Abstract
Contamination of retail foods with foodborne pathogens, particularly the antimicrobial resistant ones, poses a persistent threat to human health. There is a dearth of information about the overlapping Escherichia coli (E. coli) lineages circulating among retail foods and humans in Egypt. This study aimed to determine the clonal diversity of 120 E. coli isolates from diarrheic patients (n = 32), retail chicken carcasses (n = 61) and ground beef (n = 27) from Mansoura, Egypt using pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). Simpson’s index of diversity was calculated to compare the results of both typing methods. Antimicrobial resistance phenotypes, genotypes and phylogrouping of the isolates were also determined. Higher frequencies of antimicrobial resistance were found among chicken isolates compared to beef and human isolates; regardless of isolate source, the predominant antimicrobial resistances were found against ampicillin (87/120, 72.5%), tetracycline and sulfisoxazole (82/120, 68.3%, each), and streptomycin (79/120, 65.8%). None of the isolates displayed resistance to meropenem. The prevalent genes detected were tetA (64.2%), blaTEM (62.5%), sul1 (56.7%), floR (53.3%), sul2 (50%), strB (48.3%) and strA (47.5%) corresponding with resistance phenotypes. Alarmingly, blaCTX was detected in 63.9% (39/61) of chicken isolates. The majority of E. coli isolates from humans (90.6%), beef (81.5%) and chicken (70.5%) belonged to commensal phylogroups (A, B1, C). Using PFGE analysis, 16 out of 24 clusters (66.7%) contained isolates from different sources at a similarity level ≥75%. MLST results assigned E. coli isolates into 25, 19 and 13 sequence types (STs) from chicken, human and beef isolates, respectively. Six shared STs were identified including ST1011, ST156, ST48, ST224 (chicken and beef), ST10 (human and chicken) and ST226 (human and beef). Simpson’s index of diversity was higher for MLST (0.98) than PFGE (0.94). In conclusion, the existence of common genetic determinants among isolates from retail foods and humans in Egypt as well as the circulation of shared STs indicates a possible epidemiological link with potential zoonotic hazards.
1. Introduction
Escherichia coli (E. coli), a member of Enterobacteriaceae that inhabits the gastrointestinal tract, is divided into commensal and pathogenic strains that are capable of producing infections in humans and animals [1]. In food-producing animals, both commensal and pathogenic strains of E. coli are able to contaminate meat, even in facilities with high standards, with a subsequent potential hazard to humans. The condition is exacerbated if food is contaminated with antimicrobial resistant E. coli that could disseminate resistance traits to humans from the food chain and increases the difficulty of treatment of infections in humans [2,3].
The ever-increasing range of antimicrobial resistance in food-producing animals is a global problem. This phenomenon stems from the indiscriminate use of antimicrobials in treatment of animal infections as well as antimicrobial administration in sub-therapeutic doses for prophylaxis and growth promotion [4,5]. In low-resource countries, the problem is complicated with the development of resistances either to antimicrobials that are not licensed for use in animals or to antimicrobials that are prioritized for use in humans only, leading to a subsequent decrease in possible therapeutic choices for the treatment of human illnesses [6,7]. According to World Health Organization (WHO), many European countries and the US banned the use of antimicrobials for animal growth promotion, particularly antimicrobials that are classified as critically important antimicrobials for treatment of human infections [8].
Generally, bacterial mechanisms for antimicrobial resistance are usually associated with either chromosomal mutations and/or acquisition of resistance genes that are commonly linked to mobile genetic elements (MGE) such as plasmids, transposons and integron cassettes [4,9]. To monitor the potential sources of the acquired resistance genes, molecular typing tools are required to identify the common clones of antimicrobial resistant bacteria circulated among different sources. While whole-genome sequencing (WGS) would be the method of choice, it is not always available for use. Of the molecular typing tools, pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) are commonly used [10,11,12]. PFGE has been known to have high discriminatory power and is considered the gold standard in E. coli sub-typing [13], whereas MLST has the advantage of providing unambiguous sequences from which sequence types (STs) are generated, that could be easily tracked both at national and international levels.
In Egypt, characterization of antimicrobial resistant E. coli isolates from food animals has been the subject of many studies [14,15,16,17]; however, most of those studies have focused on conventional phenotypic and genotypic characterization methods without performing a higher resolution molecular typing of such strains. Furthermore, to the best of our knowledge, there have been no reports in Egypt that analyzed E. coli from both humans and retail foods, which might articulate the extent of potential transmission sources. Therefore, this study was conducted to determine the genetic diversity of E. coli from humans, chicken carcasses and ground beef using PFGE and MLST; antimicrobial resistances for these isolates were also determined.
