M. E., García-Heredia, A., Rivas-Estilla, A. M., Lozano-Sepúlveda, S. A., García, S., Flores-Arechiga, A., & Heredia, N. (2020). Norovirus Is the Most Frequent Cause of Diarrhea in Hospitalized Patients in Monterrey, Mexico. Pathogens, 9(9), 672. https://doi.org/10.3390/pathogens9090672





