Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns)
Abstract
1. Introduction
2. Results
2.1. MDA Titer and RNA Copy Number
2.2. Clinical Scores, Leucocyte Counts, Body Temperature Measurement, and Mortality
2.3. Detection of CSFV RNA in Organs
2.4. CSFV Erns and BVDV Erns Antibody Titers
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Serum Neutralization Test and qRT-PCR
4.3. CSFV Erns and BVDV BErns ELISAs
4.4. Ethical Approval
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edwards, S.; Fukusho, A.; Lefèvre, P.C.; Lipowski, A.; Pejsak, Z.; Roehe, P.; Westergaard, J. Classical swine fever: The global situation. Vet. Microbiol. 2000, 73, 103–119. [Google Scholar] [CrossRef]
- Moennig, V. The control of classical swine fever in wild boar. Front. Microbiol. 2015, 6, 1211. [Google Scholar] [CrossRef] [PubMed]
- Fritzemeier, J.; Teuffert, J.; Greiser-Wilke, I.; Staubach, C.; Schlüter, H.; Moennig, V. Epidemiology of classical swine fever in Germany in the 1990s. Vet. Microbiol. 2000, 77, 29–41. [Google Scholar] [CrossRef]
- Lim, S.I.; Choe, S.; Kim, K.S.; Jeoung, H.Y.; Cha, R.M.; Park, G.S.; Shin, J.; Park, G.N.; Cho, I.S.; Song, J.Y.; et al. Assessment of the efficacy of an attenuated live marker classical swine fever vaccine (Flc-LOM-BErns) in pregnant sows. Vaccine 2019, 37, 3598–3604. [Google Scholar] [CrossRef]
- Insel, R.A.; Amstey, M.; Woodin, K.; Pichichero, M. Maternl immunization to prevent infectious diseases in the neonate or infant. Int. J. Technol. Assess. Health Care 1994, 10, 143–153. [Google Scholar] [CrossRef]
- Pravieux, J.J.; Poulet, H.; Charreyre, C.; Juillard, V. Protection of newboarn animals through maternal immunization. J. Comp Pathol. 2007, 137 (Suppl.1), S32–S34. [Google Scholar] [CrossRef]
- Mierzejewska, M.; Tereszczuk, S.; Corthier, G.; Aynaud, J.M. Hog cholera virus: Influence of colostral passive antibody on immune response of pig following vaccination with the rabbit adapted Chinese strain (author’s transl). Ann. Rech Vet. 1977, 8, 227–240. [Google Scholar]
- Précausta, P.; Kato, F.; Brun, A. Hog cholera: Active immunity conferred by the Chinese strain vaccine to young pigs born to immune sows. Dev. Biol. Stand. 1978, 41, 367–379. [Google Scholar]
