Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts
Abstract
1. Introduction
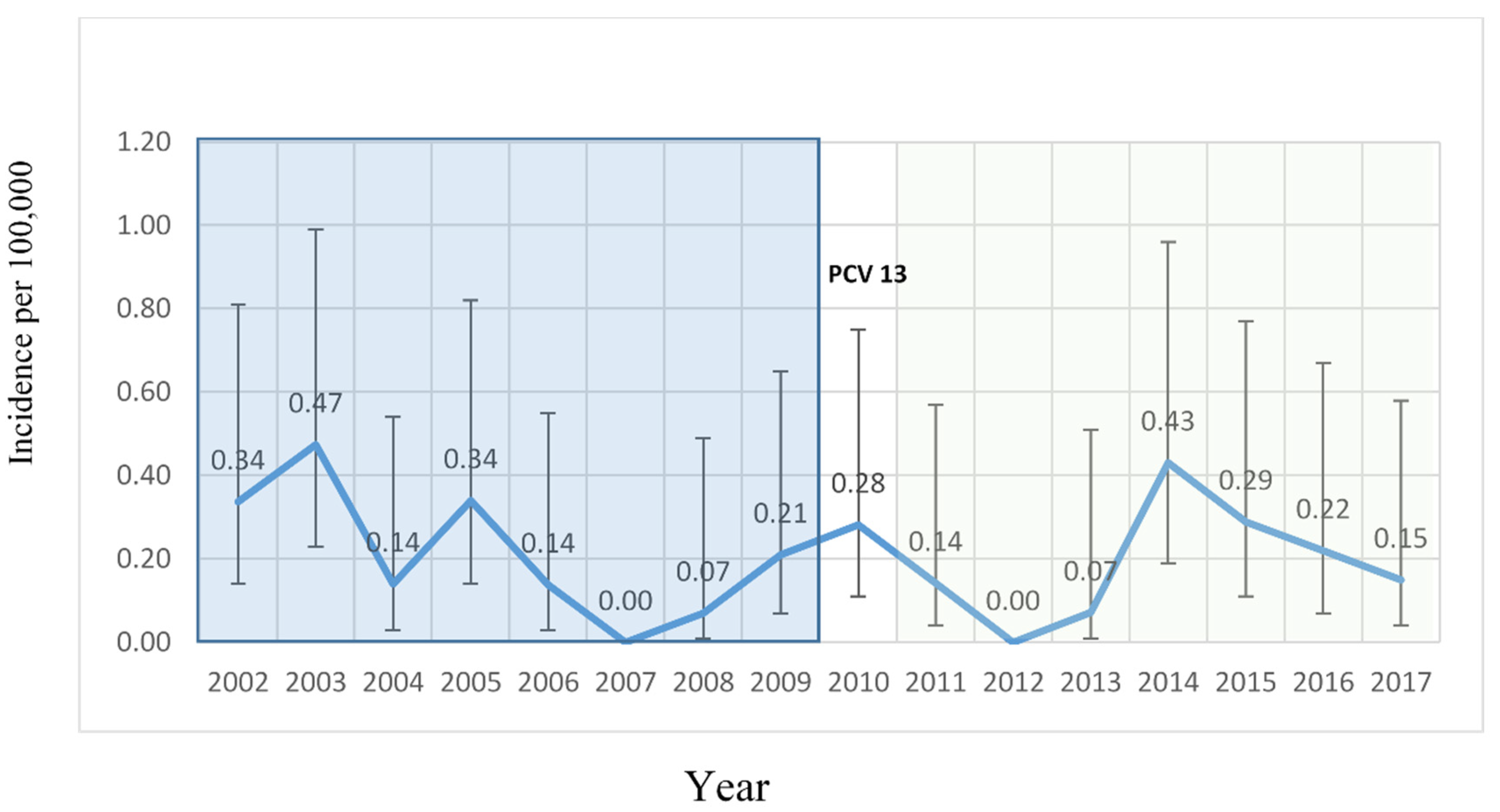
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Assessment, R. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire Sommaire. 2019, Volume 8, pp. 85–104. Available online: https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1 (accessed on 20 May 2020).
- CDC Pneumococcal Disease Surveillance and Reporting. Available online: https://www.cdc.gov/pneumococcal/surveillance.html (accessed on 10 September 2019).
