Toxoplasma gondii Monitoring in Liver Transplantation Patients: A Single Center Cross-Sectional Study in an Italian Hospital
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
2.2. Prevalence of T. gondii Antibodies in Transplant Recipients According to Age and Serostatus
2.3. Toxoplasma gondii Prevalence in Native and Foreign LT Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Patient Serological Testing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AISF | Italian Association for the study of liver disease |
| CI | confidence intervals |
| CNS | central nervous system |
| HSCT | allogeneic hematopoietic stem cell transplantation |
| Ig | Immunoglobulin |
| LT | liver transplants |
| OR | odds ratios |
| PCR | polymerase chain reaction |
| SOT | solid-organ transplant |
| TE | toxoplasmic encephalitis |
| TG | Toxoplasma gondii |
| TMP/SMX | trimethoprim/sulfamethoxazole |
| WB | Western blot |
References
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, A.; van Pelt, W.; van Duynhoven, Y.T.; Nijhuis, C.D.; Mollema, L.; van der Klis, F.R.; Havelaar, A.H.; Kortbeek, L.M. Decreased prevalence and age-specific risk factors for Toxoplasma gondii IgG antibodies in The Netherlands between 1995/1996 and 2006/2007. Epidemiol. Infect. 2011, 139, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Castagna, B.; Mattei, R.; Bruzzi, R.; Chiumiento, L.; Cristofani, R.; Buffolano, W.; Bruschi, F. Seroprevalence for toxoplasmosis in individuals living in north west Tuscany: Access to Toxo-test in central Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Mosti, M.; Pinto, B.; Giromella, A.; Fabiani, S.; Cristofani, R.; Panichi, M.; Bruschi, F. Short Report: A 4-year evaluation of toxoplasmosis seroprevalence in the general population and in women of reproductive age in central Italy. Epidemiol. Infect. 2013, 141, 2192–2195. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Khabaz, M.N.; Elkhateeb, L.; Al-Alami, J. Reactivation of latent Toxoplasma gondii in immunocompromised cancer patients. Comp. Clin. Pathol. 2011, 20, 183–186. [Google Scholar] [CrossRef]
- Abdoli, A.; Barati, M.; Dalimi, A.; Pirestani, M.; Javad, S.; Shokouh, H. Toxoplasmosis among Patients with Immunocompromising Conditions: A Snapshot. J. Arch. Mil. Med. 2016, 4, e41832. [Google Scholar] [CrossRef]