2. Results
2.1. Resistance Phenotypes and Genotypes of Human, Chicken and Beef E. coli Isolates
In the present study, antimicrobial susceptibilities of the examined 120 isolates to 14 antimicrobials were identified. Regardless of isolate source, the highest resistance was found against ampicillin (87/120, 72.5%), followed by tetracycline and sulfisoxazole (82/120, 68.3%, each), and streptomycin (79/120, 65.8%). Antimicrobial resistances ranging from 40% to 65% were observed to chloramphenicol (60.8%), quinolones (nalidixic acid, 55%; ciprofloxacin, 43.3%), trimethoprim/sulfamethoxazole (49.2%), ceftriaxone (42.5%), and gentamicin (40.8%). Low frequencies of resistance were detected against cefoxitin (2/120, 1.7%) and amoxicillin/clavulanic acid (4/120, 3.3%); none of the isolates displayed resistance to meropenem. Multidrug resistance (MDR, resistance to at least three antimicrobials in different classes) was identified in 69.1% of the examined isolates (83/120) representing 98.4%, 44.4% and 34.4% of chicken, beef and human isolates, respectively (Table 1). Some of the antimicrobials belonging to the same class exhibited different activity against the examined E. coli isolates. For example, cefoxitin and ceftriaxone, both cephalosporins, were inactive against two (1.7%) and 51 (42.5%) E. coli isolates, respectively. The resistance phenotype pattern against the majority of examined antimicrobials (11/14, 78.5%) was significantly different (p-value < 0.05) with higher frequencies identified among chicken isolates compared to beef and human isolates.

Table 1.
Frequencies of antimicrobial resistance phenotypes among E. coli isolates from humans, chicken carcasses and ground beef in Egypt.
Among the 19 resistance genes examined, tetA, blaTEM, sul1, floR, sul2, strB and strA were the prevalent genes identified among the examined isolates from retail foods and humans. blaCTX was found in 39.2% of the examined isolates (47/120) representing 63.9% (39/61), 18.8% (6/32) and 7.4% (2/27) of chicken, human and beef isolates, respectively. Similar to resistance phenotypes, the distribution of the majority of resistance genes was significantly higher among chicken isolates compared to beef and human isolates (Table 2).

Table 2.
Frequencies of resistance genes among E. coli isolates from humans, chicken carcasses and ground beef in Egypt.
2.2. Correlation and Principal Component Analyses of Resistance Phenotypes and Genotypes in E. coli Isolates
The association of resistance phenotypes and genotypes among the examined E. coli isolates was determined using correlation analysis. The analysis revealed positive and significant associations between resistance genes, either those conferring resistance to the same antimicrobial class or to different classes, except for blaSHV and blaCTX that were negatively correlated in a significant manner. Regarding phenotypic/genotypic correlation, in most of the cases, there were significant positive correlations between resistance genes and their corresponding antimicrobials. For instance, blaCTX, blaTEM and blaCMY resistance genes were correlated positively and significantly with ceftriaxone. The analysis also showed significant positive correlation between resistance genes and antimicrobials other than the corresponding ones (e.g., blaCTX and blaTEM genes with sulfisoxazole, streptomycin and tetracycline; floR gene with ampicillin, nalidixic acid, sulfisoxazole, streptomycin and tetracycline); however, the only significant negative correlation was detected between dhfr5 and ciprofloxacin (Figure S1). As shown in the principal component analysis (PCA) plot (Figure S2), the phenotypic as well as genotypic resistance trait could not segregate E. coli isolates and the isolates from various sources overlapped largely. The E. coli isolates were much closer to each other (less diverse) when analyzing their phenotypic features (Eucladean distance = 2.0) than when analyzing their genotype (Eucladean distance = 2.5).
2.3. Phylogrouping of Human, Chicken and Beef E. coli Isolates
The frequency distributions of different E. coli phylogroups among the examined E. coli isolates are summarized in Table 3. Commensal phylogroups represented by phylogroups A, B1, and C were identified in 90.6% (29/32) of human isolates, 81.5% (22/27) of beef isolates and 70.5% (43/61) of chicken isolates. Meanwhile, pathogenic phylogroups E, B2, and F were detected in chicken isolates (17/61, 27.9%), phylogroups D and E in human isolates (3/32, 9.4%) and phylogroups B2 and E in beef isolates (5/27, 18.5%).

Table 3.
Frequencies of phylogroups among E. coli isolates from humans, chicken carcasses and ground beef in Egypt.
2.4. PFGE and MLST Typing of the Examined E. coli Isolates
Based on PFGE analysis, clustering of the isolates with ≥75% similarity showed E. coli isolates from humans, chicken and beef were distributed into 34 PFGE types including 24 clusters assigned from C1 to C24 and 10 singletons (S1–S10). The 24 clusters encompassed at least two isolates that either belonged to one source (n = 8) or multiple sources (n = 16), known as mixed clusters; meanwhile, a singleton represented one isolate. Of the 16 mixed clusters, only three (C7, C10, C18) clusters contained isolates from all three sources. Meanwhile, the other 13 clusters included isolates from two different sources and were distributed as follows: seven clusters (C2, C4, C12, C13, C15, C19, C23) for isolates from humans and chicken, four clusters (C1, C3, C17, C20) for chicken and beef isolates, and two clusters for human and beef isolates (C11, C16). Cluster C10 was the largest cluster composed of 24 isolates (10 from humans, 9 from chickens and 5 from beef); the human isolate (20ST) harbored similar resistance genes to beef isolates (1M, 2M, 3M, 8M and 9M) except for the presence of blaCTX in human isolate and floR gene in beef isolates (Figure 1). In some mixed clusters, isolates from different sources were assigned to similar phylogroups (e.g., C1, C3, C10, C11, C18, C23).

Figure 1.