- Yildirim, I.; Little, B.A.; Finkelstein, J.; Lee, G.; Hanage, W.P.; Shea, K.; Pelton, S.I. Surveillance of pneumococcal colonization and invasive pneumococcal disease reveals shift in prevalent carriage serotypes in Massachusetts’ children to relatively low invasiveness. Vaccine 2017, 35, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.; Hanage, W.P.; Lipsitch, M.; Shea, K.M.; Stevenson, A.; Finkelstein, J.; Huang, S.S.; Lee, G.M.; Kleinman, K.; Pelton, S.I. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 2010, 29, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Varon, E.; Cohen, R.; Béchet, S.; Doit, C.; Levy, C. Invasive disease potential of pneumococci before and after the 13-valent pneumococcal conjugate vaccine implementation in children. Vaccine 2015, 33, 6178–6185. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Harboe, Z.B.; Sanders, E.A.M.; Ndiritu, M.; Klugman, K.P.; Rückinger, S.; Dagan, R.; Adegbola, R.; Cutts, F.; Johnson, H.L.; et al. Association of Serotype with Risk of Death Due to Pneumococcal Pneumonia: A Meta-Analysis. Clin. Infect. Dis. 2010, 51, 692–699. [Google Scholar] [CrossRef]
- Azzari, C.; Cortimiglia, M.; Nieddu, F.; Moriondo, M.; Indolfi, G.; Mattei, R.; Zuliani, M.; Adriani, B.; Degl’Innocenti, R.; Consales, G.; et al. Pneumococcal serotype distribution in adults with invasive disease and in carrier children in Italy: Should we expect herd protection of adults through infants’ vaccination? Hum. Vaccines Immunother. 2016, 12, 344–350. [Google Scholar] [CrossRef]
- No, W.V. Invasive Pneumococcal Disease in Young Children Before Licensure of 13-Valent Pneumococcal Conjugate Vaccine—United States, 2007. Morb. Mortal. Wkly. Rep. 2010, 59, 262–267. [Google Scholar]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Lee, G.M.; Kleinman, K.; Pelton, S.; Lipsitch, M.; Huang, S.S.; Lakoma, M.; Dutta-Linn, M.; Rett, M.; Hanage, W.P.; Finkelstein, J.A. Immunization, antibiotic use, and pneumococcal colonization over a 15-year period. Pediatrics 2017, 140. [Google Scholar] [CrossRef]
- Sings, H.L. Pneumococcal conjugate vaccine use in adults—Addressing an unmet medical need for non-bacteremic pneumococcal pneumonia. Vaccine 2017, 35, 5406–5417. [Google Scholar] [CrossRef]
- Yildirim, I.; Pelton, S.I. Infants at Risk for Invasive Pneumococcal Disease in the 13-Valent Pneumococcal Conjugate Vaccine Era. Clin. Infect. Dis. 2019, 69, 91–92. [Google Scholar] [CrossRef]
- Harboe, Z.B.; Dalby, T.; Weinberger, D.M.; Benfield, T.; Mølbak, K.; Slotved, H.C.; Suppli, C.H.; Konradsen, H.B.; Valentiner-Branth, P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin. Infect. Dis. 2014, 59, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Greenberg, D.; Givon-Lavi, N.; Schlesinger, Y.; Somekh, E.; Aviner, S.; Miron, D.; Dagan, R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: An active prospective nationwide surveillance. Vaccine 2014, 32, 3452–3459. [Google Scholar]
- Camilli, R.; D’Ambrosio, F.; Del Grosso, M.; de Araujo, F.P.; Caporali, M.G.; Del Manso, M.; Gherardi, G.; D’Ancona, F.; Pantosti, A. Impact of pneumococcal conjugate vaccine (PCV7 and PCV13) on pneumococcal invasive diseases in Italian children and insight into evolution of pneumococcal population structure. Vaccine 2017, 35, 4587–4593. [Google Scholar] [CrossRef] [PubMed]
- Sings, H.L.; De Wals, P.; Gessner, B.D.; Isturiz, R.; Laferriere, C.; Mclaughlin, J.M.; Pelton, S.; Schmitt, H.J.; Suaya, J.A.; Jodar, L. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine against Invasive Disease Caused by Serotype 3 in Children: A Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2019, 68, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Ehman, E.C.; Johnson, G.B.; Villanueva-meyer, J.E.; Cha, S.; Leynes, A.P.; Eric, P.; Larson, Z.; Hope, T.A.; Ladhani, S.N.; Collins, S.; et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Open Forum Infect. Dis. 2018, 3, 1247–1262. [Google Scholar]