- Meers, S.; Lagrou, K.; Theunissen, K.; Dierickx, D.; Delforge, M.; Devos, T.; Janssens, A.; Meersseman, W.; Verhoef, G.; Van Eldere, J.; et al. Myeloablative conditioning predisposes patients for Toxoplasma gondii reactivation after allogeneic stem cell transplantation. Clin. Infect. Dis. 2010, 50, 1127–1134. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Sterkers, Y.; Yera, H.; Accoceberry, I.; Menotti, J.; Cassaing, S.; Brenier-Pinchart, M.P.; Hennequin, C.; Delhaes, L.; Bonhomme, J.; et al. Molecular diagnosis of toxoplasmosis in immunocompromised patients: A 3-year multicenter retrospective study. J. Clin. Microbiol. 2015, 53, 1677–1684. [Google Scholar] [CrossRef]
- Wang, Z.D.; Liu, H.H.; Ma, Z.X.; Ma, H.Y.; Li, Z.Y.; Yang, Z.B.; Zhu, X.Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8, 389–400. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Jacob, J.T.; Hidron, A.; Rodriguez-Morales, A.J.; Kuhar, D.; Caliendo, A.M. Transplantation and tropical infectious diseases. Int. J. Infect. Dis. 2010, 14, e189–e196. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Batra, N. Toxoplasmosis in organ transplant recipients: Evaluation, implication, and prevention. Trop. Parasitol. 2016, 6, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Gomes, C.A. Toxoplasmosis After Solid OrganTransplantation. In Transplant Infections; Ljungman, P., Bowden, R.A., Snydman, D.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 781–793. [Google Scholar]
- Gajurel, K.; Dhakal, R.; Montoya, J.G. Toxoplasmosis in hematopoietic cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12734. [Google Scholar] [CrossRef] [PubMed]
- Derouin, F.; Pelloux, H. ESCMID Study Group on ClinicalParasitology. Prevention of toxoplasmosis in transplantpatients. Clin. Microbiol. Infect. 2008, 14, 1089–1101. [Google Scholar] [CrossRef]
- Castagnini, M.; Bernazzali, S.; Ginanneschi, C.; Marchi, B.; Maccherini, M.; Tsioulpas, C.; Tanganelli, P. Fatal disseminated toxoplasmosis in a cardiac transplantation with seropositive match for Toxoplasma: Should prophylaxis be extended? Transpl. Immunol. 2007, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, S.; Fortunato, S.; Petrini, M.; Bruschi, F. Allogeneic hematopoietic stem cell transplant recipients and parasitic diseases: A review of the literature of clinical cases and perspectives to screen and follow-up active and latent chronic infections. Transpl. Infect. Dis. 2017, 19, e12669. [Google Scholar] [CrossRef] [PubMed]
