The Clinical Infection with Pigeon Circovirus (PiCV) Leads to Lymphocyte B Apoptosis But Has No Effect on Lymphocyte T Subpopulation
Abstract
1. Introduction
2. Results
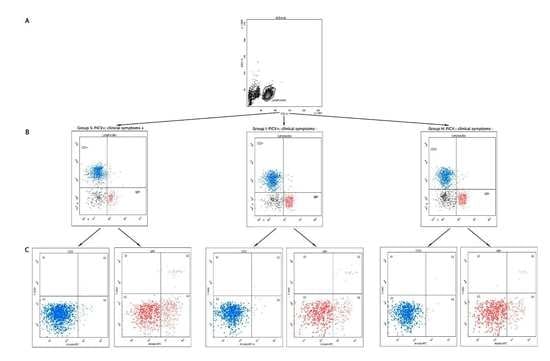
2.1. Flow Cytometry Analyses
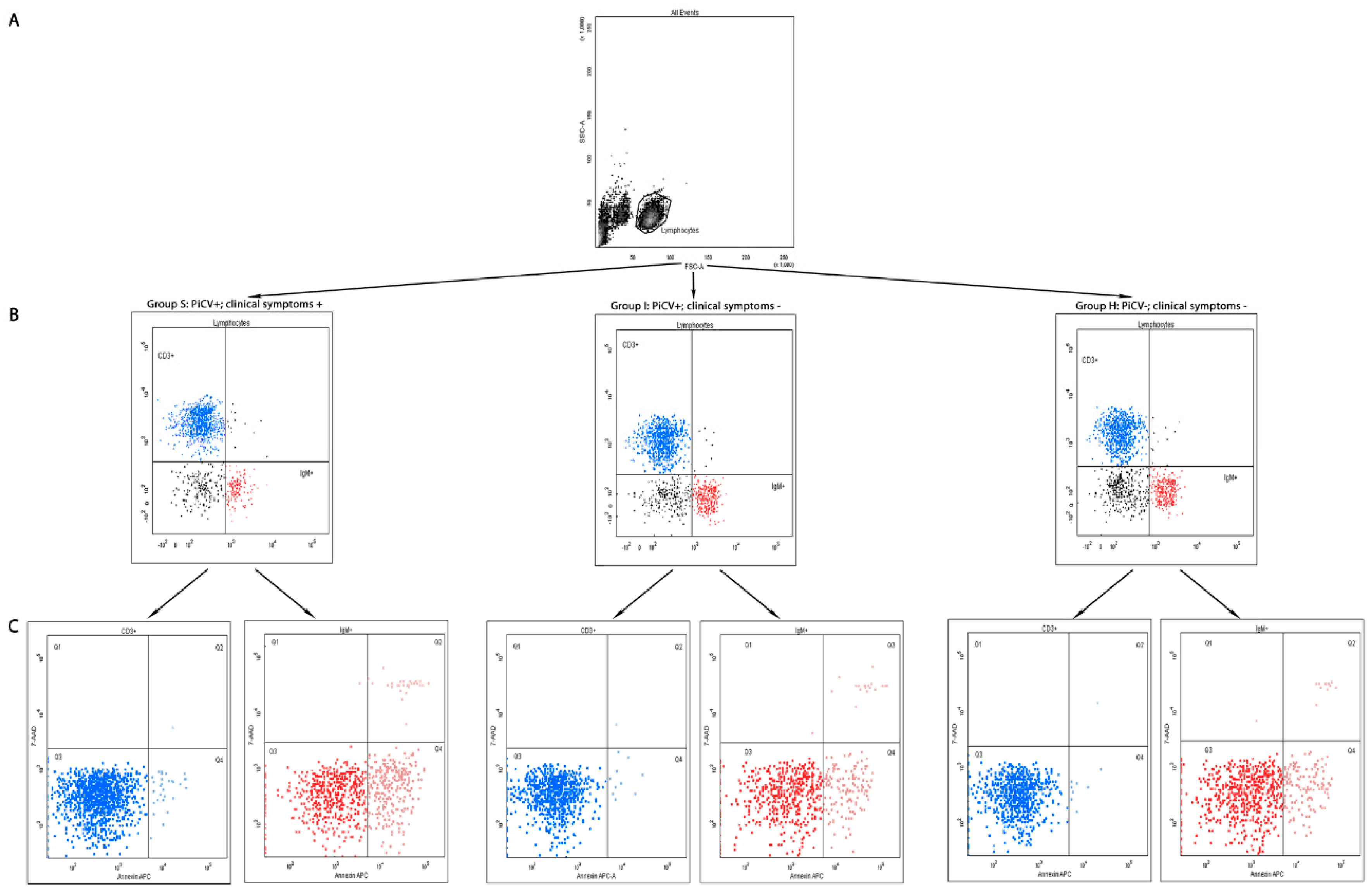
2.1.1. The Percentage of T CD3+ and B IgM+ Lymphocytes in the Spleen
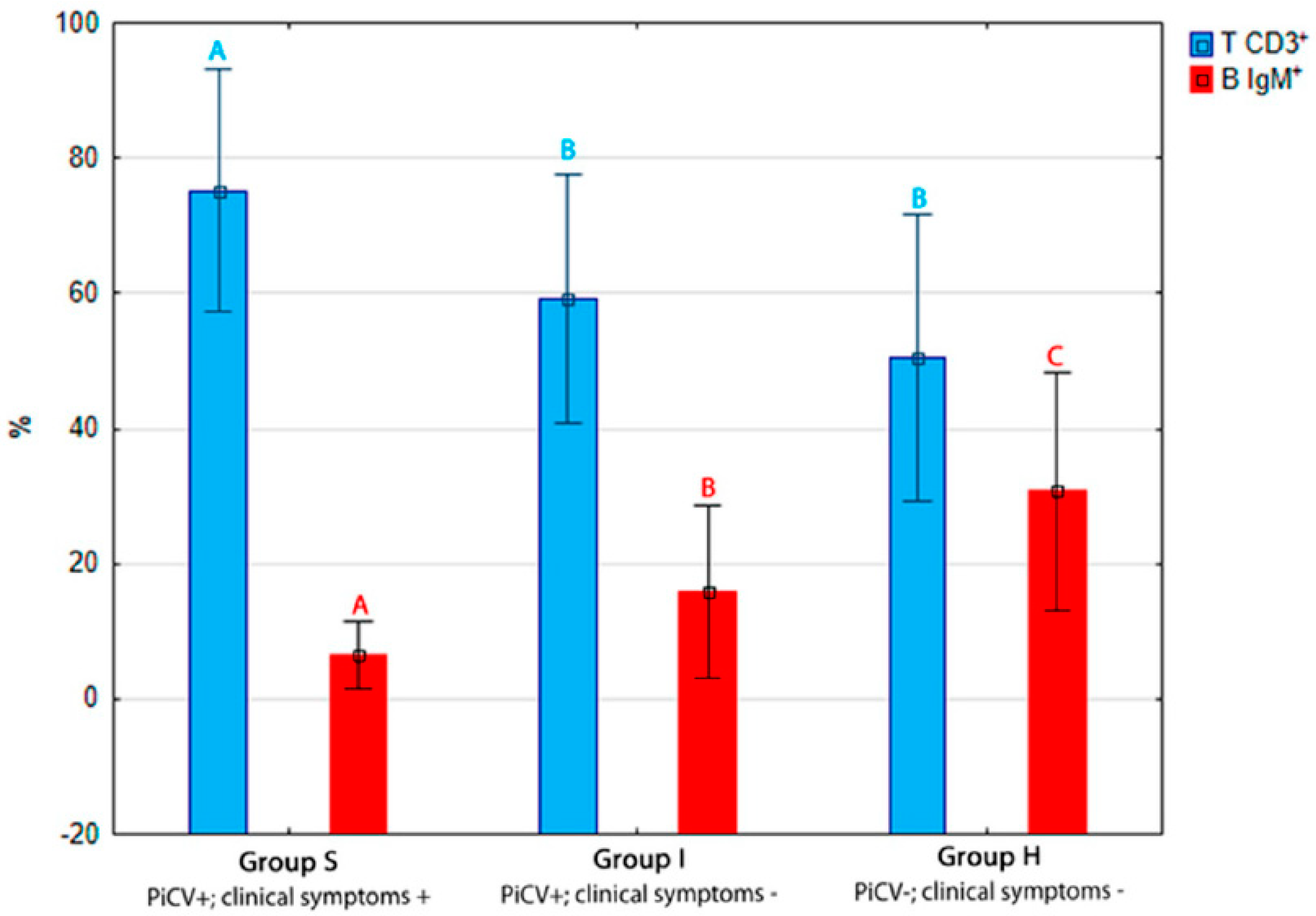
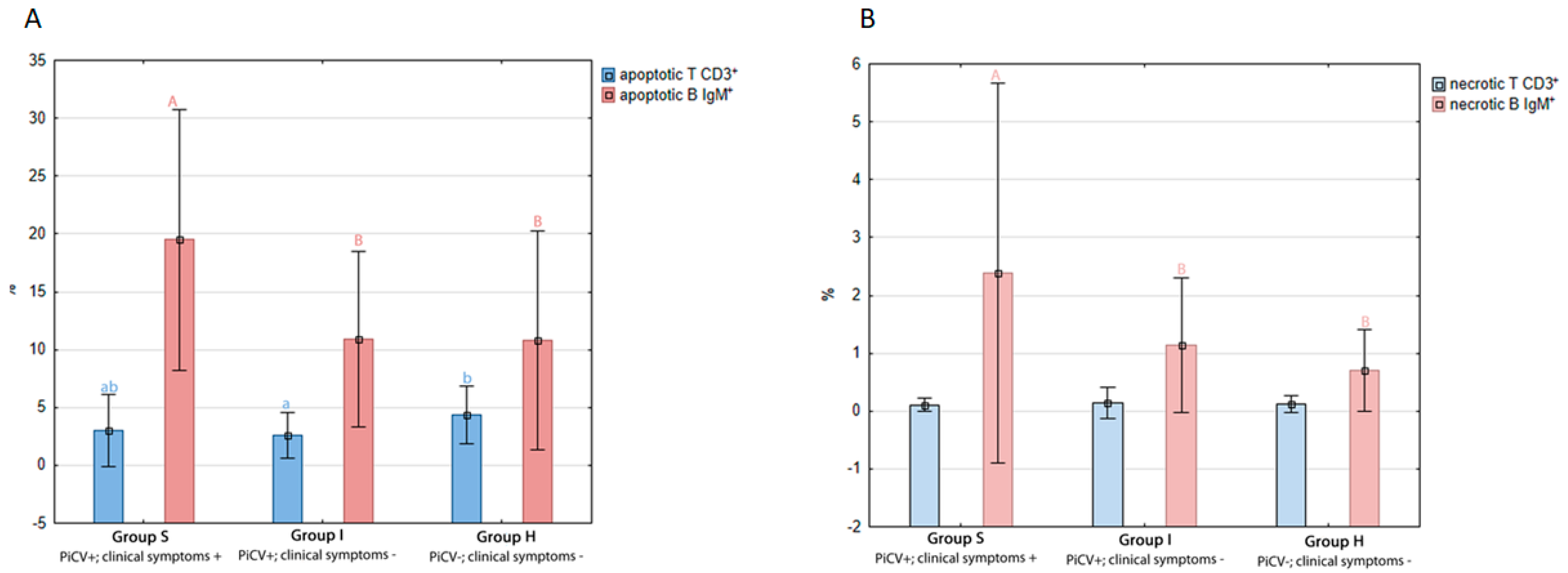
2.1.2. Apoptosis and Necrosis in Splenic T and B Lymphocytes
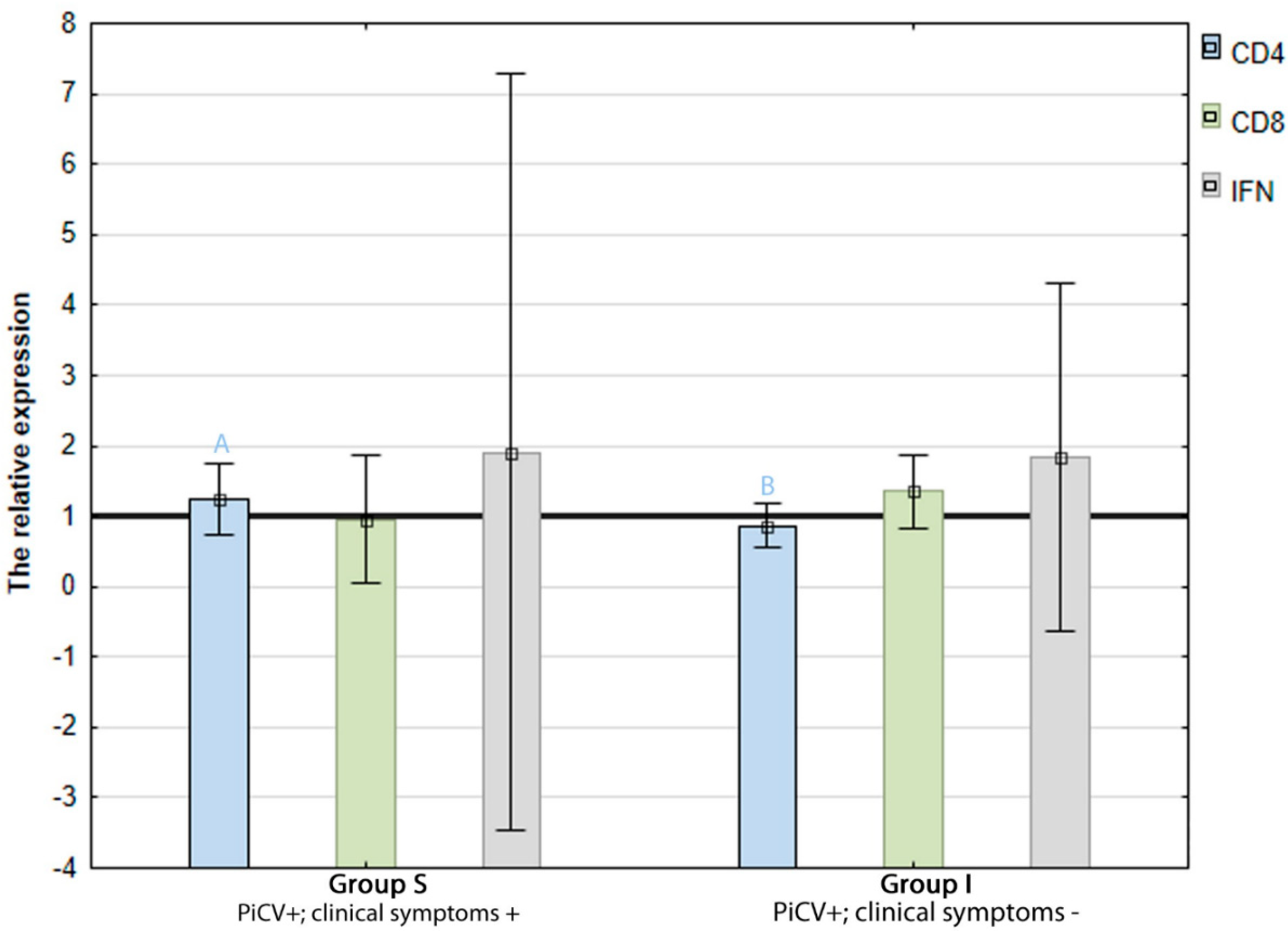
2.2. The Expression of CD4, CD8, and IFN-γ Genes
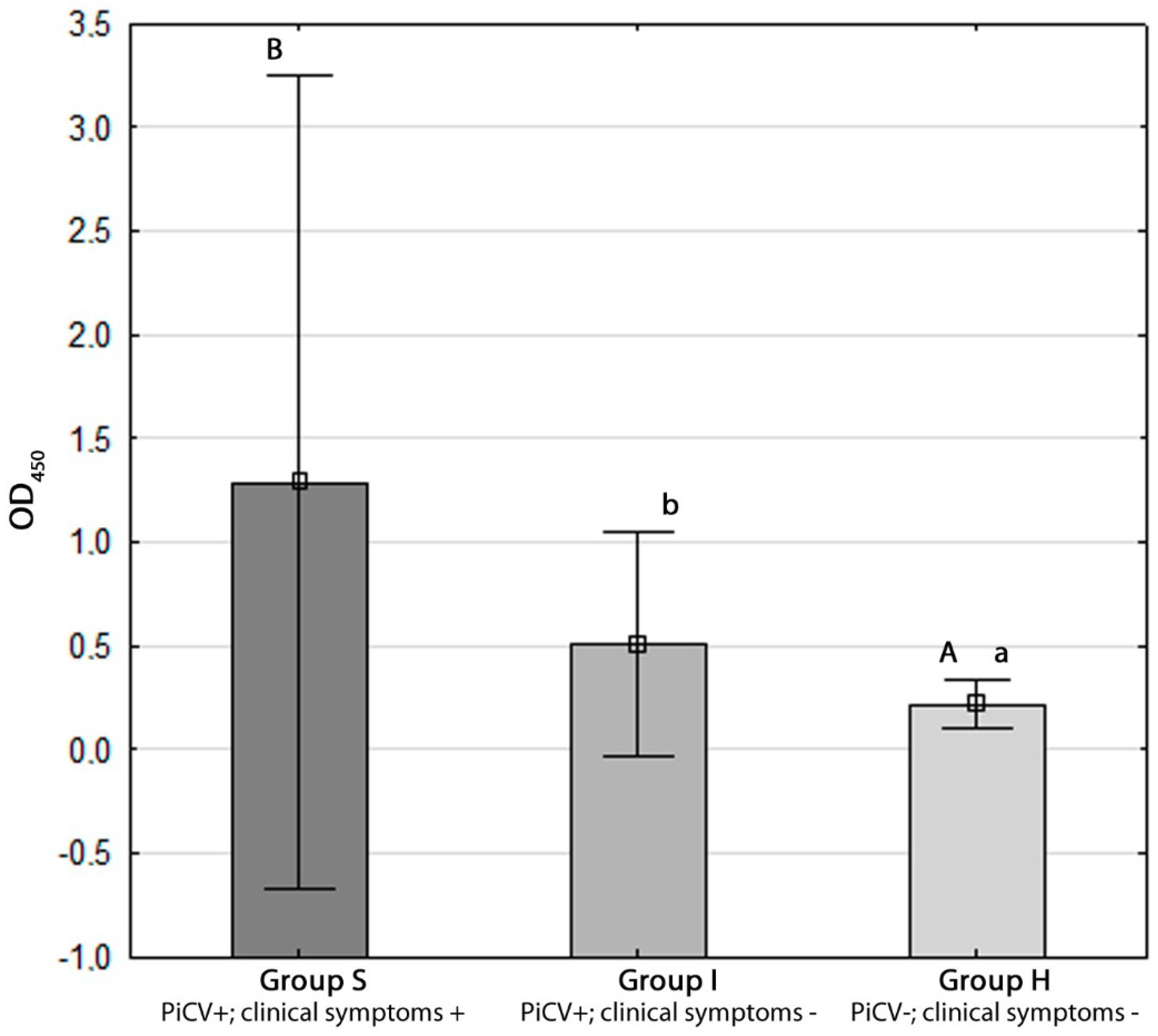
2.3. Determination of Anti-PiCV IgY
2.4. PiCV Viral Loads in the Bursa of Fabricius Samples
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Pigeons
4.3. Isolation of Mononuclear Cells from the Spleen
4.4. Flow Cytometry Analyses
4.4.1. Extracellular Staining for TCD3+ and B IgM+ Lymphocytes
4.4.2. Staining for Apoptosis and Necrosis Evaluation
4.5. RNA Isolation and qPCR for Selected Genes Expression
4.6. In-House ELISA for Determination of Anti-PiCV IgY
4.7. Digital Droplet PCR (ddPCR) for PiCV Viral Loads in the Bursa of Fabricius Samples
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mankertz, A.; Hattermann, K.; Ehlers, B.; Soike, D. Cloning and sequencing of columbid circovirus (CoCV), a new circovirus from pigeons. Arch. Virol. 2000, 145, 2469–2479. [Google Scholar] [CrossRef]
