Genotyping, Assessment of Virulence and Antibacterial Resistance of the Rostov Strain of Mycobacterium tuberculosis Attributed to the Central Asia Outbreak Clade
Abstract
1. Introduction
2. Results
2.1. Genetic and Phenotypic Characteristics of the Strain
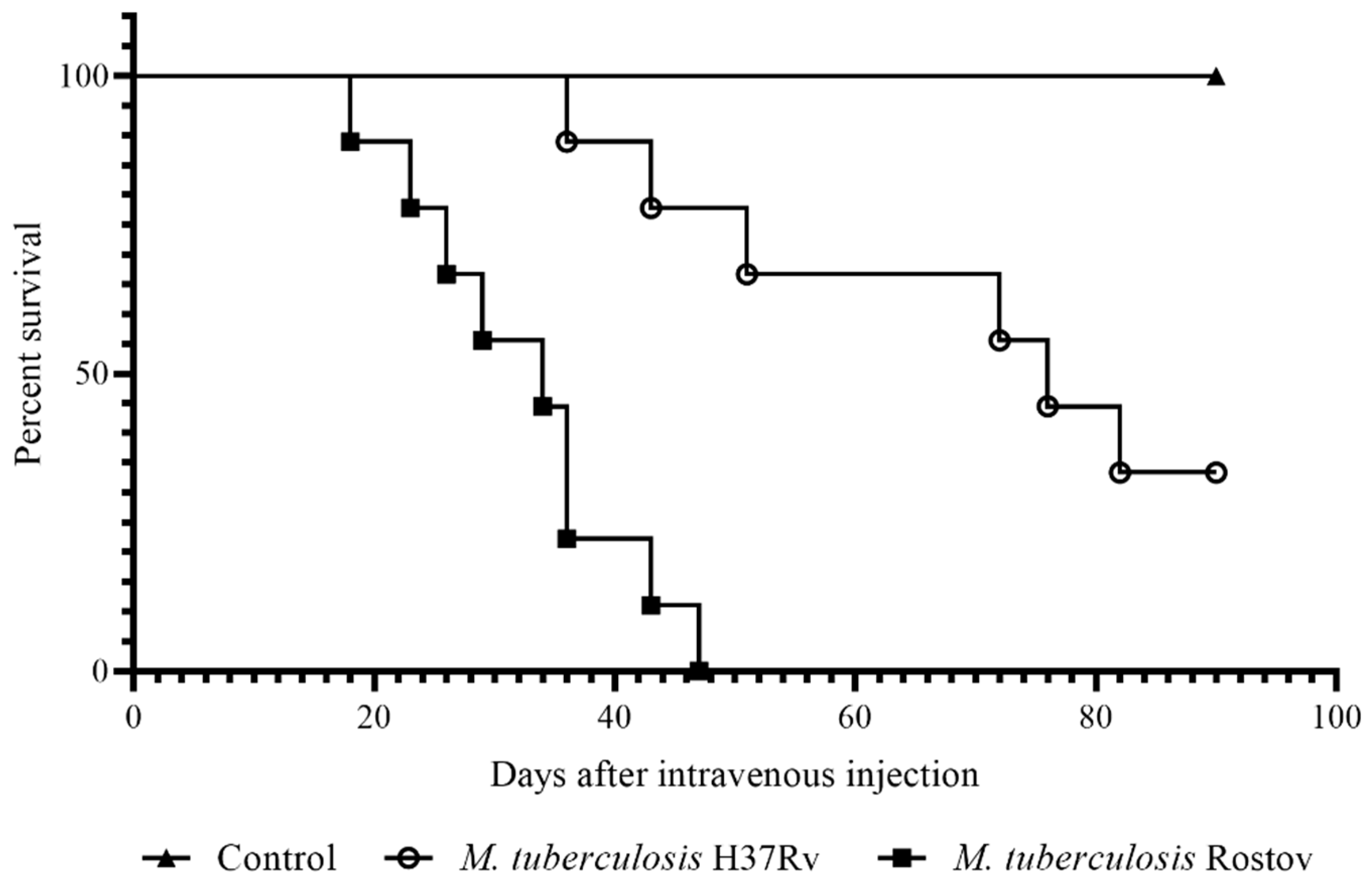
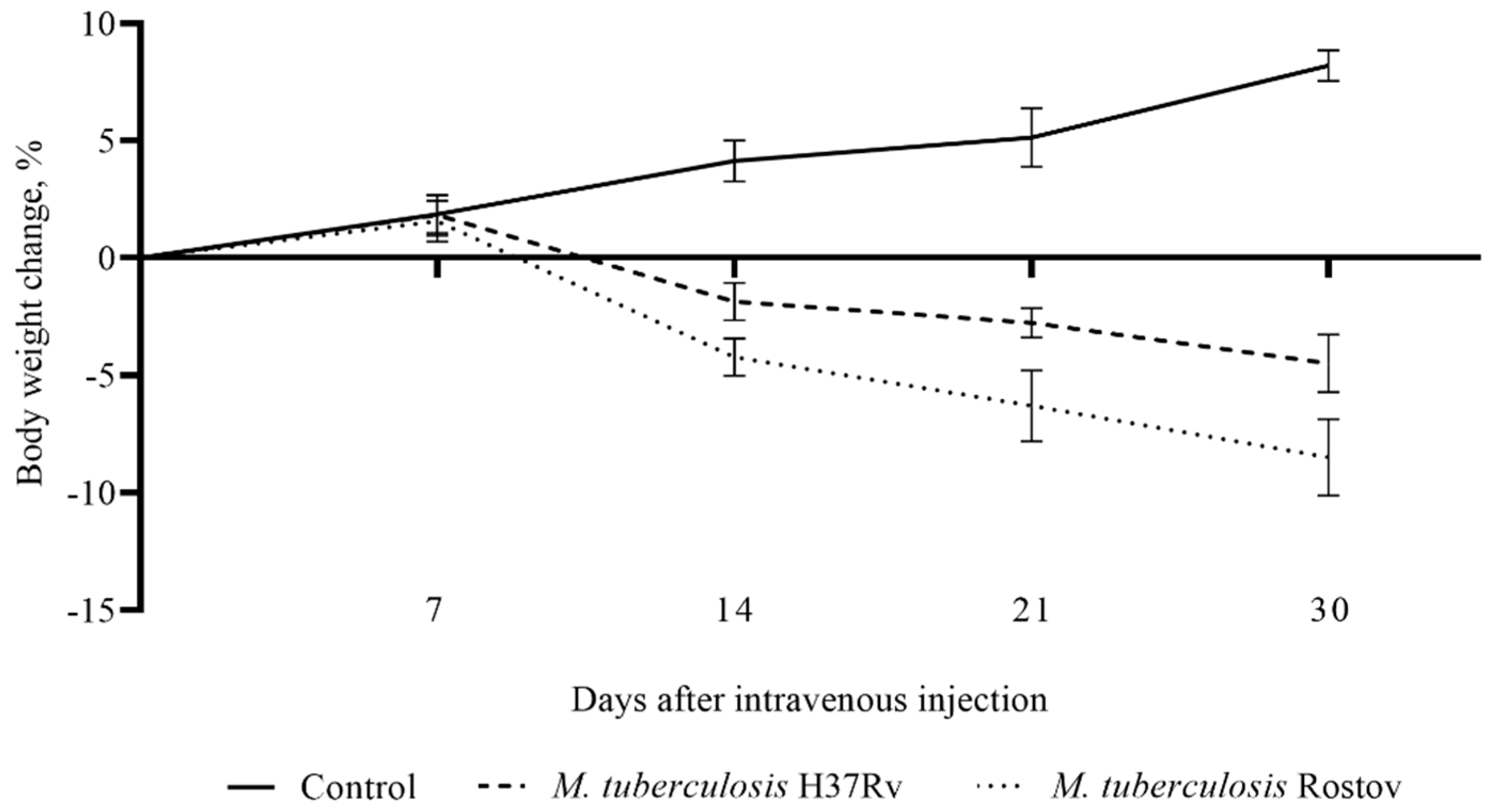
2.2. Mice Survival Rate and Bodyweight Dynamic
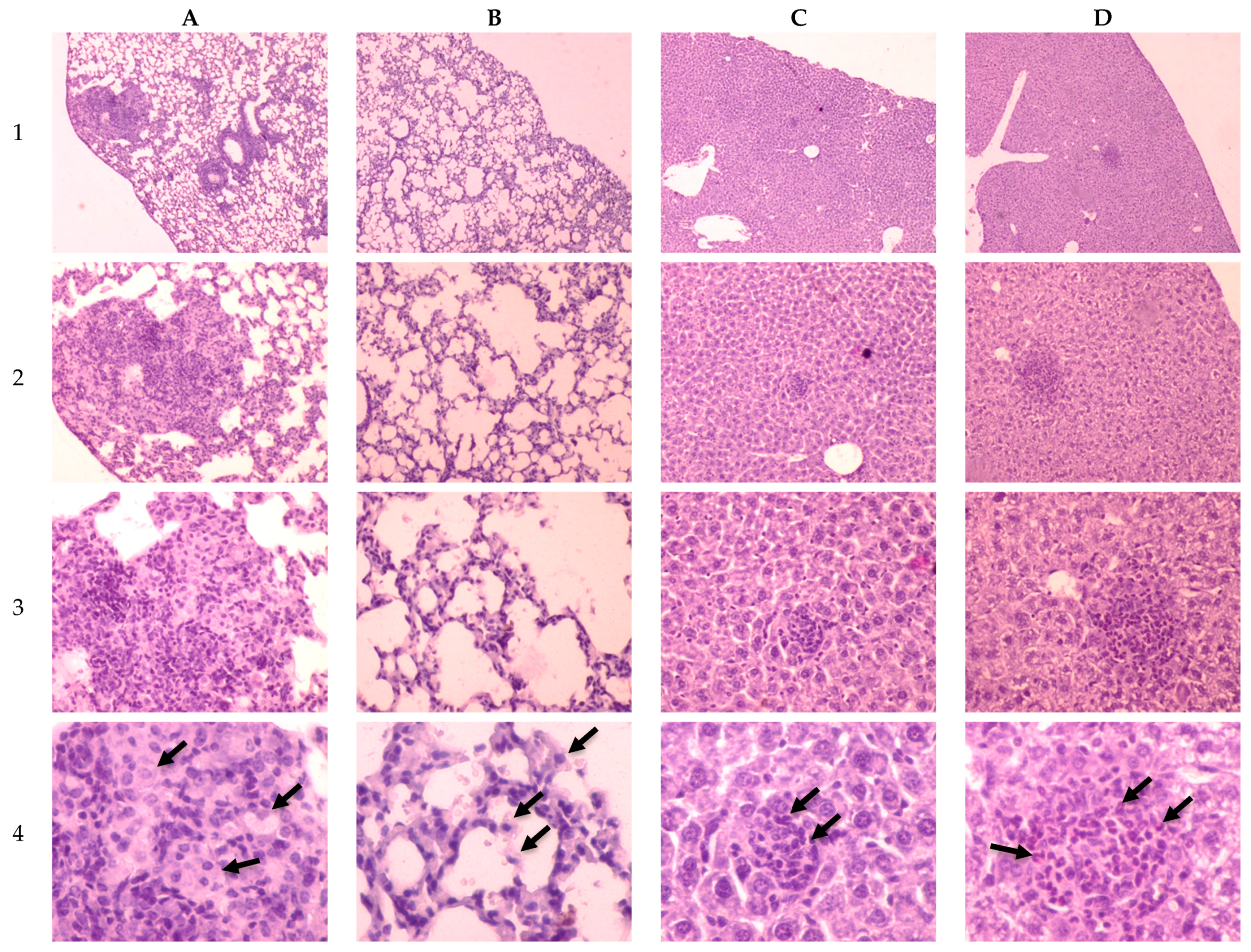
2.3. Investigation of Tuberculosis Process on the 30th Day of Infection
3. Discussion
4. Materials and Methods
4.1. M. tuberculosis Strains
4.2. Antibacterial Susceptibility
4.3. Genomic Analysis
4.4. Bioethical Requirements
4.5. Mice Infection
4.6. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019.