- Terpstra, C.; Wensvoort, G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet. Microbiol. 1988, 16, 123–128. [Google Scholar] [CrossRef]
- Eblé, P.L.; Quak, S.; Geurts, Y.; Moonen-Leusen, H.W.; Loeffen, W.L. Efficacy of CSF vaccine CP7_E2alf in piglets with maternally derived antibodies. Vet. Microbiol. 2014, 174, 27–38. [Google Scholar] [CrossRef]
- Xia, S.L.; Xiang, G.T.; Lei, J.L.; Du, M.; Wang, Y.; Zhou, M.; Liu, Y.; Ji, S.; Wang, Y.L.; Luo, Y.; et al. Efficacy of the marker vaccine rAdV-SFV-E2 against classical swine fever in the presence of maternally derived antibodies to rAdV-SFV-E2 or C-strain. Vet. Microbiol. 2016, 196, 50–54. [Google Scholar] [CrossRef]
- Schröder, C.; Dräger, C.; Aebischer, A.; Dähnert, L.; Breidenstein, C.; Mamerow, S.; Leidenberger, S.; Beer, M.; Blome, S. Kinetics of maternally derived antibodies upon intramuscular vaccination against classical swine fever with Suvaxyn® CSF Marker (CP7_E2alf). Vet. Microbiol. 2016, 196, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Farsang, A.; Lévai, R.; Barna, T.; Fábián, K.; Blome, S.; Belák, K.; Bálint, Á.; Koenen, F.; Kulcsár, G. Pre-registration efficacy study of a novel marker vaccine against classical swine fever on maternally derived antibody positive (MDA+) target animals. Biologicals 2017, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.L.; Du, M.; Lei, J.L.; Liu, Y.; Wang, Y.; Ji, S.; Xiang, G.T.; Li, L.F.; Cong, X.; Luo, Y.; et al. Piglets with maternally derived antibodies from sows immunized with rAdV-SFV-E2 were completely protected against lethal CSFV challenge. Vet. Microbiol. 2016, 190, 38–42. [Google Scholar] [CrossRef]
- Curtis, J.; Bourne, F.J. Half-lives of immunoglobulins IgG, IgA and IgM in the serum of new-born pigs. Immunology 1973, 24, 147–155. [Google Scholar]
- Reimann, I.; Depner, K.; Utke, K.; Leifer, I.; Lange, E.; Beer, M. Characterization of a new chimeric marker vaccine candidate with a mutated antigenic E2- epitope. Vet. Microbiol. 2010, 142, 45–50. [Google Scholar] [CrossRef]
- Reimann, I.; Depner, K.; Trapp, S.; Beer, M. An avirulent chimeric pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology 2004, 322, 143–157. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, S.L.; Lei, J.L.; Cong, X.; Xiang, G.T.; Luo, Y.; Sun, Y.; Qiu, H.J. Dose-dependent pathogenicity of a pseudorabies virus variant in pigs inoculated via intranasal route. Vet. Immunol. Immunopathol. 2015, 168, 147–152. [Google Scholar] [CrossRef]
