Preclinical Evidence of Nanomedicine Formulation to Target Mycobacterium tuberculosis at Its Bone Marrow Niche
Abstract
1. Introduction
2. Results
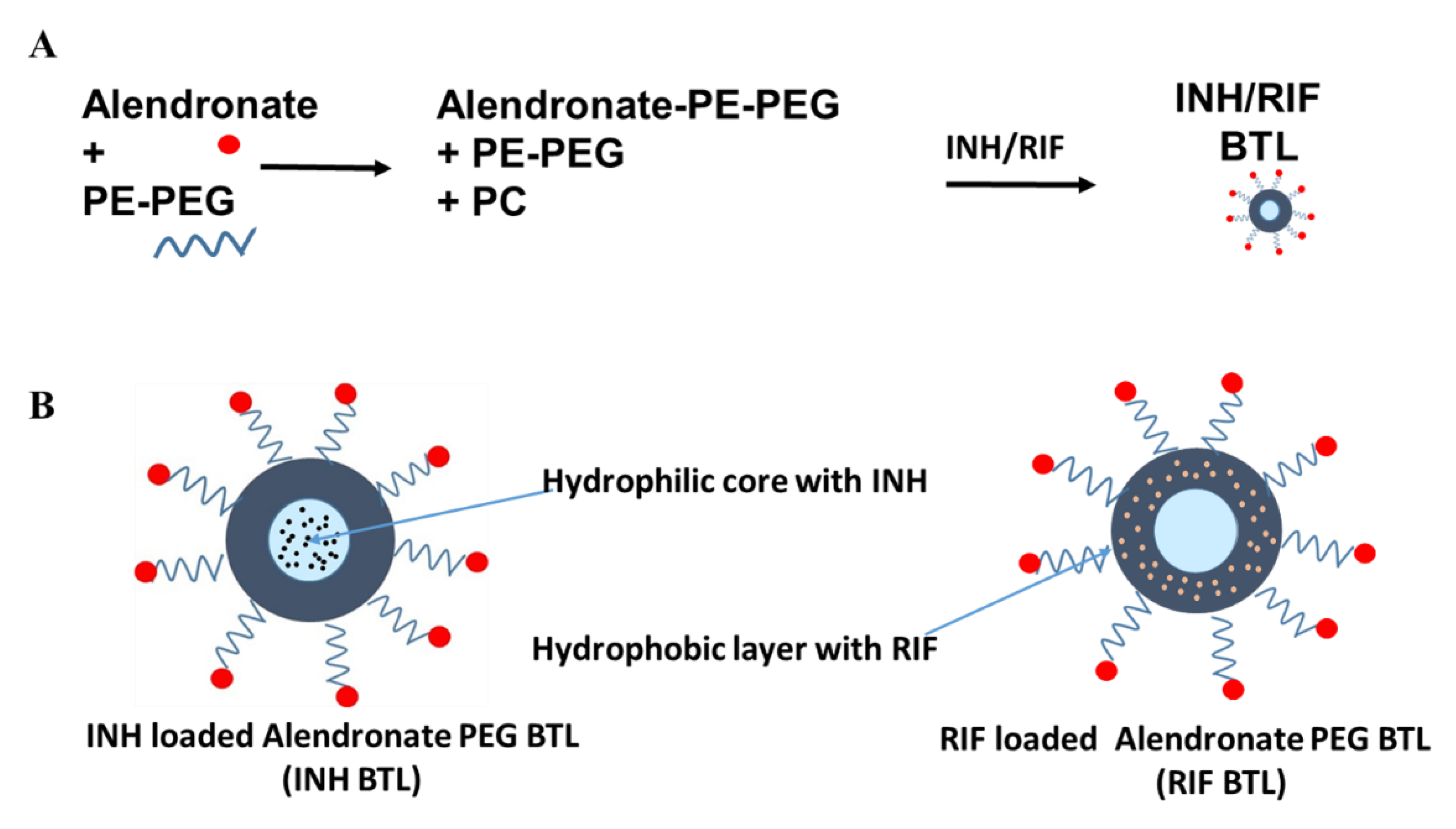
2.1. Designing of Alendronate BTL-NPs and Their Characterization
2.2. Binding Assay of Alendronate BTL-NPs
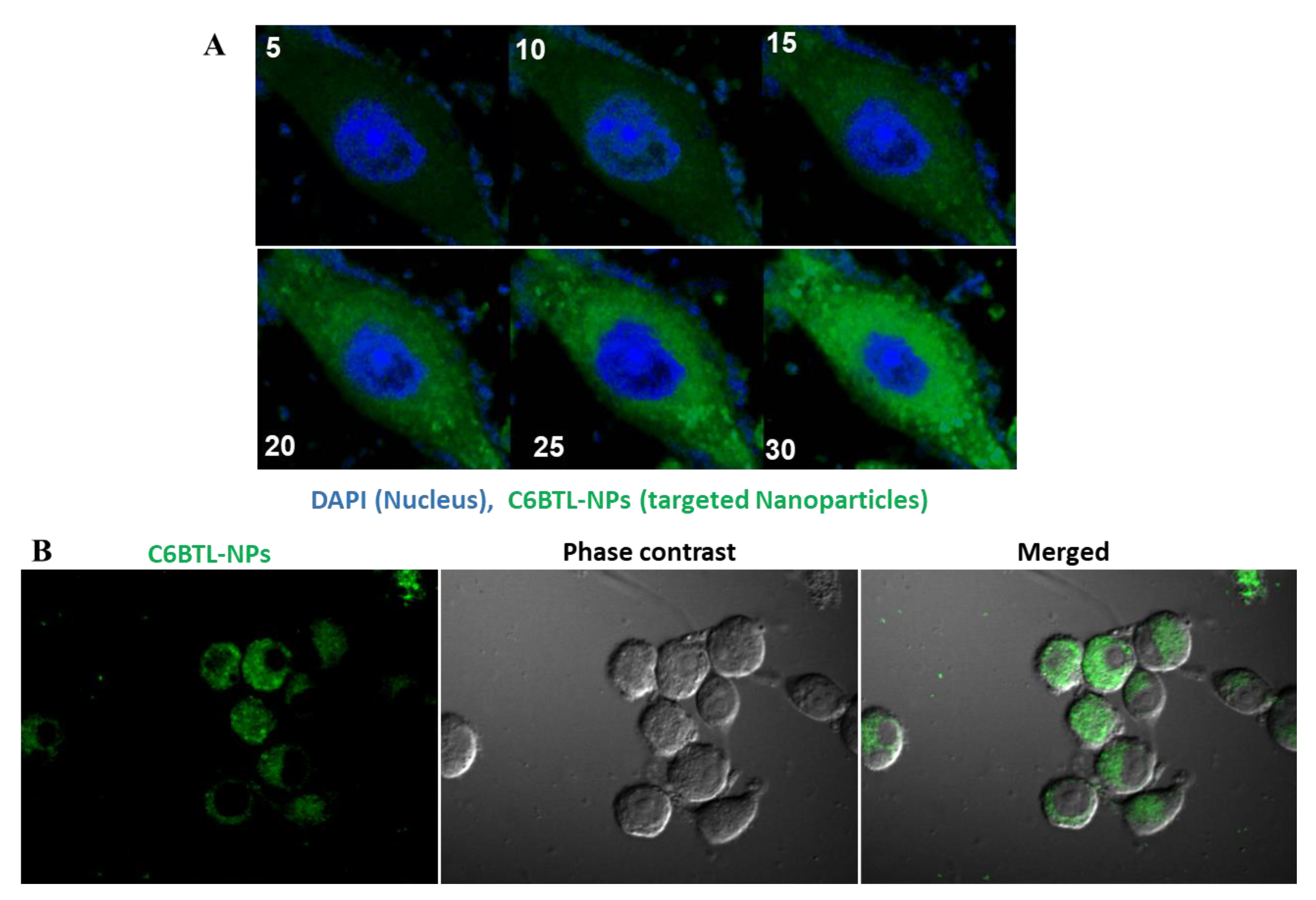