- Woods, L.W.; Latimer, K.S.; Barr, B.C.; Niagro, F.D.; Campagnoli, R.P.; Nordhausen, R.W.; Castro, A.E. Circovirus-like infection in a pigeon. J. Vet. Diagn Invest. 1993, 5, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Paré, J.A.; Brash, M.L.; Hunter, D.B.; Hampson, R.J. Observations on pigeon circovirus infection in Ontario. Can. Vet. J. 1999, 40, 659–662. [Google Scholar] [PubMed]
- Stenzel, T.; Koncicki, A. The epidemiology, molecular characterization and clinical pathology of circovirus infections in pigeons—Current knowledge. Vet. Q. 2017, 37, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Piasecki, T.; Chrząstek, K.; Julian, L.; Muhire, B.M.; Golden, M.; Martin, D.P.; Varsani, A. Pigeon circoviruses display patterns of recombination, genomic secondary structure and selection similar to those of beak and feather disease viruses. J. Gen. Virol. 2014, 95, 1338–1351. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Woźniakowski, G.; Pestka, D.; Choszcz, D.; Tykałowski, B.; Śmiałek, M.; Koncicki, A. Application of pigeon circovirus recombinant capsid protein for detecting anti-PiCV antibodies in the sera of asymptomatic domestic pigeons and the potential use of a combination of serological and molecular tests for controlling circovirus infections in pigeon breeding flocks. Poult. Sci. 2017, 96, 303–308. [Google Scholar] [PubMed]