- Terpstra, C. The immunity against challenge with swine fever virus of piglets from sows vaccinated with C-strain virus. Tijdschr. Diergeneeskd. 1977, 102, 1293–1298. [Google Scholar]
- Rangelova, D.; Nielsen, J.; Strandbygaard, B.; Koenen, F.; Blome, S.; Uttenthal, Å. Efficacy of marker vaccine candidate CP7_E2alf in piglets with maternally derived C-strain antibodies. Vaccine 2012, 30, 6376–6381. [Google Scholar] [CrossRef]
- An, D.J.; Lim, S.I.; Choe, S.; Kim, K.S.; Cha, R.M.; Cho, I.S.; Song, J.Y.; Hyun, B.H.; Park, B.K. Evolutionary dynamics of classical swine fever virus in South Korea: 1987–2017. Vet. Microbiol. 2018, 225, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Lim, S.I.; Jeoung, H.Y.; Choi, E.J.; Hyun, B.H.; Kim, B.; Kim, J.J.; Shin, Y.K.; dela Pena, R.C.; Kim, J.B.; et al. Prevalence of Classical Swine Fever Virus in Domestic Pigs in South Korea: 1999–2011. Transbound Emerg. Dis. 2013, 60, 546–551. [Google Scholar] [CrossRef]
- Mittelholzer, C.; Moser, C.; Tratschin, J.; Hofmann, M.A. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 2000, 74, 293–308. [Google Scholar] [CrossRef]
- Drew, T. Classical swine fever (hog cholera). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees, 6th ed.; Office International des Epizooties (OIE), Ed.; OIE: Paris, France, 2008; pp. 1092–1106. [Google Scholar]
- Choe, S.; Kim, J.H.; Kim, K.S.; Song, S.; Cha, R.M.; Kang, W.C.; Kim, H.J.; Park, G.N.; Shin, J.; Jo, H.N.; et al. Adverse Effects of Classical Swine Fever Virus LOM Vaccine and Jeju LOM Strains in Pregnant Sows and Specific Pathogen-Free Pigs. Pathogens 2019, 23, 18. [Google Scholar] [CrossRef] [PubMed]
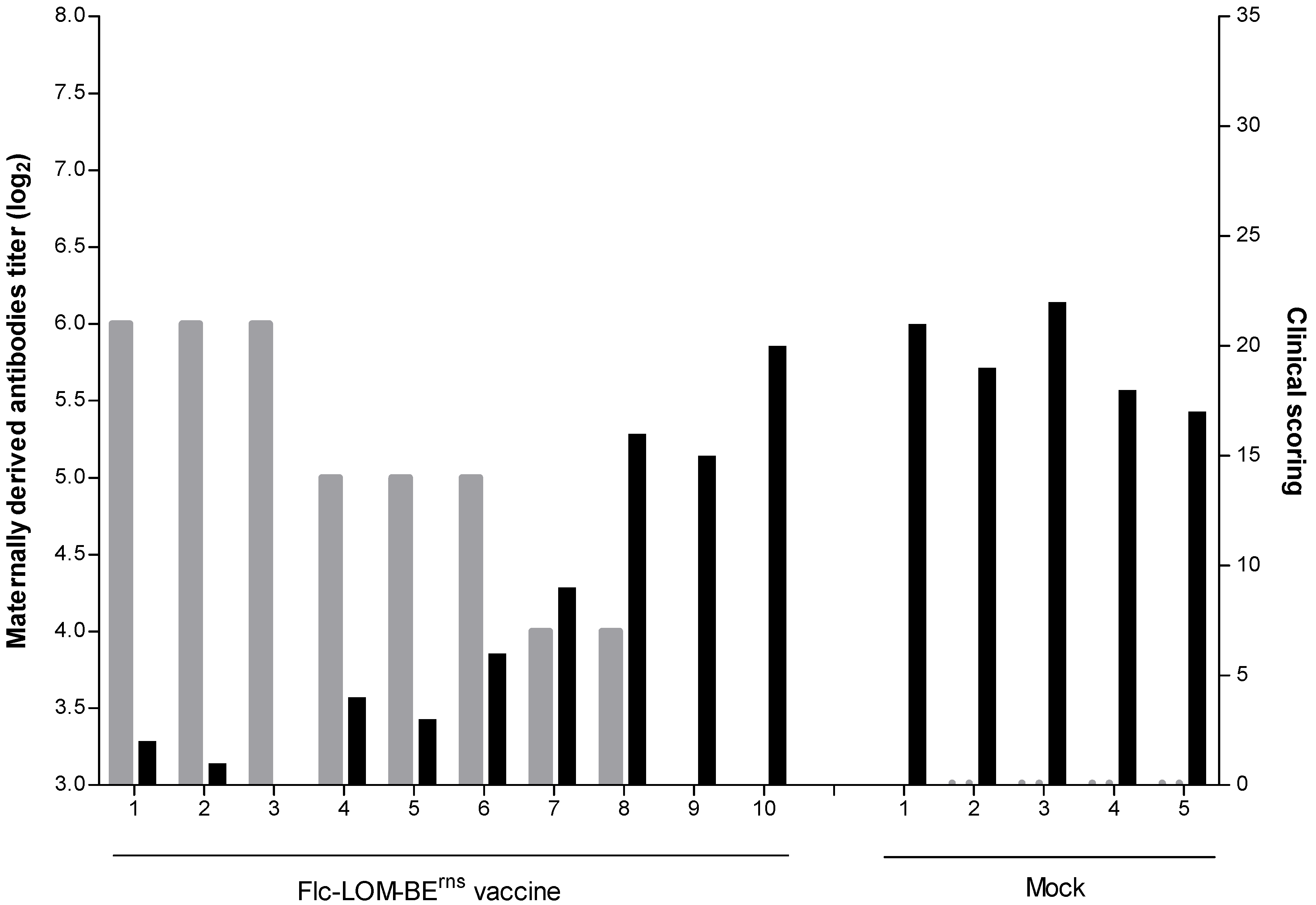
| Group a | Sub Group | Pig ID | MDA (log2) of Pigs b | Pig Age c | RNA Copy Number (log10) from Samples (Blood/Nasal/Feces) on Different Days Post-CSFV Inoculation (dpi) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 10 | 14 | 18 | 21 | |||||
| Flc-LOM-BErns | V1 | 1 | 6 | 42 | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- |
| 2 | 6 | 42 | -/-/- | -/-/- | 2.1/-/- | -/-/- | -/-/- | -/-/- | -/-/- | ||