2.3. Cellular Uptake of Alendronate BTL-NPs by CD271+BM-MSCs
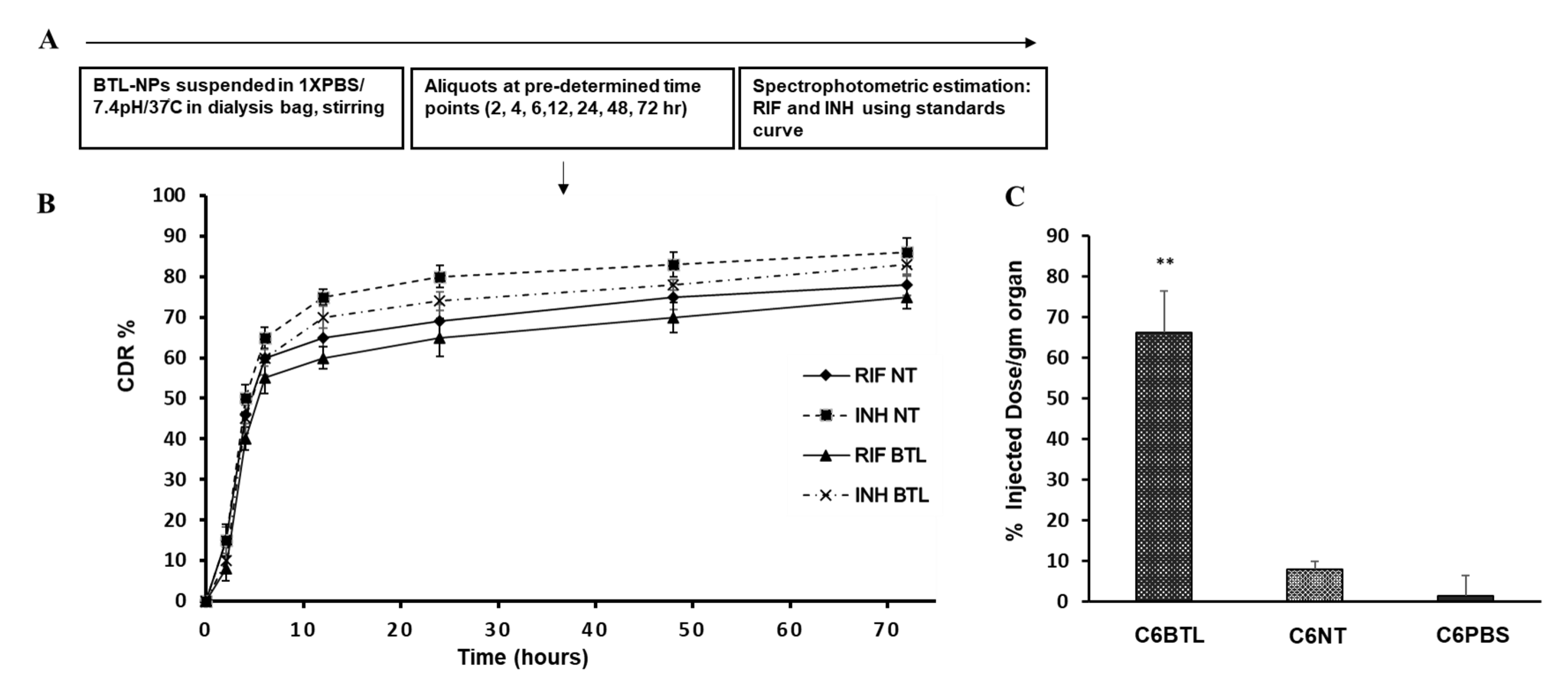
2.4. In Vitro Drug Release Profile of INH and RIF BTL-NPs and Tissue Uptake
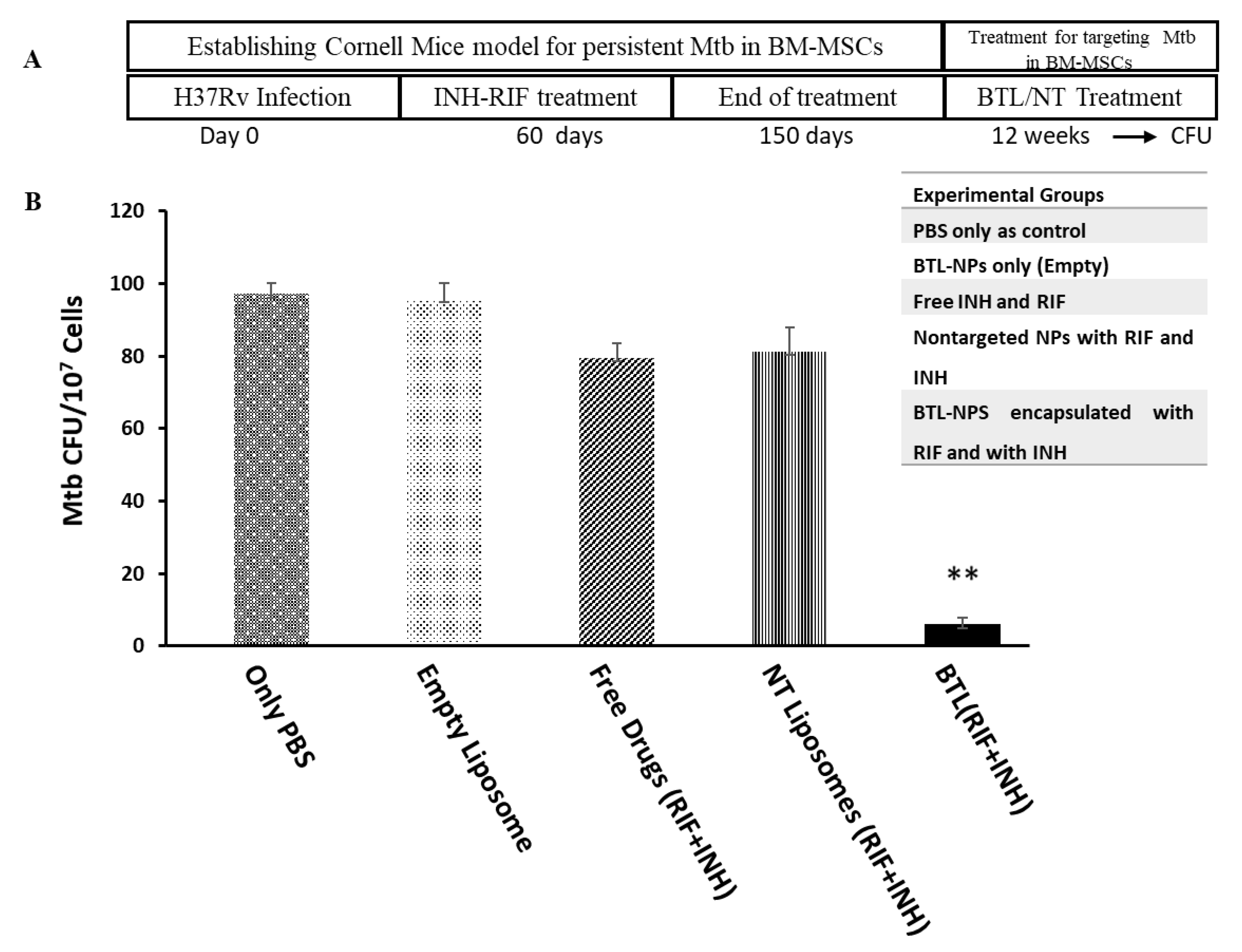
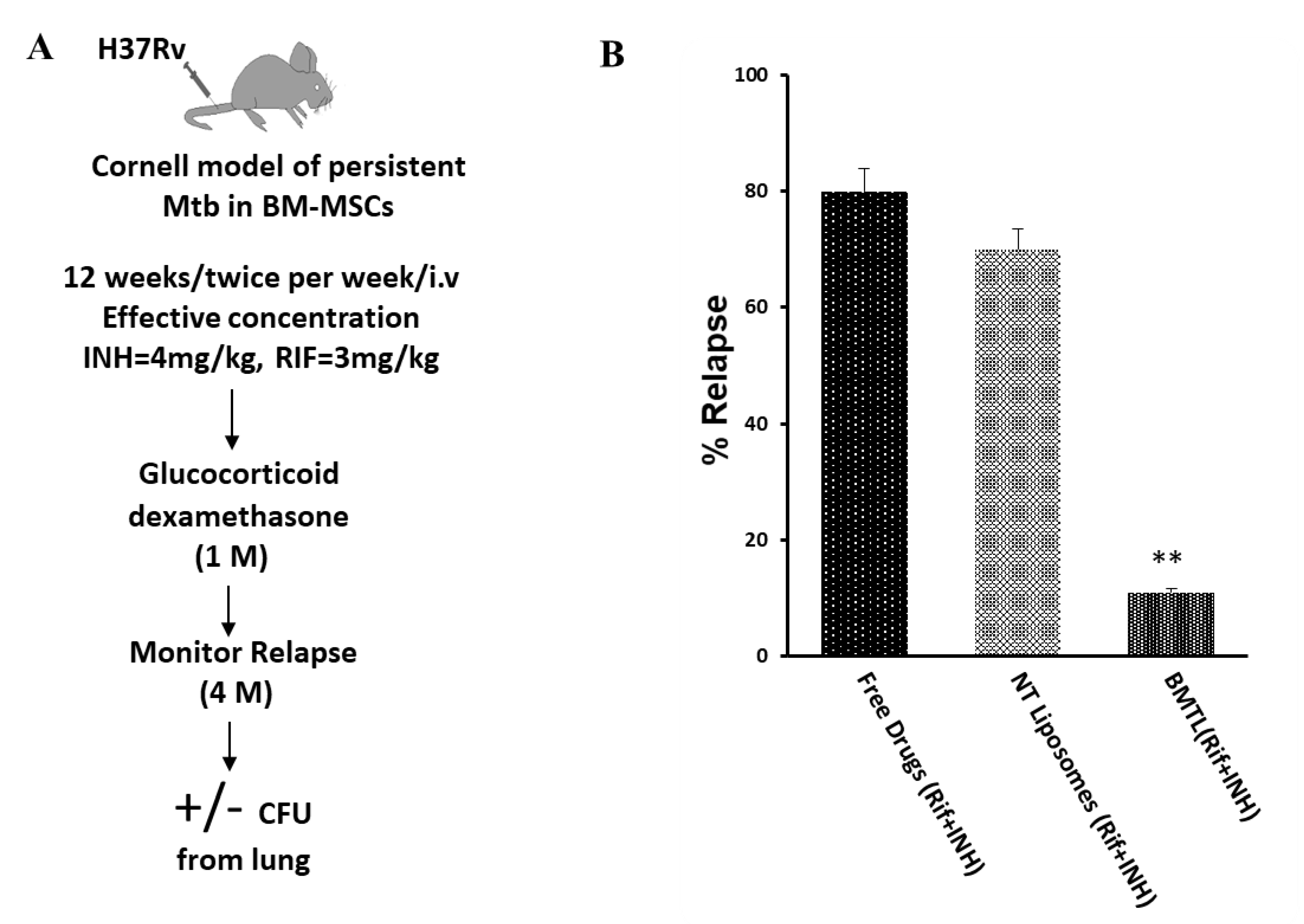
2.5. Efficacy of INH and RIF BTL-NPs in H37Rv Infected Mice
3. Discussion
4. Materials and Methods
4.1. Alendronate Tagging of DSPE-PEG
4.2. Preparation of PEG Liposome and BTL PEGylated Liposome NPs with Ald Tagged PEG-PE and PC (with INH and RIF Encapsulation)
4.3. TEM and Size Distribution
4.4. Drug Concentration Estimation and Encapsulation Efficiency
4.5. Cellular Uptake by Mouse CD271+ BM-MSCs
4.6. NP Binding Assay with Bone Chips
4.7. In Vitro Release from BTL-NPs and Tissue Uptake
4.8. In Vivo Administration and Assessment of BTL-NPs Efficacy
4.9. Enumeration of Mtb CFU from CD271+BM-MSCs
4.10. TB Relapse Assay
4.11. Statistical Analysis
Supplementary Materials
Ethical Statement
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- A.O. Global Tuberculosis Report 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 20 March 2020).