Resistance genotype, phenotype, phylogrouping, pulsed-field gel electrophoresis (PFGE), and multilocus sequence typing (MLST) analyses of Escherichia coli isolates from humans, chicken carcasses and ground beef. PFGE clustering of isolates was performed based on band-based Dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA). At a similarity ≥75%, the isolates were clustered into 34 PFGE types including 24 clusters assigned from C1 to C24 and 10 singletons (S1–S10). For resistance genotype and phenotype, black squares indicate the presence of resistance genes and resistance phenotype. Antimicrobials are amoxicillin/clavulanate (AM/CLA), ampicillin (AMP), azithromycin (AZI), cefoxitin (FOX), ceftriaxone (AXO), chloramphenicol (CHL), ciprofloxacin (CIP), gentamicin (GEN), meropenem (MERO), nalidixic acid (NAL), streptomycin (STR), sulfisoxazole (FIS), tetracycline (TET) and trimethoprim/sulphamethoxazole (SXT).
Using MLST typing, 119 E. coli isolates were assigned into 51 different STs, and one isolate from humans was non-typeable. Only 16 STs representing 43.7% (52/119) of isolates were grouped into 13 clonal complexes (CCs). Irrespective of the shared STs among different sources, E. coli isolates from humans, chicken carcasses and ground beef were assigned into 19, 25 and 13 STs, respectively (Table S1). Approximately 50% of chicken isolates belonged to six STs: ST1196 (n = 7), ST162 (n = 6), ST189 (n = 5), ST1011 (n = 5), ST69 (n = 4) and ST117 (n = 4). The most prevalent STs among human isolates were ST43 (n = 6) and ST10 (n = 3). Of the 13 STs identified from beef isolates, ST156 (n = 5), ST4623 (n = 5) and ST9611 (n = 4) were the predominant STs that accounted for 52% of beef isolates. Four STs (ST1011, ST156, ST48, ST224) were shared between chicken and beef isolates; whereas, two STs circulated among human and food animal isolates including ST10 (human and chicken isolates) and ST226 (human and beef isolates) (Figure 1 and Figure 2).
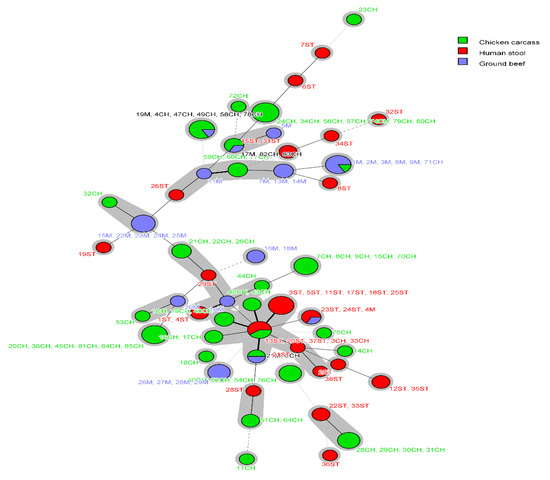
Figure 2.
Minimum spanning tree (MST) based on MLST analysis of E. coli from humans, chicken carcasses and ground beef. Each circle corresponds to an individual sequence type (ST), and circle size is relevant to the number of isolates assigned to the same ST. The color of the circle denotes the isolate source. Connecting lines (solid and dashed) between circles denote allelic variations between STs, and grey shadowing indicates no more than two different loci between STs.
Clonal complex (CC)10 was the only CC found in E. coli isolates from humans, chicken and beef; it was represented in human isolates by ST43 (n = 6) and ST10 (n = 3), in chicken isolates by ST10 (n = 2) and ST48 (n = 1), and in beef isolates by ST48 (n = 1). Other CCs were identified among isolates from two different sources such as CC155 and CC156 (chicken and beef) and CC226 (human and beef) (Table S1).
Our findings also showed that majority of E. coli assigned to similar STs belonged to similar phylogroups. For instance, the overlapping STs (ST1011, ST156, ST48 and ST224) circulating among chicken and beef belonged to phylogroups E, B1, C and B1, respectively, in both sources. Similarly, the human and beef E. coli ST226 isolates were assigned to phylogroup A. Moreover, the shared ST10 that was identified in both human and chicken isolates, belonged to phylogroup C in chickens and phylogroup A in humans; both are commensal phylogroups. Likewise, all the identical isolates and sets of isolates that exhibited ≥ 90% similarity within each PFGE cluster were assigned to the same ST.
Simpson’s index of diversity (D) was used to compare between MLST and PFGE as to their ability to discriminate E. coli isolates. For both typing methods, the diversity index (discriminatory ability) exceeded 90%, and irrespective of isolate source, MLST had a higher index than PFGE, at approximately 4%. This also remained true when diversity was measured on human and chicken isolates with MLST exhibiting a 5% and 2% increase in discriminatory ability compared to PFGE, respectively, whereas PFGE had a higher discriminatory ability than MLST for beef isolates (Figure S3).
3. Discussion
There is a need to monitor the potential sources of human infections using high discriminatory molecular typing methods. This is especially true considering the increase in antimicrobial resistance and the possibility of dissemination of resistance traits, especially those mediated by MGE through the food chain to humans [11,18,19]. In Egypt, the available information about the clonality of antimicrobial resistant E. coli from humans and food-producing animals is still minimal. This study provides insight into the overlapping E. coli lineages and shared PFGE pulsotypes among isolates from humans and food-producing animals.