| 3 | 6 | 40 | -/-/- | -/-/- | 1.5/-/- | -/-/- | -/-/- | -/-/- | -/-/- | ||
| V2 | 4 | 5 | 40 | -/-/- | -/-/- | 1.2/-/- | 2.3/-/- | -/-/- | -/-/- | -/-/- | |
| 5 | 5 | 46 | -/-/- | -/-/- | 2.3/-/- | 1.0/-/- | -/-/- | -/-/- | -/-/- | ||
| 6 | 5 | 46 | -/-/- | -/-/- | 2.5/-/- | 2.8/-/1.5 | 2.4/1.8/- | 2.1/-/- | 2.3/1.7/- | ||
| V3 | 7 | 4 | 53 | -/-/- | -/-/- | 2.5/1.8/2.1 | 3.1/3.7/- | 4.3/1.5/2.3 | 3.5/-/2.5 | 3.8/2.6/3.1 | |
| 8 | 4 | 53 | -/-/- | -/-/- | 3.5/2.1/- | 4.1/3.2/4.4 | 5.3/2.5/2.9 | 4.5/3.3/3.1 | D | ||
| V4 | 9 | ≤3 | 60 | -/-/- | -/-/- | 4.4/2.9/1.6 | 5.7/3.5/4.2 | 3.8/2.7/2.6 | D | ||
| 10 | ≤3 | 60 | -/-/- | -/-/- | 3.5/-/2.8 | 5.2/3.3/3.7 | 4.6/3.4/2.9 | 3.2/3.6/3.4 | D | ||
| Unvaccinated | Mock | 1 | ≤3 | 48 | -/-/- | 2.6/2.1/1.5 | 5.1/3.4/3.6 | 4.4/3.6/3.2 | 4.5/3.3/3.8 | D | |
| 2 | ≤3 | 48 | -/-/- | 3.4/-/2.7 | 4.2/2.4/3.8 | 5.5/3.8/3.1 | 5.1/3.1/2.4 | 3.7/4.2/2.5 | D | ||
| 3 | ≤3 | 55 | -/-/- | 2.9/2.4/- | 4.4/3.4/2.2 | 4.6/3.7/3.6 | 5.3/2.6/3.4 | D | |||
| 4 | ≤3 | 55 | -/-/- | 3.6/2.4/2.7 | 4.7/2.5/4.2 | 5.3/2.4/3.7 | D | ||||
| 5 | ≤3 | 55 | -/-/- | 2.8/3.2/2.5 | 3.4/3.7/2.5 | 5.4/4.2/3.5 | 4.7/4.4/2.8 | D | |||
| Group | Sub Group | Pig ID | MDA (log 2) of Pigs | Pig Age a | RNA Copy Number (log 10) from Organ Samples on the Necropsy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To b | Lu | He | Li | Ki | Il | Ce | Sp | ML | LN | Br | |||||
| Flc-LOM-BErns | V1 | 1 2 3 | 6 6 6 | 42 42 40 | - | - | - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - | - | - | |||||
| - | - | - | - | - | - | - | - | - | - | - | |||||
| V2 | 4 5 6 | 5 5 5 | 40 46 46 | - | - | - | - | - | - | - | - | - | - | - | |
| 2.2 | - | - | - | - | - | - | - | - | - | - | |||||
| 2.5 | - | - | - | 2.9 | - | - | 2.1 | - | - | - | |||||
| V3 | 7 8 | 4 4 | 53 53 | 3.1 | - | - | - | - | - | - | 3.4 | 1.5 | 1.9 | - | |
| 2.7 | - | - | - | 2.6 | - | 2.1 | - | - | 1.5 | - | |||||
| V4 | 9 10 | ≤3 ≤3 | 60 60 | 3.6 | - | 2.7 | - | 3.5 | - | - | - | 2.3 | 2.8 | 2.4 | |
| 2.9 | 2.8 | - | - | 4.2 | - | - | 2.5 | 4.2 | 3.7 | - | |||||
| Unvaccinated | Mock | 1 2 3 4 5 | ≤3 ≤3 ≤3 ≤3 ≤3 | 48 48 55 55 55 | 3.7 | - | - | - | 3.4 | - | - | 4.2 | - | 4.1 | 2.9 |
| 4.6 | 2.8 | 1.4 | - | 4.5 | - | - | - | - | - | - | |||||