- Chaw, L.; Chien, L.C.; Wong, J.; Takahashi, K.; Koh, D.; Lin, R.T. Global trends and gaps in research related to latent tuberculosis infection. BMC Public Health 2020, 20, 352. [Google Scholar] [CrossRef] [PubMed]
- Escalante, P.; Arias-Guillen, M.; Palacios Gutierrez, J.J. New Research Strategies in Latent Tuberculosis Infection. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Knight, G.M.; McQuaid, C.F.; Dodd, P.J.; Houben, R. Global burden of latent multidrug-resistant tuberculosis: Trends and estimates based on mathematical modelling. Lancet Infect. Dis 2019, 19, 903–912. [Google Scholar] [CrossRef]
- Barry, C.E., 3rd; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Turetz, M.L.; Ma, K.C. Diagnosis and management of latent tuberculosis. Curr. Opin. Infect. Dis. 2016, 29, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Scanga, C.A.; Mohan, V.P.; Joseph, H.; Yu, K.; Chan, J.; Flynn, J.L. Reactivation of latent tuberculosis: Variations on the Cornell murine model. Infect. Immun. 1999, 67, 4531–4538. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Vatankhah, R.; Sharifi, M. Nonlinear adaptive control of tuberculosis with consideration of the risk of endogenous reactivation and exogenous reinfection. J. Theor. Biol. 2020, 486, 110081. [Google Scholar] [CrossRef]
- Dippenaar, A.; De Vos, M.; Marx, F.M.; Adroub, S.A.; van Helden, P.D.; Pain, A.; Sampson, S.L.; Warren, R.M. Whole genome sequencing provides additional insights into recurrent tuberculosis classified as endogenous reactivation by IS6110 DNA fingerprinting. Infect. Genet. Evol. 2019, 75, 103948. [Google Scholar] [CrossRef]
- Pai, M. Spectrum of latent tuberculosis—Existing tests cannot resolve the underlying phenotypes. Nat. Rev. Microbiol. 2010, 8, 242. [Google Scholar] [CrossRef]
- Beamer, G.; Major, S.; Das, B.; Campos-Neto, A. Bone marrow mesenchymal stem cells provide an antibiotic-protective niche for persistent viable Mycobacterium tuberculosis that survive antibiotic treatment. Am. J. Pathol. 2014, 184, 3170–3175. [Google Scholar] [CrossRef]
- Das, B.; Kashino, S.S.; Pulu, I.; Kalita, D.; Swami, V.; Yeger, H.; Felsher, D.W.; Campos-Neto, A. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci. Transl. Med. 2013, 5, 170ra13. [Google Scholar] [CrossRef]
- Garhyan, J.; Bhuyan, S.; Pulu, I.; Kalita, D.; Das, B.; Bhatnagar, R. Preclinical and Clinical Evidence of Mycobacterium tuberculosis Persistence in the Hypoxic Niche of Bone Marrow Mesenchymal Stem Cells after Therapy. Am. J. Pathol. 2015, 185, 1924–1934. [Google Scholar] [CrossRef]
- Fatima, S.; Kamble, S.S.; Dwivedi, V.P.; Bhattacharya, D.; Kumar, S.; Ranganathan, A.; Van Kaer, L.; Mohanty, S.; Das, G. Mycobacterium tuberculosis programs mesenchymal stem cells to establish dormancy and persistence. J. Clin. Investig. 2020, 130, 655–661. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef]
- Cohen, K.A.; Abeel, T.; Manson McGuire, A.; Desjardins, C.A.; Munsamy, V.; Shea, T.P.; Walker, B.J.; Bantubani, N.; Almeida, D.V.; Alvarado, L.; et al. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med. 2015, 12, e1001880. [Google Scholar] [CrossRef]