- Zhang, Z.; Lu, C.; Wang, Y.; Wang, S.; Dai, D.; Chen, Z.; Fan, H. Molecular characterization and epidemiological investigation of Pigeon circovirus isolated in eastern China. J. Vet. Diagn Invest. 2011, 23, 665–672. [Google Scholar] [CrossRef]
- Franciosini, M.P.; Fringuelli, E.; Tarhuni, O.; Guelfi, G.; Todd, D.; Casagrande Proietti, P.; Falocci, N.; Asdrubali, G. Development of polymerase chain reaction-based in vivo method in the diagnosis of subclinical pigeon circovirus infection. Avian Dis. 2005, 49, 340–343. [Google Scholar] [CrossRef]
- Julian, L.; Piasecki, T.; Chrząstek, K.; Walters, M.; Muhire, B.; Harkins, G.W.; Martin, D.P.; Varsani, A. Extensive recombination detected among beak and feather disease virus isolates from breeding facilities in Poland. J. Gen. Virol. 2013, 94, 1086–1095. [Google Scholar] [CrossRef]
- Loiko, M.R.; Junqueira, D.M.; Varela, A.P.M.; Tochetto, C.; Scheffer, C.M.; Lima, D.A.; Morel, A.P.; Cerva, C.; Paim, W.P.; Quoos Mayer, F.; et al. Columbid circoviruses detected in free ranging pigeons from Southern Brazil: Insights on PiCV evolution. Arch. Virol. 2018, 163, 3083–3090. [Google Scholar] [CrossRef]
- Sarker, S.; Das, S.; Ghorashi, S.A.; Forwood, J.K.; Raidal, S.R. Pigeon circoviruses from feral pigeons in Australia demonstrate extensive recombination and genetic admixture with other circoviruses. Avian Pathol. 2019, 48, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Dolka, B.; Dolka, I.; Ledwoń, A.; Sapierzyński, R.; Koralewski, A.; Szeleszczuk, P. Investigations into feather abnormalities in racing pigeons. Med. Water 2016, 72, 693–698. [Google Scholar] [CrossRef]