In the current study, an overall high prevalence of MDR among the isolates (69.1%) was observed irrespective of their source and when analyzing the entire E. coli population within various hosts. The existence of MDR in 98.4% and 44.4% of retail chicken and beef isolates, respectively, with higher resistances against ampicillin, tetracycline, sulfisoxazole and streptomycin, was in concordance with previous studies not only in Egypt [15,16,17], but also worldwide [20,21,22], indicating the selective pressure of these antimicrobials in the treatment of E. coli infections in poultry and livestock. Antimicrobial resistances were also detected against chloramphenicol, nalidixic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, ceftriaxone and gentamicin, leaving few therapeutic choices. Fortunately, all the isolates in the current study were sensitive to meropenem, which could be an alternative for the treatment of infections with MDR bacteria. Nonetheless, recent reports from Egypt revealed the existence of carbapenem resistance in E. coli isolates from both food producing [14,23] and companion animals [24]. This could provide an evidence about the veterinary use of critically important antimicrobials to humans (e.g., ciprofloxacin, ceftriaxone, cefepime, meropenem, colisitin) in Egypt.
Molecular identification of resistance genes from the examined isolates showed higher frequencies of tetA, blaTEM, sul1, sul2, strA, and strB genes that matched higher resistance phenotypes to the corresponding antimicrobials including tetracycline, ampicillin, sulfisoxazole, and streptomycin. Although blaCTX, which confers resistance to third-generation cephalosporins, is not the predominant β-lactamase in this study, its presence in about 64% of chicken isolates is alarming. This was markedly higher than that previously reported in Egypt from either live birds [25] or chicken meat [14,16,26], with potential hazards to humans, since blaCTX is carried on conjugative plasmids and possibly disseminated to humans through food.
The observation that antimicrobials belonging to the same class exhibited variable activity against E. coli isolates might indicate discrepancies in the gene content of respective isolates or differences in the use of a particular antimicrobial and thus the selection of resistance. The current study also revealed significant host-dependent differences in phenotypic and genotypic antimicrobial resistance traits. In this, we observed that chicken E. coli showed a higher resistance than ground beef isolates, which agrees with a previous study done in Egypt [16]. This could reflect the high usage of antimicrobials for treatment, prophylaxis and growth promotion in chicken than livestock [27].
From correlation analysis, the significant association of resistance genes to the corresponding antimicrobials was expected. However, the existence of significant correlations between resistance genes and other unrelated antimicrobials belonging to different classes could signal co-localization and genetic linkage of these genes either on the chromosome and/or on MGE [28,29]. Despite the differences among the studied sources in E. coli isolate profiles, the PCA plot shown in Figure S2 suggests that neither exhibiting a particular resistance phenotype nor harboring certain resistance genes could segregate the sources of E. coli into distinct groups. This indicates the high extent of resistance phenotypes and genotypes overlapping among the studied E. coli population from humans and food animals.
Molecular subtyping of E. coli isolates from humans, chicken and beef was determined using PFGE and MLST. PFGE grouped isolates into 34 distinguishable profiles at a similarity of ≥75%, including 24 clusters and 10 singletons. In 33.3% (8/24) of the clusters, isolates belonging to the same source were grouped together; whereas the clustering of isolates from various sources in mixed clusters (16/24, 67.7%) suggests the existence of shared genetic determinants among these isolates. Likely, the presence of similar phylogroups in some mixed clusters signifies that isolates from different sources might have the same origin at potential animal-to-human transmission.
E. coli genotyping using MLST showed a wide diversity of STs; chicken isolates displayed a higher variety of STs followed by human and beef isolates. The predominant STs from chicken isolates differed from the prevalent STs from humans and beef isolates which was in accordance with different studies that showed diversified distribution of E. coli lineages among different sources. For instance, in a study performed by Yamaji et al. [11], human and retail meat isolates were assigned into 61 and 95 STs, respectively, and only 12 STs were shared between both isolate sources. In another study from Germany, whole genome sequence analyses revealed the heterogeneous population of blaCMY-2-producing E. coli from humans, livestock animals and food that were assigned into 31, 29 and 20 different STs, respectively [30]. A recent study from Ghana showed the existence of 22 and 13 STs among 34 and 45 E. coli isolates from humans and broilers, respectively, with five overlapping STs [31].
Unfortunately, there are limited data about MLST genotyping of E. coli from Egypt. A study performed by Fam et al. [32], showed that ST131 was identified in 75% of group B2 clinical E. coli isolates from human patients, a pandemic ST that was not identified in the present study. However, other STs that have been previously reported in Egypt were circulating among the current study isolates. For instance, ST1011 that was identified in our study from chicken and beef isolates, was previously recovered from a human patient hospitalized in the intensive care unit of a Cairo City hospital [33]. The predominant ST lineages ST162, ST189, ST1011, and ST117 as well as other STs (ST155, ST93, and ST57) among chicken isolates in this study, have been reported previously in association with avian pathogenic E. coli (APEC) and avian fecal E. coli (AFEC) from poultry in Egypt [34], indicating the persistent circulation of these clones in poultry in Egypt.
MLST findings also showed the existence of overlapping STs among different sources in the present study with ST1011, ST156, ST48 and ST224 in chicken and beef isolates, ST10 in human and chicken and ST226 in human and beef isolates. Both ST10 and ST48 that belonged to CC10, one of the major CC associated with diarrheagenic E. coli infections in humans worldwide [35], were identified in our study from chicken and beef isolates. This suggests the adaptability of certain STs to different hosts with a possible inter-species transmission of these clones.