- Velayati, A.A.; Masjedi, M.R.; Farnia, P.; Tabarsi, P.; Ghanavi, J.; ZiaZarifi, A.H.; Hoffner, S.E. Emergence of new forms of totally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 2009, 136, 420–425. [Google Scholar] [CrossRef]
- Jacobs, R.E.; Gu, P.; Chachoua, A. Reactivation of pulmonary tuberculosis during cancer treatment. Int. J. Mycobacteriol. 2015, 4, 337–340. [Google Scholar] [CrossRef]
- Motta, I.; Calcagno, A.; Bonora, S. Pharmacokinetics and pharmacogenetics of anti-tubercular drugs: A tool for treatment optimization? Expert Opin. Drug Metab. Toxicol. 2018, 14, 59–82. [Google Scholar] [CrossRef]
- Singh, D.; Goel, D.; Bhatnagar, R. Recombinant L7/L12 protein entrapping PLGA (poly lactide-co-glycolide) micro particles protect BALB/c mice against the virulent B. abortus 544 infection. Vaccine 2015, 33, 2786–2792. [Google Scholar] [CrossRef]
- Goel, D.; Rajendran, V.; Ghosh, P.C.; Bhatnagar, R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine 2013, 31, 1231–1237. [Google Scholar] [CrossRef]
- Mata-Espinosa, D.; Molina-Salinas, G.M.; Barrios-Payan, J.; Navarrete-Vazquez, G.; Marquina, B.; Ramos-Espinosa, O.; Bini, E.I.; Baeza, I.; Hernandez-Pando, R. Therapeutic efficacy of liposomes containing 4-(5-pentadecyl-1,3,4-oxadiazol-2-yl)pyridine in a murine model of progressive pulmonary tuberculosis. Pulm. Pharmacol. Ther. 2015, 32, 7–14. [Google Scholar] [CrossRef]
- Manish, M.; Rahi, A.; Kaur, M.; Bhatnagar, R.; Singh, S. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS ONE 2013, 8, e61885. [Google Scholar] [CrossRef]
- Malik, A.; Gupta, M.; Mani, R.; Gogoi, H.; Bhatnagar, R. Trimethyl Chitosan Nanoparticles Encapsulated Protective Antigen Protects the Mice against Anthrax. Front. Immunol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Gogoi, H.; Mani, R.; Bhatnagar, R. A niosome formulation modulates the Th1/Th2 bias immune response in mice and also provides protection against anthrax spore challenge. Int. J. Nanomed. 2018, 13, 7427–7440. [Google Scholar] [CrossRef]
- Jahagirdar, P.S.; Gupta, P.K.; Kulkarni, S.P.; Devarajan, P.V. Intramacrophage delivery of dual drug loaded nanoparticles for effective clearance of Mycobacterium tuberculosis. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the Management of Microbial Infection—Overview and Perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef]
- Poste, G.; Bucana, C.; Raz, A.; Bugelski, P.; Kirsh, R.; Fidler, I.J. Analysis of the fate of systemically administered liposomes and implications for their use in drug delivery. Cancer Res. 1982, 42, 1412–1422. [Google Scholar]
- Sou, K.; Goins, B.; Takeoka, S.; Tsuchida, E.; Phillips, W.T. Selective uptake of surface-modified phospholipid vesicles by bone marrow macrophages in vivo. Biomaterials 2007, 28, 2655–2666. [Google Scholar] [CrossRef]
- Sou, K.; Goins, B.; Leland, M.M.; Tsuchida, E.; Phillips, W.T. Bone marrow-targeted liposomal carriers: A feasibility study in nonhuman primates. Nanomedicine 2010, 5, 41–49. [Google Scholar] [CrossRef]
- Swami, A.; Reagan, M.R.; Basto, P.; Mishima, Y.; Kamaly, N.; Glavey, S.; Zhang, S.; Moschetta, M.; Seevaratnam, D.; Zhang, Y.; et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl. Acad. Sci. USA 2014, 111, 10287–10292. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Grobler, A.; Rath, G.; Goyal, A.K.; Jain, A.K.; Mehta, A. Pulmonary Delivery of Anti-Tubercular Drugs Using Ligand Anchored pH Sensitive Liposomes for the Treatment of Pulmonary Tuberculosis. Curr. Drug Deliv. 2016, 13, 909–922. [Google Scholar] [CrossRef]