- Huang, Y.L.; Castaneda, O.A.; Thongchan, D.; Khatri-Chhetri, R.; Tsai, S.S.; Wu, H.Y. Pigeon circovirus infection in disqualified racing pigeons from Taiwan. Avian Pathol. 2017, 46, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Coletti, M.; Franciosini, M.P.; Asdrubali, G.; Passamonti, F. Atrophy of the primary lymphoid organs of meat pigeons in Italy associated with circoviruslike particles in the bursa of Fabricius. Avian Dis. 2000, 44, 454–459. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Groizeleau, C.; Amenna, N.; Fernandez, B.; Guereaud, C.; Guigand, L.; Robart, P.; Lefebvre, B.; Wyers, M. Pigeon circovirus infection: Pathological observations and suggested pathogenesis. Avian Pathol. 2001, 30, 149–158. [Google Scholar] [CrossRef]
- Stenzel, T.; Pestka, D.; Tykałowski, B.; Śmiałek, M.; Koncicki, A. Epidemiological investigation of selected pigeon viral infections in Poland. Vet. Rec. 2012, 171, 562. [Google Scholar] [CrossRef]
- Stenzel, T.; Pestka, D.; Choszcz, D. The prevalence and genetic characterization of Chlamydia psittaci from domestic and feral pigeons in Poland and the correlation between infection rate and incidence of pigeon circovirus. Poult. Sci. 2014, 93, 3009–3016. [Google Scholar] [CrossRef]
- Lv, Y.; Dai, L.; Han, H.; Zhang, S. PCV2 induces apoptosis and modulates calcium homeostasis in piglet lymphocytes in vitro. Res. Vet. Sci. 2012, 93, 1525–1530. [Google Scholar] [CrossRef]