- Rasti, S.; Hassanzadeh, M.; Soliemani, A.; Hooshyar, H.; Mousavi, S.G.; Nikoueinejad, H.; Abdoli, A. Serological and molecular survey of toxoplasmosis in renal transplant recipients and hemodialysis patients in Kashan and Qom regions, central Iran. Ren. Fail. 2016, 38, 970–973. [Google Scholar] [CrossRef]
- Campbell, A.L.; Goldberg, C.L.; Magid, M.S.; Gondolesi, G.; Rumbo, C.; Herold, B.C. First case of toxoplasmosis following small bowel transplantation and systematic review of tissue-invasive toxoplasmosis following noncardiac solid organ transplantation. Transplantation 2006, 81, 408–417. [Google Scholar] [CrossRef]
- Fernandez-Sabe, N.; Cervera, C.; Farinas, M.C.; Bodro, M.; Muñoz, P.; Gurguí, M.; Torre-Cisneros, J.; Martín-Dávila, P.; Noblejas, A.; Len, O.; et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: A matched case-control study. Clin. Infect. Dis. 2012, 54, 355–361. [Google Scholar] [CrossRef]
- Coster, L.O. Parasitic infections in solid organ transplant recipients. Infect. Dis. Clin. N. Am. 2013, 27, 395–427. [Google Scholar] [CrossRef]
- Nosotti, M.; Tarsia, P.; Morlacchi, L.C. Infections after lung transplantation. J. Thorac. Dis. 2018, 10, 3849–3868. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Meroni, V.; Dupont, D.; Botterel, F.; Garcia, J.M.A.; Brenier-Pinchart, M.P.; Accoceberry, I.; Akan, H.; Abbate, I.; Boggian, K.; et al. Toxoplasmosis in Transplant Recipients, Europe, 2010–2014. Emerg. Infect. Dis. 2018, 24, 1497–1504. [Google Scholar] [CrossRef]
- Autier, B.; Dion, S.; Robert-Gangneux, F. The liver as an organ at risk for Toxoplasma transmission during transplantation: Myth or reality? J. Clin. Pathol. 2018, 71, 763–766. [Google Scholar] [CrossRef]
- Puccio, G.; Cajozzo, C.; Canduscio, L.A.; Cino, L.; Romano, A.; Schimmenti, M.G.; Giuffrè, M.; Corsello, G. Epidemiology of Toxoplasma and CMV serology and of GBS colonization in pregnancy and neonatal outcome in a Sicilian population. Ital. J. Pediatr. 2014, 40, 23. [Google Scholar] [CrossRef]