Simpson’s index of diversity (D) has been used to provide a numerical description, and thus an assessment of the discriminatory ability of bacterial typing methods [36]. In general, both MLST and PFGE were useful approaches in typing E. coli and performed well in discriminating E. coli isolates as indicated by the above-90% D index upon including or excluding the hosts. This agrees with previous studies of human ESBL producing E. coli [37] as well as other bacteria [38,39]. In the current study, we observed an overall 4% increase in the discriminatory ability of MLST compared to that of PFGE. This agrees with a previous study on human E. coli isolates, which stated that MLST also exceeds PFGE diversity [37]. The observed difference in D index between both typing schemes is not uncommon and is thought to be partially attributed to the mechanism employed by both methods to pinpoint the genetic change. It is worth mentioning that there is no consensus in the literature regarding the preference of MLST over PFGE since MLST showed less discriminatory power than PFGE in other bacteria [38,40]. Therefore, we do believe that our conclusion is specific to the studied E. coli and not universal to other bacterial species.
4. Materials and Methods
4.1. E. coli Isolates from Humans and Retail Chicken and Beef
One hundred and twenty E. coli isolates were randomly chosen for this study from a set of E. coli (n = 163) isolated during the period from April to July 2017. Of these, 32 isolates were recovered from human samples and 88 isolates from retail food including 61 isolates from whole chicken carcasses and 27 isolates from ground beef (one isolate per each human and retail food sample). Whole chicken carcasses and ground beef were purchased from retail live chicken shops and supermarkets, respectively in Mansoura, Egypt, and were immediately transported in cooled boxes (4–8 °C) to the laboratory of Hygiene and Zoonoses Department, Faculty of Veterinary Medicine, Mansoura University for bacteriological analysis. Stool samples were provided arbitrarily in sterile specimen cups from diarrheic patients attending Mansoura University Hospitals (MUH), Mansoura, Egypt. Authors were not involved in the sampling process of stool from patients.
Whole chicken carcasses were rinsed thoroughly with tryptic soy broth (TSB; Oxoid, UK) in sterile polyethylene bags and 250 mL of chicken rinsate was incubated at 37 °C for 18 h. Ground beef samples (25 g) were individually homogenized in TSB (225 mL) using a stomacher, and the homogenate was incubated overnight at 37 °C. One gram of each stool sample was suspended in 9 mL of TSB and incubated at 37 °C for 18 h. From the incubated broth of all sample types, a loopful was streaked onto eosin methylene blue (EMB; Oxoid, UK) agar plates and incubated overnight at 37 °C. Typical metallic green sheen colonies were picked from EMB plates and sub-cultured onto blood agar (Oxoid, UK) plates [41]. Presumptive E. coli were biochemically confirmed with the automated VITEK2 System (bioMérieux, Durham, NC) according to the manufacturer’s directions. Whole cell template was prepared from each isolate by suspending one colony into 200 μL of sterile nuclease free water, and confirmed as E. coli using PCR targeting the uidA gene [42].
4.2. Antimicrobial Susceptibility Testing of E. coli
Antimicrobial susceptibilities of the recovered E. coli isolates from humans and retail food were determined via the microbroth dilution method using the SensititreTM semi-automated susceptibility system (TREK Diagnostic Systems, Inc., Westlake, OH, USA) and the National Antimicrobial Resistance Monitoring System (NARMS) plate CMV3AGNF, according to Clinical and Laboratory Standards Institute (CLSI) guidelines [43]. Minimum inhibitory concentrations (MICs) were determined based on the breakpoints listed in CLSI standards as follows: ampicillin (≥32 μg/mL), amoxicillin/clavulanic acid (≥32/16 μg/mL), cefoxitin (≥32 μg/mL), ceftriaxone (≥4 μg/mL), meropenem (≥4 μg/mL), chloramphenicol (≥32 μg/mL), azithromycin (≥32 μg/mL), ciprofloxacin (≥4 μg/mL), nalidixic acid (≥32 μg/mL), sulfisoxazole (≥512 μg/mL), tetracycline (≥16 μg/mL), trimethoprim/sulfamethoxazole (≥4/76 μg/mL), and gentamicin (≥16 μg/mL). For streptomycin, which does not have a breakpoint determined by CLSI, result interpretation was determined according to the NARMS breakpoint (≥64 μg/mL). Reference strains, E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212, were used for quality control.
4.3. Resistance Genotypes and E. coli Phylogrouping
PCR with whole cell templates of all 120 E. coli isolates was used to screen for the presence of genes encoding resistance to β-lactams (blaCTX, blaTEM, blaCMY, blaSHV, blaOXA), chloramphenicol (cat1, cat2, floR), tetracycline (tetA, tetB), sulfisoxazole (sul1, sul2), streptomycin (strA, strB), trimethoprim/sulfamethoxazole (dhfr1, dhfr5, dhfr12, dhfr13), and azithromycin (mphA) as previously described [19,44,45]. PCR assay for each resistance gene was performed in 25 μL reaction mixture containing 2.5 μL of each 10 pmole primer (Eurofins Genomics, Huntsville, AL, USA), 1.5 μL MgCl2 (30 mM), 0.5 μL dNTP (2 mM) (Roche, Indianapolis, IN, USA), 0.25 μL Taq polymerase (5 U/μL) (Roche, Indianapolis, IN, USA) and 3 μL of DNA template. Positive and negative controls were included in each PCR run. E. coli phylogrouping into one of 8 phylogroups: A, B1, C, B2, D, F, E, and Escherichia cryptic clade I, was performed according to Clermont et al. [46].