- Xiang, Q.W.; Wang, X.; Xie, Z.J.; Sun, Y.N.; Zhu, Y.L.; Wang, S.J.; Liu, H.J.; Jiang, S.J. ORF3 of duck circovirus: A novel protein with apoptotic activity. Vet. Microbiol. 2012, 159, 251–256. [Google Scholar] [CrossRef]
- Stenzel, T.; Dziewulska, D.; Śmialek, M.; Tykałowski, B.; Kowalczyk, J.; Koncicki, A. Comparison of the immune response to vaccination with pigeon circovirus recombinant capsid protein (PiCV rCP) in pigeons uninfected and subclinically infected with PiCV. PLoS ONE 2019, 14, e0219175. [Google Scholar] [CrossRef]
- Stenzel, T.; Dziewulska, D.; Tykałowski, B.; Śmiałek, M.; Kowalczyk, J.; Koncicki, A. Immunogenicity of Pigeon Circovirus Recombinant Capsid Protein in Pigeons. Viruses 2018, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Dziewulska, D.; Stenzel, T.; Śmiałek, M.; Tykałowski, B.; Koncicki, A. The impact of Aloe vera and licorice extracts on selected mechanisms of humoral and cell-mediated immunity in pigeons experimentally infected with PPMV-1. BMC Vet. Res. 2018, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, P.; Jia, R.; Wang, M.; Yin, Z.; Cheng, A. Regulation of Apoptosis during Porcine Circovirus Type 2 Infection. Front. Microbiol. 2018, 9, 2086. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tian, J.; Tan, X.; Yu, H.; Ding, S.; Sun, H.; Yu, X. Pathological observations of an experimental infection of geese with goose circovirus. Avian Pathol. 2011, 40, 55–61. [Google Scholar] [CrossRef]