- Picazo, J.J.; Ruiz-Contreras, J.; Casado-Flores, J.; Negreira, S.; Baquero-Artigao, F.; Hernández-Sampelayo, T.; Otheo, E.; del Amo, M.; Méndez, C. Impact of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children under 15 years old in Madrid, Spain, 2007 to 2016: The HERACLES clinical surveillance study. Vaccine 2019, 37, 2200–2207. [Google Scholar] [CrossRef]
- Goettler, D.; Streng, A.; Kemmling, D.; Schoen, C.; von Kries, R.; Rose, M.A.; van der Linden, M.; Liese, J.G. Increase in Streptococcus pneumoniae serotype 3 associated parapneumonic pleural effusion/empyema after the introduction of PCV13 in Germany. Vaccine 2020, 38, 570–577. [Google Scholar] [CrossRef]
- Ho, P.L.; Law, P.Y.T.; Chiu, S.S. Increase in incidence of invasive pneumococcal disease caused by serotype 3 in children eight years after the introduction of the pneumococcal conjugate vaccine in Hong Kong. Hum. Vaccines Immunother. 2019, 15, 455–458. [Google Scholar] [CrossRef]
- Poolman, J.; Kriz, P.; Feron, C.; Di-Paolo, E.; Henckaerts, I.; Miseur, A.; Wauters, D.; Prymula, R.; Schuerman, L. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 2009, 27, 3213–3222. [Google Scholar] [CrossRef]
- Choi, E.H.; Zhang, F.; Lu, Y.J.; Malley, R. Capsular Polysaccharide (CPS) Release by Serotype 3 Pneumococcal Strains Reduces the Protective Effect of Anti-Type 3 CPS Antibodies. Clin. Vaccine Immunol. 2016, 23, 162–167. [Google Scholar] [CrossRef]
- Linley, E.; Bell, A.; Gritzfeld, J.F.; Borrow, R. Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines 2019, 7, 4. [Google Scholar] [CrossRef]
- Song, J.Y.; Moseley, M.A.; Burton, R.L.; Nahm, M.H. Pneumococcal vaccine and opsonic pneumococcal antibody. J. Infect. Chemother. 2013, 19, 412–425. [Google Scholar] [CrossRef]
- Van Der Linden, M.; Falkenhorst, G.; Perniciaro, S.; Fitzner, C.; Imöhl, M. Effectiveness of pneumococcal conjugate vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Weinberger, R.; van der Linden, M.; Imöhl, M.; von Kries, R. Vaccine effectiveness of PCV13 in a 3 + 1 vaccination schedule. Vaccine 2016, 34, 2062–2065. [Google Scholar] [CrossRef]
- Miller, E.; Andrews, N.J.; Waight, P.A.; Slack, M.P.E.; George, R.C. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011, 29, 9127–9131. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir. Med. 2016, 4, 399–406. [Google Scholar] [CrossRef]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.P.; et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef]
- Said, M.A.; Johnson, H.L.; Nonyane, B.A.S.; Deloria-Knoll, M.; OBrien, K.L. Estimating the Burden of Pneumococcal Pneumonia among Adults: A Systematic Review and Meta-Analysis of Diagnostic Techniques. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Lee, G.M.; Kleinman, K.; Pelton, S.I.; Hanage, W.; Huang, S.S.; Lakoma, M.; Dutta-Linn, M.; Croucher, N.J.; Stevenson, A.; Finkelstein, J.A. Impact of 13-valent pneumococcal conjugate vaccination on Streptococcus pneumoniae carriage in young children in Massachusetts. J. Pediatric Infect. Dis. Soc. 2014, 3, 23–32. [Google Scholar] [CrossRef]
- Dagan, R.; Patterson, S.; Juergens, C.; Greenberg, D.; Givon-Lavi, N.; Porat, N.; Gurtman, A.; Gruber, W.C.; Scott, D.A. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: A randomized double-blind trial. Clin. Infect. Dis. 2013, 57, 952–962. [Google Scholar] [CrossRef]
- Cohen, R.; Varon, E.; Doit, C.; Schlemmer, C.; Romain, O.; Thollot, F.; Béchet, S.; Bonacorsi, S.; Levy, C. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine 2015, 33, 5118–5126. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005, 54, 893–897. [Google Scholar]
- Rubin, J.L.; McGarry, L.J.; Strutton, D.R.; Klugman, K.P.; Pelton, S.I.; Gilmore, K.E.; Weinstein, M.C. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010, 28, 7634–7643. [Google Scholar] [CrossRef]