4.4. Pulsed-Field Gel Electrophoresis (PFGE) Analysis
PFGE was performed to determine the genetic relatedness among E. coli isolates from humans, retail chicken and beef according to PulseNet [13]. Briefly, E. coli genomic DNA was embedded in 1% Seakem Gold agarose (BioWhittaker Molecular Applications, Rockland, ME, USA) and agarose plugs were digested with 10 U of XbaI (Roche Molecular Biochemicals, Indianapolis, IN, USA). Digested fragments were electrophoresed using the CHEF-DRII system (Bio-Rad, Hercules, California, CA, USA) at 6 V with a ramped pulse time of 2.16–54.17 s for 19 h at 14 °C. Gel images were imported into BioNumerics software (Applied Maths, Austin, TX, USA, version 7.6) and the dendrogram was generated using the band-based Dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA).
4.5. Multilocus Sequence Typing (MLST)
PCR amplification of seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) was performed according to Wirth et al. [47] to identify the sequence types (STs) of the examined E. coli isolates. The amplified products for each gene were purified with a QIAquick PCR Purification Kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s instructions. Sanger sequencing of the purified amplicons using ABI 3730XL sequencer (Applied Biosystems Inc., Foster City, CA, USA) was performed at the USDA Genomics and Bioinformatics Research Unit, Stoneville, MS, USA. Sequences were imported into BioNumerics software (Applied Maths, Austin, TX, USA, version 7.6), assembled and trimmed; the allelic profiles of the seven housekeeping genes, STs and clonal complexes (CCs) were obtained from the E. coli MLST database (https://pubmlst.org/bigsdb?db=pubmlst_mlst_seqdef). Minimum spanning tree (MST) was generated using BioNumerics software (Applied Maths, Austin, TX, USA, version 7.6).
4.6. Statistical Analyses
To compare the discriminatory ability of PFGE and MLST of E. coli typing, Simpson’s index of diversity (D) [48] was calculated using the following formula 1-D. In this equation, D = , where = summation, n = number of bacterial isolates showing a particular PFGE or MLST type and N = total number of bacterial strains in the respective source. To determine if the differences in the frequency of resistance phenotype, resistance genes or phylogroups in E. coli was significant among investigated hosts, these frequencies were used as inputs to create contingency tables and the significance was determined by X2 test (Chi-square or Fisher exact test, whenever needed), with a cutoff level for p-value equal to 0.05.
To determine the extent of correlation between the antibiotic-resistance phenotype and the presence of resistance genes, the profile of resistance against an antibiotic and the presence of resistance genes were entered as 1, whereas sensitivity to an antibiotic and absence of resistance genes were scored as 0. To calculate the correlation, the “cor” and “cor.test” functions from the software “R, v. 3.6.1” were utilized. Significant correlation was visualized using “corrplot” function from the “corrplot” package in the software “R, v. 3.6.1” [49]. To visualize the overlap between human and retail food isolates, binary data representing the isolates phenotypic and genotypic profiles were used to generate a principal component analysis (PCA) plot based on the Euclidean distances among isolate pairs. This analysis was done using PC-ORD Software (v. 5, MjM Software, Gleneden Beach, OR, USA).
5. Conclusions
This study determined the existence of shared antimicrobial resistances, phylogroups, PFGE types and ST clones among E. coli isolates from retail foods and humans. While the knowledge gained from this study complements previous reports in Egypt, the provided STs would be valuable for epidemiological surveillances of foodborne outbreaks in Egypt, and possibly other countries by highlighting potential transmission sources. Further study is planned to apply whole-genome analyses for the shared ST lineages from retail food and humans, which will improve our knowledge about the possible mechanisms of potential animal-to-human transmission and help to set up control interventions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/5/357/s1, Figure S1: Correlation matrix showing the significant (p-value < 0.05) correlation (r) between resistance phenotypes and genotypes among the examined E. coli isolates, Figure S2: Principle component analysis (PCA) of resistance phenotypes and genotypes among the examined E. coli isolates, Figure S3: Discriminatory power of PFGE and MLST as measured by Simpson’s index of diversity (D) among the examined E. coli isolates, Table S1: Distribution of specific sequence types (STs), shared STs and clonal complexes (CCs) among E. coli isolates from humans, chicken carcasses and ground beef in Egypt.
Author Contributions
Conceptualization, H.R. and C.R.J.; Methodology, H.R., L.M.H., A.A. and T.A.W.; Validation, H.R. and C.R.J.; Investigation, H.R., J.G.F. and C.R.J.; Software, H.R. and C.R.J.; Formal Analysis, H.R., M.S. and C.R.J.; Data Curation, H.R. and C.R.J.; Resources, J.G.F. and C.R.J.; Writing—Original Draft Preparation, H.R.; Writing—Review and Editing, H.R., M.S., J.G.F. and C.R.J.; Supervision, C.R.J.; Project Administration, C.R.J.; Funding Acquisition, J.G.F. and C.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by the U.S. Department of Agriculture (USDA) project 6040-32000-009-00D.