- Robino, P.; Grego, E.; Rossi, G.; Bert, E.; Tramuta, C.; Stella, M.C.; Bertoni, P.; Nebbia, P. Molecular analysis and associated pathology of beak and feather disease virus isolated in Italy from young Congo African grey parrots (Psittacus erithacus) with an “atypical peracute form” of the disease. Avian Pathol. 2014, 43, 333–344. [Google Scholar] [CrossRef]
- Liu, J.; Chen, I.; Kwang, J. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 2005, 79, 8262–8274. [Google Scholar] [CrossRef] [PubMed]
- Raue, R.; Schmidt, V.; Freick, M.; Reinhardt, B.; Johne, R.; Kamphausen, L.; Kaleta, E.F.; Müller, H.; Krautwald-Junghanns, M.E. A disease complex associated with pigeon circovirus infection, young pigeon disease syndrome. Avian Pathol. 2005, 34, 418–425. [Google Scholar] [CrossRef]
- Todd, D.; Duchatel, J.P.; Weston, H.; Ball, N.W.; Borghmans, B.J.; Moffett, D.A.; Smyth, J.A. Evaluation of polymerase chain reaction and dot blot hybridisation tests in the diagnosis of pigeon circovirus infections. Vet. Microbiol. 2002, 89, 1–16. [Google Scholar] [CrossRef]
- Duchatel, J.P.; Todd, D.; Willeman, C.; Losson, B. Quantification of pigeon circovirus in serum, blood, semen and different tissues of naturally infected pigeons using a real-time polymerase chain reaction. Avian Pathol. 2009, 38, 143–148. [Google Scholar] [CrossRef]