- Billi, P.; Della Strada, M.; Pierro, A.; Semprini, S.; Tommasini, N.; Sambri, V. Three-year retrospective analysis of the incidence of Toxoplasma gondii infection in pregnant women living in the Greater Romagna Area (northeastern Italy). Clin. Microbiol. Infect. 2016, 22, e1–e3. [Google Scholar] [CrossRef]
- Condoleo, R.; Rinaldi, L.; Sette, S.; Mezher, Z. Risk Assessment of Human Toxoplasmosis Associated with the Consumption of Pork Meat in Italy. Risk Anal. 2018, 38, 1202–1222. [Google Scholar] [CrossRef]
- Contini, C. Clinical and diagnostic management of toxoplasmosis in the immunocompromised patient. Parassitologia 2008, 50, 45–50. [Google Scholar]
- Gallino, A.; Maggiorini, M.; Kiowski, W.; Martin, X.; Wunderli, W.; Schneider, J.; Turina, M.; Follath, F. Toxoplasmosis in heart transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 389–393. [Google Scholar] [CrossRef]
- Shaffer, A. Pretransplant Evaluation for Infections in Donors and Recipients of Solid Organs. Clin. Infect. Dis. 2001, 33, S9–S14. [Google Scholar]
- Villard, O.; Cimon, B.; L’Ollivier, C.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I.; Candolfi, E. Serological diagnosis of Toxoplasma gondii infection: Recommendations from the French National Reference Center for Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2016, 84, 22–33. [Google Scholar] [CrossRef]
- Vaessen, N.; Verweij, J.J.; Spijkerman, I.J.; van Hoek, B.; van Lieshout, L. Fatal disseminated toxoplasmosis after liver transplantation: Improved and early diagnosis by PCR. Neth. J. Med. 2007, 65, 222–223. [Google Scholar]
- Fabiani, S.; Fortunato, S.; Bruschi, F. Solid organ transplant and parasitic diseases: A review of the clinical cases in the last two decades. Pathogens 2018, 7, 65. [Google Scholar] [CrossRef]
- Galvan-Ramírez, M.D.L.L.; Sanchez-Orozco, L.V.; Gutierrez-Maldonado, A.F.; Rodriguez Perez, L.R. Does Toxoplasma gondii infection impact liver transplantation outcomes? A systematic review. J. Med. Microbiol. 2018, 67, 499–506. [Google Scholar] [CrossRef]
- Santiago, E.V.; Silveira, M.R.; Araújo, V.E.; Farah, K.P.; Acurcio, F.A.; Ceccato, M. Sex and gender considerations in transplantation research: Protocol for a scoping review. Rev. Saude Publica 2015, 49, 68. [Google Scholar]