- Franklin, R.K.; Marcus, S.A.; Talaat, A.M.; KuKanich, B.K.; Sullivan, R.; Krugner-Higby, L.A.; Heath, T.D. A Novel Loading Method for Doxycycline Liposomes for Intracellular Drug Delivery: Characterization of in Vitro and in Vivo Release Kinetics and Efficacy in a J774A.1 Cell Line Model of Mycobacterium smegmatis Infection. Drug Metab. Dispos. 2015, 43, 1236–1245. [Google Scholar] [CrossRef]
- Singh, J.; Garg, T.; Rath, G.; Goyal, A.K. Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis—A critical review. Drug Deliv. 2016, 23, 1676–1698. [Google Scholar] [CrossRef]
- Labana, S.; Pandey, R.; Sharma, S.; Khuller, G.K. Chemotherapeutic activity against murine tuberculosis of once weekly administered drugs (isoniazid and rifampicin) encapsulated in liposomes. Int. J. Antimicrob. Agents 2002, 20, 301–304. [Google Scholar] [CrossRef]
- Deol, P.; Khuller, G.K.; Joshi, K. Therapeutic efficacies of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrob. Agents Chemother. 1997, 41, 1211–1214. [Google Scholar] [CrossRef][Green Version]
- Pandey, R.; Sharma, S.; Khuller, G.K. Lung specific stealth liposomes as antitubercular drug carriers in guinea pigs. Indian J. Exp. Biol. 2004, 42, 562–566. [Google Scholar]
- Farrell, K.B.; Karpeisky, A.; Thamm, D.H.; Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018, 9, 47–60. [Google Scholar] [CrossRef]
- Adjei, I.M.; Temples, M.N.; Brown, S.B.; Sharma, B. Targeted Nanomedicine to Treat Bone Metastasis. Pharmaceutics 2018, 10, 205. [Google Scholar] [CrossRef]
- Adachi, J.D. Alendronate for osteoporosis. Safe and efficacious nonhormonal therapy. Can. Fam. Physician 1998, 44, 327–332. [Google Scholar]
- Chou, M.Y.; Yan, D.; Jafarov, T.; Everett, E.T. Modulation of murine bone marrow-derived CFU-F and CFU-OB by in vivo bisphosphonate and fluoride treatments. Orthod. Craniofac. Res. 2009, 12, 141–147. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Liu, X.; Shmeeda, H.; Shudde, C.; Gabizon, A.A. Repurposing amino-bisphosphonates by liposome formulation for a new role in cancer treatment. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Devogelaer, J.P. A risk-benefit assessment of alendronate in the treatment of involutional osteoporosis. Drug Saf. 1998, 19, 141–154. [Google Scholar] [CrossRef]
- Scheen, A.J. Drug clinics. The drug of the month. Alendronate (Fosamax). Rev. Med. Liege 1998, 53, 220–222. [Google Scholar]
- Sou, K.; Goins, B.; Oyajobi, B.O.; Travi, B.L.; Phillips, W.T. Bone marrow-targeted liposomal carriers. Expert Opin. Drug Deliv. 2011, 8, 317–328. [Google Scholar] [CrossRef]
- Marques-Gallego, P.; de Kroon, A.I. Ligation strategies for targeting liposomal nanocarriers. Biomed. Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef]
- Pinheiro, M.; Lucio, M.; Lima, J.L.; Reis, S. Liposomes as drug delivery systems for the treatment of TB. Nanomedicine 2011, 6, 1413–1428. [Google Scholar] [CrossRef]
- Tandel, N.; Joseph, A.Z.; Joshi, A.; Shrama, P.; Mishra, R.P.; Tyagi, R.K.; Bisen, P.S. An evaluation of liposome-based diagnostics of pulmonary and extrapulmonary tuberculosis. Expert Rev. Mol. Diagn 2020. [Google Scholar] [CrossRef]