- Schmidt, V.; Schlömer, J.; Lüken, C.; Johne, R.; Biere, B.; Müller, H.; Krautwald-Junghanns, M.E. Experimental infection of domestic pigeons with pigeon circovirus. Avian Dis. 2008, 52, 380–386. [Google Scholar] [CrossRef]
- McCowan, C.; Crameri, S.; Kocak, A.; Shan, S.; Fegan, M.; Forshaw, D.; Rubbenstroth, D.; Chen, H.; Holmes, C.; Harper, J.; et al. A novel group A rotavirus associated with acute illness and hepatic necrosis in pigeons (Columba livia), in Australia. PLoS ONE 2018, 13, e0203853. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Peus, E.; Schramm, E.; Kottmann, D.; Bartels, H.; McCowan, C.; Schulze, C.; Akimkin, V.; Fischer, N.; Wylezich, C.; et al. Identification of a novel clade of group A rotaviruses in fatally diseased domestic pigeons in Europe. Transbound. Emerg. Dis. 2019, 66, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Ulrich, R.; Wylezich, C.; Rautenschlein, S.; Beer, M.; Mohr, L. First experimental proof of Rotavirus A (RVA) genotype G18P [17] inducing the clinical presentation of ‘young pigeon disease syndrome’ (YPDS) in domestic pigeons (Columba livia). Transbound. Emerg. Dis. 2020, 67, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Maślanka, T.; Jaroszewski, J.J.; Markiewicz, W.; Jasiecka, A.; Ziółkowski, H.; Jędrzkiewicz, D. Effects of dexamethasone and meloxicam on bovine CD25+ CD8+ and CD25- CD8+ T cells-in vitro study. Res. Vet. Sci. 2013, 94, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