| - | - | - | - | 2.1 | - | 3.5 | 5.3 | 4.6 | 3.2 | - | |||||
| 5.3 | - | - | - | - | - | - | 4.6 | 3.8 | - | 3.2 | |||||
| 2.9 | 4.2 | - | - | 3.3 | - | - | 4.1 | 2.8 | 3.6 | - | |||||
| Group | Subgroup | Pig ID | Pig Age a | Day of Challenge (0 dpi) | Day of Necropsy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDA b | CSF Erns | BVDV Erns | SN | CSF Erns | BVDV Erns | dpi | ||||
| Flc-LOM-BErns | V1 | 1 | 42 | 6 | 0.81 | 0.76 | 6 | 0.36 | 0.68 | 21 |
| 2 | 42 | 6 | 0.73 | 0.85 | 7 | 0.42 | 0.73 | 21 | ||
| 3 | 40 | 6 | 0.76 | 0.77 | 6 | 0.32 | 0.60 | 21 | ||
| V2 | 4 | 40 | 5 | 0.68 | 0.83 | 6 | 0.45 | 0.72 | 21 | |
| 5 | 46 | 5 | 0.71 | 0.75 | 5 | 0.39 | 0.58 | 21 | ||
| 6 | 46 | 5 | 0.67 | 0.84 | ≤3 | 0.73 | 0.36 | 21 | ||
| V3 | 7 | 53 | 4 | 0.79 | 0.55 | ≤3 | 0.81 | 0.31 | 21 | |
| 8 | 53 | 4 | 0.69 | 0.63 | ≤3 | 0.62 | 0.45 | 20 | ||
| V4 | 9 | 60 | ≤3 | 0.77 | 0.40 | ≤3 | 0.85 | 0.18 | 18 | |
| 10 | 60 | ≤3 | 0.72 | 0.51 | ≤3 | 0.79 | 0.33 | 21 | ||
| Unvaccinated | Mock | 1 | 48 | ≤3 | 0.89 | 0.42 | ≤3 | 0.76 | 0.25 | 17 |
| 2 | 48 | ≤3 | 0.92 | 0.31 | ≤3 | 0.85 | 0.33 | 19 | ||
| 3 | 55 | ≤3 | 0.73 | 0.18 | ≤3 | 0.87 | 0.42 | 16 | ||
| 4 | 55 | ≤3 | 0.90 | 0.36 | ≤3 | 0.82 | 0.19 | 12 | ||
| 5 | 55 | ≤3 | 0.82 | 0.28 | ≤3 | 0.93 | 0.32 | 15 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, S.; Shin, J.; Kim, K.-S.; Song, S.; Cha, R.M.; Jung, B.-I.; Hyun, B.-H.; Park, B.-K.; An, D.-J. Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns). Pathogens 2020, 9, 608. https://doi.org/10.3390/pathogens9080608
Choe S, Shin J, Kim K-S, Song S, Cha RM, Jung B-I, Hyun B-H, Park B-K, An D-J. Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns). Pathogens. 2020; 9(8):608. https://doi.org/10.3390/pathogens9080608
Chicago/Turabian StyleChoe, SeEun, Jihye Shin, Ki-Sun Kim, Sok Song, Ra Mi Cha, Byung-Il Jung, Bang-Hun Hyun, Bong-Kyun Park, and Dong-Jun An. 2020. "Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns)" Pathogens 9, no. 8: 608. https://doi.org/10.3390/pathogens9080608
APA StyleChoe, S., Shin, J., Kim, K.-S., Song, S., Cha, R. M., Jung, B.-I., Hyun, B.-H., Park, B.-K., & An, D.-J. (2020). Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns). Pathogens, 9(8), 608. https://doi.org/10.3390/pathogens9080608