- Laprise, C.; Sridhar, V.S.; West, L.; Foster, B.; Pilote, L.; Sapir-Pichhadze, R. Sex and gender considerations in transplantation research: Protocol for a scoping review. Syst. Rev. 2018, 6, 186. [Google Scholar] [CrossRef]
- Mıhçıokur, S.; Ayvazoğlu Soy, E.H.; Türkçelik, E.; Akın, A.; Haberal, M. Gender Imbalance and the Relationship Between Living Donors and Recipients in Liver Transplantations in an Organ Transplant Center in Turkey. Exp. Clin. Transpl. 2019, 17, 131–134. [Google Scholar] [CrossRef]
- Mıhçıokur, S.; Ayvazoğlu Soy, E.H.; Türkçelik, E.; Akın, A.; Haberal, M. Gender Disparity and the Relationship Between Living Donors and Recipients in Kidney Transplants in an Organ Transplant Center in Turkey. Exp. Clin. Transpl. 2019, 17, 246–249. [Google Scholar] [CrossRef]
- Puoti, F.; Ricci, A.; Nanni-Costa, A.; Ricciardi, W.; Malorni, W.; Ortona, E. Organ transplantation and gender differences: A paradigmatic example of intertwining between biological and sociocultural determinants. Biol. Sex. Differ. 2016, 7, 35. [Google Scholar] [CrossRef]
- Pinto, B.; Mattei, R.; Moscato, G.A.; Cristofano, M.; Giraldi, M.; Scarpato, R.; Buffolano, W.; Bruschi, F. Toxoplasma infection in individuals in central Italy: Does a gender-linked risk exist? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 739–746. [Google Scholar] [CrossRef]
- Fagiuoli, S.; Colli, A.; Bruno, R.; Craxì, A.; Gaeta, G.B.; Grossi, P.; Mondelli, M.U.; Puoti, M.; Sagnelli, E.; Stefani, S.; et al. Management of infections pre- and post-liver transplantation: Report of an AISF consensus conference. J. Hepatol. 2014, 60, 1075–1089. [Google Scholar] [CrossRef]
- De Simone, P.; Carrai, P.; Coletti, L.; Ghinolfi, D.; Petruccelli, S.; Filipponi, F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 199–209. [Google Scholar] [CrossRef]
- Caner, A.; Döşkaya, M.; Karasu, Z.; Değirmenci, A.; Guy, E.; Kiliç, M.; Zeytunlu, M.; Francis, J.; Bozoklar, A.; Gürüz, Y. Incidence and diagnosis of active Toxoplasma infection among liver transplant recipients in Western Turkey. Liver Transpl. 2008, 14, 1526–1532. [Google Scholar] [CrossRef]
- Gourishankar, S.; Doucette, K.; Fenton, J.; Purych, D.; Kowalewska-Grochowska, K.; Preiksaitis, J. The use of donor and recipient screening for Toxoplasma in the era of universal trimethoprim sulfamethoxazole prophylaxis. Transplantation 2008, 85, 980–985. [Google Scholar] [CrossRef]