Acknowledgments
Science and Technology Development Fund, Egypt (STDF) has supported Ramadan’s travel to the U.S. (Short term fellowship, STF; ID 25449). The authors acknowledge the technical help of Calvin Williams, Sandra House and Liu Fanny.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clements, A.; Young, J.C.; Constantinou, N.; Frankel, G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 2012, 3, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Padola, N.; Parma, A.; Lucchesi, P. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process. Poult. Sci. 2011, 90, 2638–2641. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; Pacholewicz, E.; Becker, E.; Correia-Carreira, G.; Bandick, N.; Kaesbohrer, A. Reviewing interventions against Enterobacteriaceae in broiler processing: Using old techniques for meeting the new challenges of ESBL E. coli? BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Frye, J.G.; Jackson, C.R. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enterococcus spp. isolated from US food animals. Front. Microbiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Adenipekun, E.O.; Jackson, C.R.; Ramadan, H.; Iwalokun, B.A.; Oyedeji, K.S.; Frye, J.G.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A.; Oluwadun, A. Prevalence and multidrug resistance of Escherichia coli from community-acquired infections in Lagos, Nigeria. J. Infect. Dev. Ctries. 2016, 10, 920–931. [Google Scholar] [CrossRef]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; World Health Organization Advisory Group; Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR); Agerso, Y.; Andremont, A.; Collignon, P.; et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar] [CrossRef]
- Dandachi, I.; Chabou, S.; Daoud, Z.; Rolain, J.-M. Prevalence and emergence of extended-spectrum cephalosporin-, carbapenem- and colistin-resistant Gram negative bacteria of animal origin in the Mediterranean Basin. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine: Ranking of Antimicrobial Agents for Risk Management of Antimicrobial Resistance due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Castellanos, L.R.; Donado-Godoy, P.; Leon, M.; Clavijo, V.; Arevalo, A.; Bernal, J.F.; Timmerman, A.J.; Mevius, D.J.; Wagenaar, J.A.; Hordijk, J. High Heterogeneity of Escherichia coli sequence types harbouring ESBL/AmpC genes on IncI1 plasmids in the Colombian poultry chain. PLoS ONE 2017, 12, e0170777. [Google Scholar] [CrossRef]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Hung-Fan, M.; Riley, L.W. A population-based surveillance study of shared genotypes of Escherichia coli isolates from retail meat and suspected cases of urinary tract infections. mSphere 2018, 3, e00179-18. [Google Scholar] [CrossRef]
- Kluytmans, J.A.J.W.; Overdevest, I.T.M.A.; Willemsen, I.; Kluytmans-van den Bergh, M.F.Q.; van der Zwaluw, K.; Heck, M.; Rijnsburger, M.; Vandenbroucke-Grauls, C.M.J.E.; Savelkoul, P.H.M.; Johnston, B.D.; et al. Extended-Spectrum β-Lactamase–producing Escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 2012, 56, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ribot, E.M.; Fair, M.; Gautom, R.; Cameron, D.; Hunter, S.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157: H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Reuland, E.A.; Wintermans, B.B.; Al Naiemi, N.; Koek, A.; Abdelwahab, A.M.; Ammar, A.M.; Mohamed, A.A.; Vandenbroucke-Grauls, C.M.J.E. Extended-Spectrum β-Lactamases and/or carbapenemases-producing Enterobacteriaceae isolated from retail chicken meat in Zagazig, Egypt. PLoS ONE 2015, 10, e0136052. [Google Scholar] [CrossRef]
- El-Shazly, D.A.; Nasef, S.A.; Mahmoud, F.F.; Jonas, D. Expanded spectrum β–lactamase producing Escherichia coli isolated from chickens with colibacillosis in Egypt. Poult. Sci. 2017, 96, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Tomaso, H.; Neubauer, H.; Hafez, H.M.; El-Adawy, H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Enany, M.E.; Algammal, A.M.; Nasef, S.A.; Abo-Eillil, S.A.M.; Bin-Jumah, M.; Taha, A.E.; Allam, A.A. The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express 2019, 9, 192. [Google Scholar] [CrossRef]
- Wang, J.; Zhi, C.-P.; Chen, X.-J.; Guo, Z.-W.; Liu, W.-L.; Luo, J.; Huang, X.-Y.; Zeng, L.; Huang, J.-W.; Xia, Y.-B.; et al. Characterization of oqxAB in Escherichia coli isolates from animals, retail meat, and human patients in Guangzhou, China. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Adenipekun, E.O.; Jackson, C.R.; Ramadan, H.; Iwalokun, B.A.; Frye, J.G.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A.; House, S.L.; McMillan, E.A.; et al. Plasmid replicons and beta-lactamase-encoding genes of multidrug-resistant Escherichia coli isolated from humans and food animals in Lagos, Southwest Nigeria. Microb. Drug Resist. 2019, 25, 1410–1423. [Google Scholar] [CrossRef]