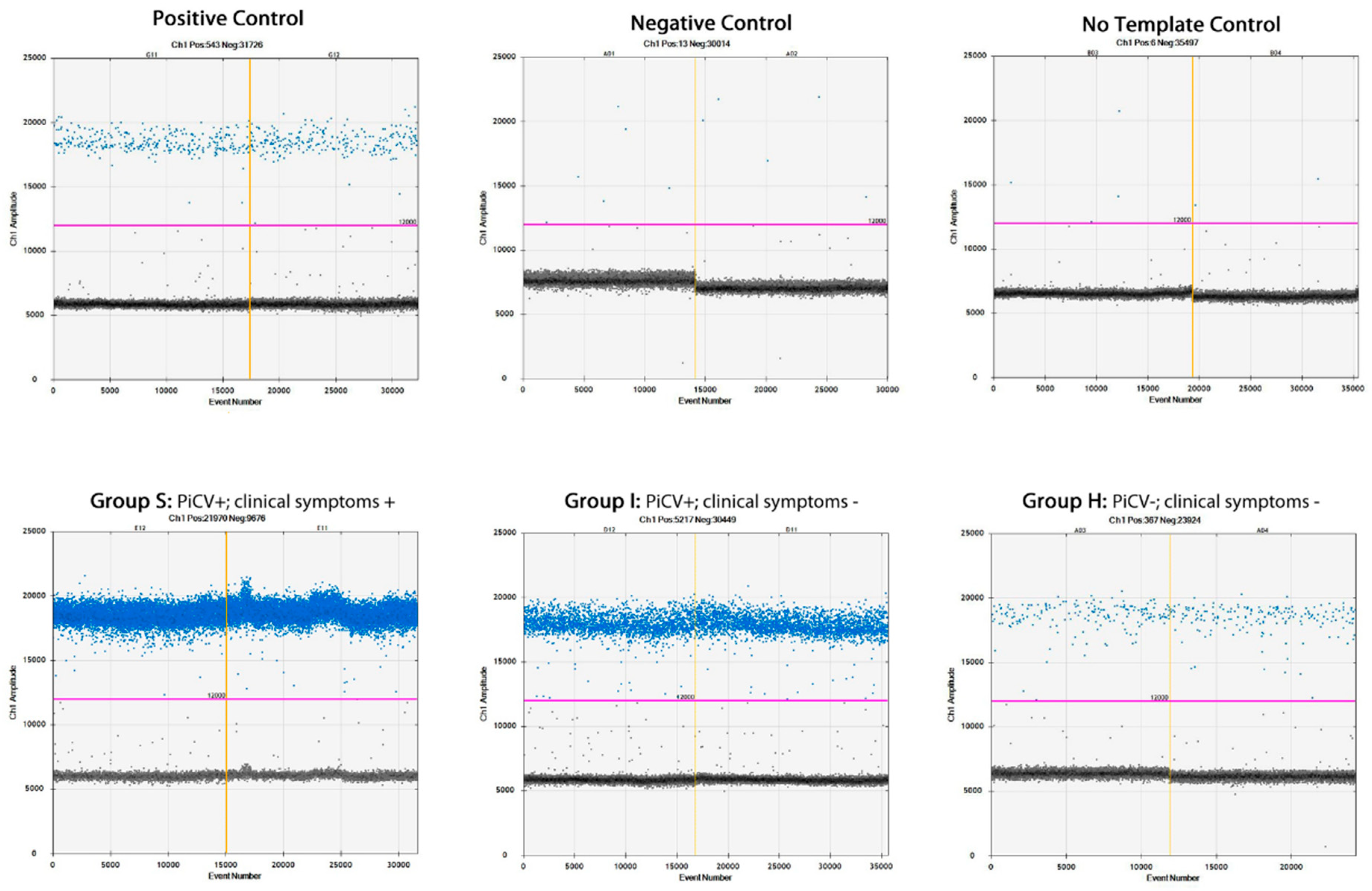
| PiCV Genome Copy Number/ 22 µL | Group | ||
|---|---|---|---|
| S | I | H | |
| Min. | 239,794.61 | 1413.61 | 533.61 |
| MN SD | 36,019,604.61 A 66,136,956.68 | 38,236.72 B 63,281.49 | 3488.94 B 3695.72 |
| Max. | 215,599,994.60 | 195,354.61 | 10,774.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenzel, T.; Dziewulska, D.; Tykałowski, B.; Koncicki, A. The Clinical Infection with Pigeon Circovirus (PiCV) Leads to Lymphocyte B Apoptosis But Has No Effect on Lymphocyte T Subpopulation. Pathogens 2020, 9, 632. https://doi.org/10.3390/pathogens9080632
Stenzel T, Dziewulska D, Tykałowski B, Koncicki A. The Clinical Infection with Pigeon Circovirus (PiCV) Leads to Lymphocyte B Apoptosis But Has No Effect on Lymphocyte T Subpopulation. Pathogens. 2020; 9(8):632. https://doi.org/10.3390/pathogens9080632
Chicago/Turabian StyleStenzel, Tomasz, Daria Dziewulska, Bartłomiej Tykałowski, and Andrzej Koncicki. 2020. "The Clinical Infection with Pigeon Circovirus (PiCV) Leads to Lymphocyte B Apoptosis But Has No Effect on Lymphocyte T Subpopulation" Pathogens 9, no. 8: 632. https://doi.org/10.3390/pathogens9080632
APA StyleStenzel, T., Dziewulska, D., Tykałowski, B., & Koncicki, A. (2020). The Clinical Infection with Pigeon Circovirus (PiCV) Leads to Lymphocyte B Apoptosis But Has No Effect on Lymphocyte T Subpopulation. Pathogens, 9(8), 632. https://doi.org/10.3390/pathogens9080632