- Gupta, R.; Rajendran, V.; Ghosh, P.C.; Srivastava, S. Assessment of anti-plasmodial activity of non-hemolytic, non-immunogenic, non-toxic antimicrobial peptides (AMPs LR14) produced by Lactobacillus plantarum LR/14. Drugs R. D 2014, 14, 95–103. [Google Scholar] [CrossRef]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, G.M.; Dutt, S.; Ghosh, P.C. Stearylamine Liposomal Delivery of Monensin in Combination with Free Artemisinin Eliminates Blood Stages of Plasmodium falciparum in Culture and P. berghei Infection in Murine Malaria. Antimicrob. Agents Chemother. 2015, 60, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Asadpour-Zeynali, K.; Saeb, E. Simultaneous Spectrophotometric Determination of Rifampicin, Isoniazid and Pyrazinamide in a Single Step. Iran. J. Pharm. Res. 2016, 15, 713–723. [Google Scholar]
- Rivolta, I.; Panariti, A.; Lettiero, B.; Sesana, S.; Gasco, P.; Gasco, M.R.; Masserini, M.; Miserocchi, G. Cellular uptake of coumarin-6 as a model drug loaded in solid lipid nanoparticles. J. Physiol. Pharmacol. 2011, 62, 45–53. [Google Scholar]
- Ramanlal Chaudhari, K.; Kumar, A.; Megraj Khandelwal, V.K.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Ramachandra Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef]
- Radaeva, T.V.; Kondratieva, E.V.; Sosunov, V.V.; Majorov, K.B.; Apt, A. A human-like TB in genetically susceptible mice followed by the true dormancy in a Cornell-like model. Tuberculosis 2008, 88, 576–585. [Google Scholar] [CrossRef]
- de Wit, D.; Wootton, M.; Dhillon, J.; Mitchison, D.A. The bacterial DNA content of mouse organs in the Cornell model of dormant tuberculosis. Tuber Lung Dis. 1995, 76, 555–562. [Google Scholar] [CrossRef]
- Gupta, S.; Tyagi, S.; Almeida, D.V.; Maiga, M.C.; Ammerman, N.C.; Bishai, W.R. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am. J. Respir. Crit. Care Med. 2013, 188, 600–607. [Google Scholar] [CrossRef]
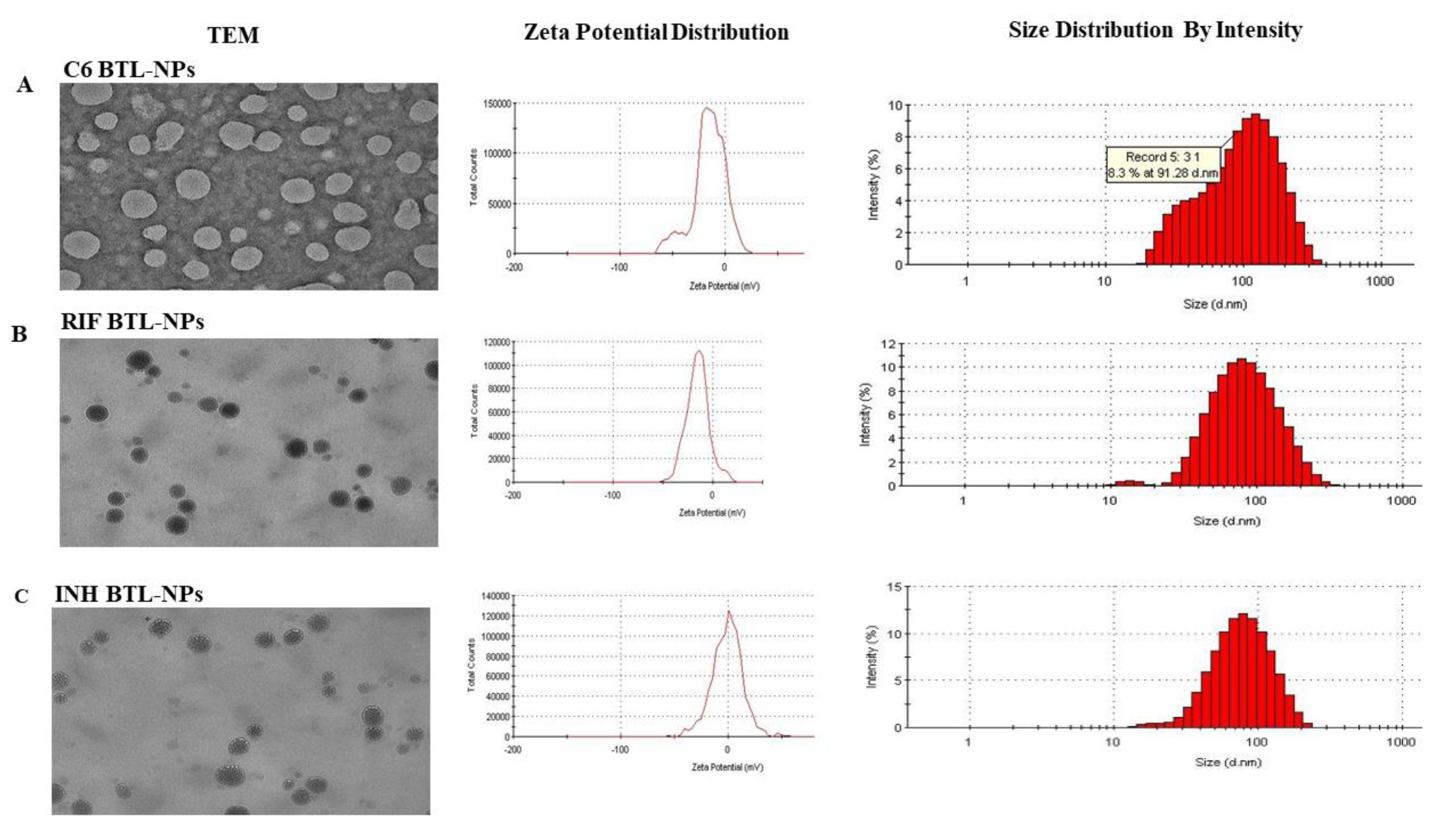

| Nanoparticle (NP) | NP Size (nm) | % Encapsulation |
|---|---|---|
| C6 BTL-NP | 109 ± 12.6 | 70 ± 3.7 |
| INH BTL-NP | 100 ± 16.3 | 69.5 ± 4.2 |
| RIF BTL-NP | 84 ± 18.4 | 70.6 ± 4.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garhyan, J.; Mohan, S.; Rajendran, V.; Bhatnagar, R. Preclinical Evidence of Nanomedicine Formulation to Target Mycobacterium tuberculosis at Its Bone Marrow Niche. Pathogens 2020, 9, 372. https://doi.org/10.3390/pathogens9050372
Garhyan J, Mohan S, Rajendran V, Bhatnagar R. Preclinical Evidence of Nanomedicine Formulation to Target Mycobacterium tuberculosis at Its Bone Marrow Niche. Pathogens. 2020; 9(5):372. https://doi.org/10.3390/pathogens9050372
Chicago/Turabian StyleGarhyan, Jaishree, Surender Mohan, Vinoth Rajendran, and Rakesh Bhatnagar. 2020. "Preclinical Evidence of Nanomedicine Formulation to Target Mycobacterium tuberculosis at Its Bone Marrow Niche" Pathogens 9, no. 5: 372. https://doi.org/10.3390/pathogens9050372
APA StyleGarhyan, J., Mohan, S., Rajendran, V., & Bhatnagar, R. (2020). Preclinical Evidence of Nanomedicine Formulation to Target Mycobacterium tuberculosis at Its Bone Marrow Niche. Pathogens, 9(5), 372. https://doi.org/10.3390/pathogens9050372