- Yassin, A.K.; Gong, J.; Kelly, P.; Lu, G.; Guardabassi, L.; Wei, L.; Han, X.; Qiu, H.; Price, S.; Cheng, D.; et al. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE 2017, 12, e0185326. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.D.; Ahmed, M.F.; El-Adawy, H.; Hotzel, H.; Engelmann, I.; Weiß, D.; Monecke, S.; Ehricht, R. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile Delta, Egypt. Front. Microbiol. 2016, 7, 1020. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.; Gupta, S.K.; Sharma, P.; Ahmed, M.; Hiott, L.M.; Barrett, J.B.; Woodley, T.A.; Frye, J.G.; Jackson, C.R. Circulation of emerging NDM-5-producing Escherichia coli among humans and dogs in Egypt. Zoonoses Public Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. IJMM 2013, 303, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.; Awad, A. Phenotypic and genetic characterization of β-lactam resistance in Klebsiella from retail chicken meat in Mansoura, Egypt. Iran J. Microbiol. 2017, 9, 74–81. [Google Scholar]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial usage in animal production: A review of the literature with a focus on low- and middle-income countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef]
- Brown, E.M.; Nathwani, D. Antibiotic cycling or rotation: A systematic review of the evidence of efficacy. J. Antimicrob. Chemother. 2005, 55, 6–9. [Google Scholar] [CrossRef]
- Wales, A.D.; Davies, R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef]
- Pietsch, M.; Irrgang, A.; Roschanski, N.; Brenner Michael, G.; Hamprecht, A.; Rieber, H.; Käsbohrer, A.; Schwarz, S.; Rösler, U.; Kreienbrock, L.; et al. Whole genome analyses of CMY-2-producing Escherichia coli isolates from humans, animals and food in Germany. BMC Genom. 2018, 19, 601. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N.; et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 2019, 9, 3358. [Google Scholar] [CrossRef]
- Fam, N.; Leflon-Guibout, V.; Fouad, S.; Aboul-Fadl, L.; Marcon, E.; Desouky, D.; El-Defrawy, I.; Abou-Aitta, A.; Klena, J.; Nicolas-Chanoine, M.H. CTX-M-15-producing Escherichia coli clinical isolates in Cairo (Egypt), including isolates of clonal complex ST10 and clones ST131, ST73, and ST405 in both community and hospital settings. Microb. Drug Resist. 2011, 17, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Elnahriry, S.S.; Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Hussein, A.M.; Shimamoto, T.; Shimamoto, T. Emergence of Plasmid-mediated colistin resistance gene mcr-1 in a clinical Escherichia coli isolate from Egypt. Antimicrob. Agents Chemother. 2016, 60, 3249. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; Ghanem, I.A.; Eid, A.A.; Ali, M.A.; Sherwood, J.S.; Li, G.; Nolan, L.K.; Logue, C.M. Molecular and phenotypic characterization of Escherichia coli isolated from broiler chicken flocks in Egypt. Avian Dis. 2013, 57, 602–611. [Google Scholar] [CrossRef]
- Yu, F.; Chen, X.; Zheng, S.; Han, D.; Wang, Y.; Wang, R.; Wang, B.; Chen, Y. Prevalence and genetic diversity of human diarrheagenic Escherichia coli isolates by multilocus sequence typing. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2018, 67, 7–13. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Nemoy, L.L.; Kotetishvili, M.; Tigno, J.; Keefer-Norris, A.; Harris, A.D.; Perencevich, E.N.; Johnson, J.A.; Torpey, D.; Sulakvelidze, A.; Morris, J.G., Jr.; et al. Multilocus sequence typing versus pulsed-field gel electrophoresis for characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates. J. Clin. Microbiol. 2005, 43, 1776–1781. [Google Scholar] [CrossRef]
- Johnson, J.K.; Arduino, S.M.; Stine, O.C.; Johnson, J.A.; Harris, A.D. Multilocus sequence typing compared to pulsed-field gel electrophoresis for molecular typing of Pseudomonas aeruginosa. J. Clin. Microbiol. 2007, 45, 3707–3712. [Google Scholar] [CrossRef]
- Henri, C.; Félix, B.; Guillier, L.; Leekitcharoenphon, P.; Michelon, D.; Mariet, J.-F.; Aarestrup, F.M.; Mistou, M.-Y.; Hendriksen, R.S.; Roussel, S. Population Genetic structure of Listeria monocytogenes strains as determined by Pulsed-Field Gel Electrophoresis and Multilocus Sequence Typing. Appl. Environ. Microbiol. 2016, 82, 5720–5728. [Google Scholar] [CrossRef]
- Machado, G.E.; Matsumoto, C.K.; Chimara, E.; Duarte, R.d.S.; de Freitas, D.; Palaci, M.; Hadad, D.J.; Lima, K.V.B.; Lopes, M.L.; Ramos, J.P.; et al. Multilocus sequence typing scheme versus pulsed-field gel electrophoresis for typing Mycobacterium abscessus isolates. J. Clin. Microbiol. 2014, 52, 2881–2891. [Google Scholar] [CrossRef]
- Quinn, P.; Markey, B.; Carter, M.; Donnelly, W.; Leonard, F. Veterinary Microbiology and Microbial Disease; Blackwell Science Company: Hoboken, NJ, USA, 2002. [Google Scholar]
- Müller, D.; Greune, L.; Heusipp, G.; Karch, H.; Fruth, A.; Tschäpe, H.; Schmidt, M.A. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 2007, 73, 3380–3390. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Seventh Informational Supplement M100-S27; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Ramadan, H.H.; Jackson, C.R.; Taha, S.A.; Moawad, A.A.; Barrett, J.B.; Woodley, T.A. Contribution of healthy chickens to antimicrobial-resistant Escherichia coli associated with human extraintestinal infections in Egypt. Vector Borne Zoonotic Dis. 2018, 18, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Nguyen, H.A.T.; McDonald, J.M.; Woodley, T.A.; Hiott, L.M.; Barrett, J.B.; Jackson, C.R.; Frye, J.G. Genetic Characterization of Antimicrobial-Resistant Escherichia coli Isolated from a Mixed-Use Watershed in Northeast Georgia, USA. Int. J. Environ. Res. Public Health 2019, 16, 3761. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Friendly, M. Corrgrams. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).