- Webb, G.J.; Shah, H.; David, M.D.; Tiew, S.; Beare, N.; Hirschfield, G.M. Post-prophylaxis Toxoplasma chorioretinitis following donor–recipient mismatched liver transplantation. Transpl. Infect. Dis. 2016, 18, 805–808. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
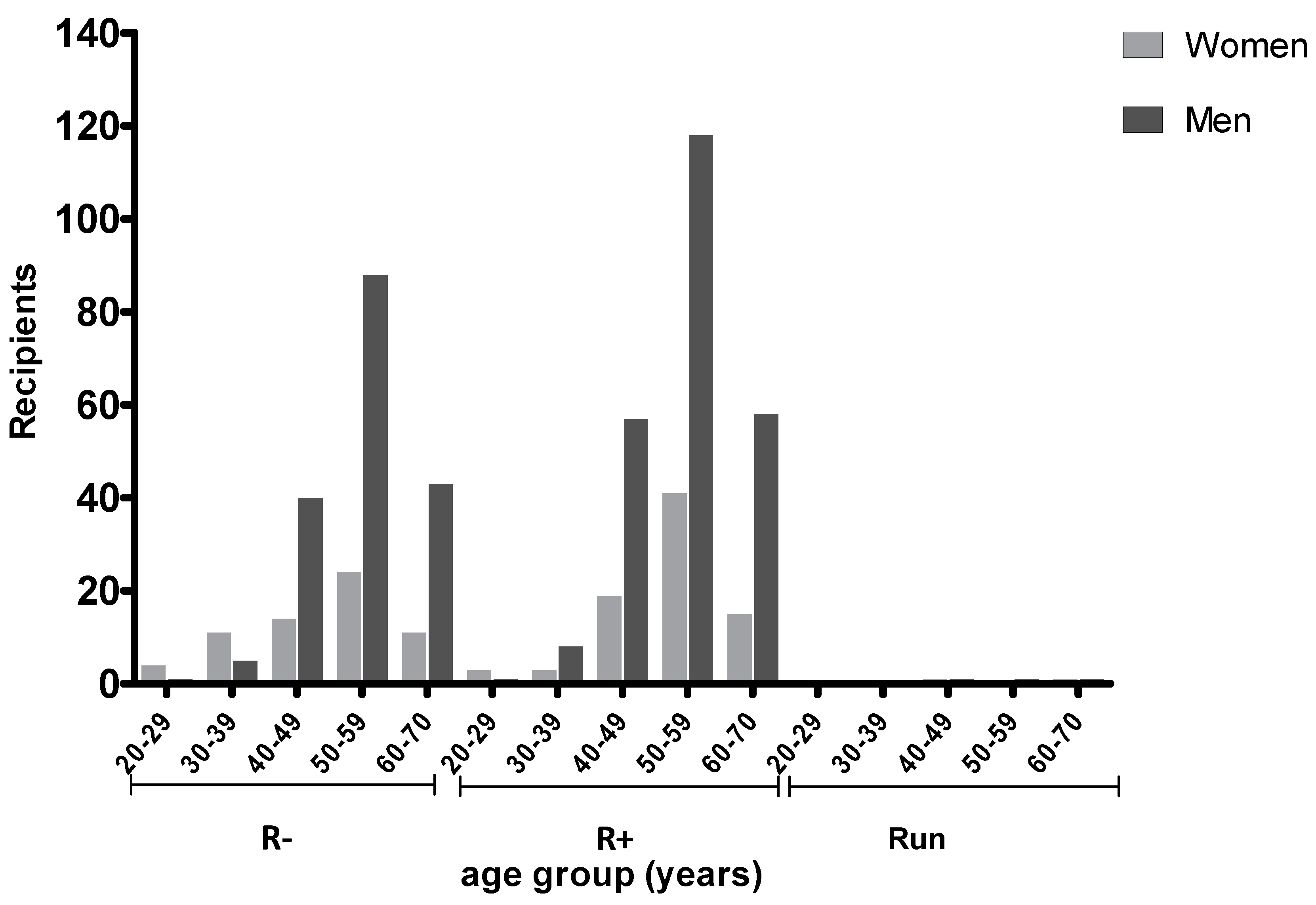
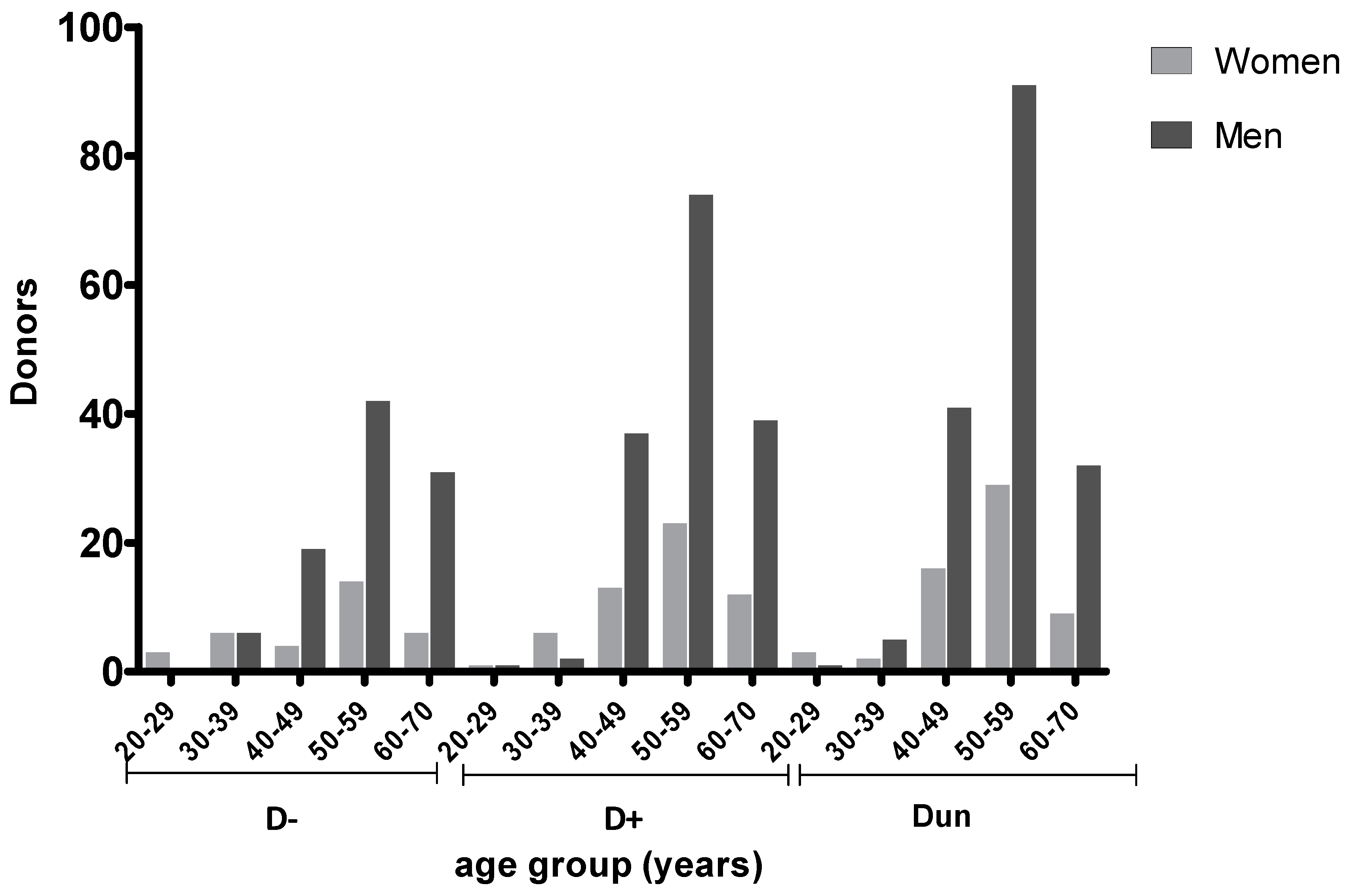
| Group | LT with Prophylaxis | LT w.o. Prophylaxis |
|---|---|---|
| D−/R− | 24 | 67 |
| D−/R+ | 1 | 39 |
| D+/R− | 122 | 10 |
| D+/R+ | 2 | 72 |
| D+/Run | 1 | 1 |
| Dun/R− | 5 | 13 |
| Dun/R+ | 3 | 204 |
| Dun/Run | 1 | 3 |
| Total | 159 | 409 |
| Patient Ethnicity and Gender | Italian N. (%) | Foreign N. (%) | Adjusted OR* (CI) | p-Value |
|---|---|---|---|---|
| Female R− | 58 (47.9) | 5 (20.0) | ||
| Female R+ | 61 (50.4) | 20 (80.0) | 3.80 (1.34–10.80) | 0.012 |
| Male R− | 163 (42.4) | 13 (34.2) | ||
| Male R+ | 216 (56.3) | 25 (65.8) | 1.45 (0.72–2.92) | 0.297 |
| Total | 498 | 63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, B.; Lotti, F.; Petruccelli, S.; Carrai, P.; De Simone, P.; Bruschi, F. Toxoplasma gondii Monitoring in Liver Transplantation Patients: A Single Center Cross-Sectional Study in an Italian Hospital. Pathogens 2020, 9, 354. https://doi.org/10.3390/pathogens9050354
Pinto B, Lotti F, Petruccelli S, Carrai P, De Simone P, Bruschi F. Toxoplasma gondii Monitoring in Liver Transplantation Patients: A Single Center Cross-Sectional Study in an Italian Hospital. Pathogens. 2020; 9(5):354. https://doi.org/10.3390/pathogens9050354
Chicago/Turabian StylePinto, Barbara, Federica Lotti, Stefania Petruccelli, Paola Carrai, Paolo De Simone, and Fabrizio Bruschi. 2020. "Toxoplasma gondii Monitoring in Liver Transplantation Patients: A Single Center Cross-Sectional Study in an Italian Hospital" Pathogens 9, no. 5: 354. https://doi.org/10.3390/pathogens9050354
APA StylePinto, B., Lotti, F., Petruccelli, S., Carrai, P., De Simone, P., & Bruschi, F. (2020). Toxoplasma gondii Monitoring in Liver Transplantation Patients: A Single Center Cross-Sectional Study in an Italian Hospital. Pathogens, 9(5), 354. https://doi.org/10.3390/pathogens9050354