- Slotved, H.C.; Dalby, T.; Hoffmann, S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine 2016, 34, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Steens, A.; Bergsaker, M.A.R.; Aaberge, I.S.; Rønning, K.; Vestrheim, D.F. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013, 31, 6232–6238. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.J.; ElSherif, M.; Ye, L.; MacKinnon-Cameron, D.; Ambrose, A.; Hatchette, T.F.; Lang, A.L.S.; Gillis, H.D.; Martin, I.; Demczuk, W.; et al. Streptococcus pneumoniae serotype 3 is masking PCV13-mediated herd immunity in Canadian adults hospitalized with community acquired pneumonia: A study from the Serious Outcomes Surveillance (SOS) Network of the Canadian immunization research Network (CIRN). Vaccine 2019, 37, 5466–5473. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Borrow, R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines 2011, 10, 307–322. [Google Scholar] [CrossRef]
- Moberley, S.; Licciardi, P.V.; Balloch, A.; Andrews, R.; Leach, A.J.; Kirkwood, M.; Binks, P.; Mulholland, K.; Carapetis, J.; Tang, M.L.K.; et al. Repeat pneumococcal polysaccharide vaccine in Indigenous Australian adults is associated with decreased immune responsiveness. Vaccine 2017, 35, 2908–2915. [Google Scholar] [CrossRef]
- Van Effelterre, T.; Moore, M.R.; Fierens, F.; Whitney, C.G.; White, L.; Pelton, S.I.; Hausdorff, W.P. A dynamic model of pneumococcal infection in the United States: Implications for prevention through vaccination. Vaccine 2010, 28, 3650–3660. [Google Scholar] [CrossRef]
- CDC Pneumococcal Vaccine Recommendations. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/recommendations.html (accessed on 10 September 2019).
| Characteristic | Serotype 3 Cases Prior to PCV 13 (n = 25) | Serotype 3 Cases Following PCV 13 (n = 18) |
|---|---|---|
| Age (month), median (range) | 26 (2–149) | 31 (0–209) |
| ≤6 | 6 (24%) | 2 (11.1%) |
| 6–≤12 | 3 (12%) | 0 (0.0%) |
| 12–≤24 | 3 (12%) | 5 (27.8%) |
| 24–≤36 | 2 (8%) | 4 22.2%) |
| >36 | 11 (44%) | 7 (38.9%) |
| Gender, Male n (%) | 14 (56%) | 11 (61%) |
| Race/ethnicity n (%) | ||
| Asian | 0 (0.0%) | 1 (5.6%) |
| African American | 1 (4.0%) | 4 (22.2%) |
| Hispanic | 7 (28.0%) | 4 (22.2%) |
| White | 11 (44.0%) | 8 (44.4%) |
| Other/Unknown | 6 (24.0%) | 1 (5.6%) |
| Immunization status with PCV 13 n (%) | ||
| Fully immunized * | 0 (0.0%) | 14 (77.8%) |
| Partially immunized + | 0 (0.0%) | 0 (0.0%) |
| No vaccination with PCV 13 | 25 (100%) | 4 (22.2%) |
| IPD syndrome n (%) | ||
| Bacteremia without a focus | 7 (28.0%) | 5 (27.7%) |
| Bacteremia with focus | 0 (0.0%) | 1 (5.6%) |
| Bacteremic pneumonia/Empyema | 14 (56.0%) | 10 (55.6%) |
| Meningitis | 3 (12%) | 2 (11.1%) |
| Osteoarthritis | 1 (4%) | 0 (0.0) |
| Mortality n (%) | 0(0.0%) | 2 (11.1%) |
| Comorbidities # n (%) | 2 (8.0%) | 3 (16.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapidot, R.; Shea, K.M.; Yildirim, I.; Cabral, H.J.; Pelton, S.I.; Department of Public Health, t.M. Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts. Pathogens 2020, 9, 396. https://doi.org/10.3390/pathogens9050396
Lapidot R, Shea KM, Yildirim I, Cabral HJ, Pelton SI, Department of Public Health tM. Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts. Pathogens. 2020; 9(5):396. https://doi.org/10.3390/pathogens9050396
Chicago/Turabian StyleLapidot, Rotem, Kimberly M. Shea, Inci Yildirim, Howard J. Cabral, Stephen I. Pelton, and the Massachusetts Department of Public Health. 2020. "Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts" Pathogens 9, no. 5: 396. https://doi.org/10.3390/pathogens9050396
APA StyleLapidot, R., Shea, K. M., Yildirim, I., Cabral, H. J., Pelton, S. I., & Department of Public Health, t. M. (2020). Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts. Pathogens, 9(5), 396. https://doi.org/10.3390/pathogens9050396