- Comas, I.; Coscolla, M.; Luo, T.; Borrell, S.; Holt, K.E.; Kato-Maeda, M.; Parkhill, J.; Malla, B.; Berg, S.; Thwaites, G.; et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 2013, 45, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Wiens, K.E.; Woyczynski, L.P.; Ledesma, J.R.; Ross, J.M.; Zenteno-Cuevas, R.; Goodridge, A.; Ullah, I.; Mathema, B.; Djoba Siawaya, J.F.; Biehl, M.H.; et al. Global variation in bacterial strains that cause tuberculosis disease: A systematic review and meta-analysis. BMC Med. 2018, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Brudey, K.; Driscoll, J.R.; Rigouts, L.; Prodinger, W.M.; Gori, A.; Al-Hajoj, S.A.; Allix, C.; Aristimuño, L.; Arora, J.; Baumanis, V.; et al. Mycobacterium tuberculosis complex genetic diversity: Mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Parwati, I.; van Crevel, R.; van Soolingen, D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 2010, 10, 103–111. [Google Scholar] [CrossRef]
- Merker, M.; Blin, C.; Mona, S.; Duforet-Frebourg, N.; Lecher, S.; Willery, E.; Blum, M.G.B.; Rüsch-Gerdes, S.; Mokrousov, I.; Aleksic, E.; et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat. Genet. 2015, 47, 242–249. [Google Scholar] [CrossRef]
- Casali, N.; Nikolayevskyy, V.; Balabanova, Y.; Harris, S.R.; Ignatyeva, O.; Kontsevaya, I.; Corander, J.; Bryant, J.; Parkhill, J.; Nejentsev, S.; et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat. Genet. 2014, 46, 279–286. [Google Scholar] [CrossRef]
- Aguilar, D.; Hanekom, M.; Mata, D.; Gey Van Pittius, N.C.; Van Helden, P.D.; Warren, R.M.; Hernandez-Pando, R. Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis 2010, 90, 319–325. [Google Scholar] [CrossRef]
- López, B.; Aguilar, D.; Orozco, H.; Burger, M.; Espitia, C.; Ritacco, V.; Barrera, L.; Kremer, K.; Hernandez-Pando, R.; Huygen, K.; et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 2003, 133, 30–37. [Google Scholar] [CrossRef]
- Li, Q.; Whalen, C.C.; Albert, J.M.; Larkin, R.; Zukowski, L.; Cave, M.D.; Silver, R.F. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 2002, 70, 6489–6493. [Google Scholar] [CrossRef]
- Theus, S.A.; Cave, M.D.; Eisenach, K.D. Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J. Infect. Dis. 2005, 191, 453–460. [Google Scholar] [CrossRef]
- Bifani, P.J.; Mathema, B.; Kurepina, N.E.; Kreiswirth, B.N. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002, 10, 45–52. [Google Scholar] [CrossRef]
- Hanekom, M.; Van Der Spuy, G.D.; Streicher, E.; Ndabambi, S.L.; McEvoy, C.R.E.; Kidd, M.; Beyers, N.; Victor, T.C.; Van Helden, P.D.; Warren, R.M. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J. Clin. Microbiol. 2007, 45, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Mokrousov, I. Insights into the origin, emergence, and current spread of a successful Russian clone of Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2013, 26, 342–360. [Google Scholar] [CrossRef]
- Shitikov, E.; Kolchenko, S.; Mokrousov, I.; Bespyatykh, J.; Ischenko, D.; Ilina, E.; Govorun, V. Evolutionary pathway analysis and unified classification of East Asian lineage of Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, T.; Dong, X.; Sun, G.; Liu, Z.; Gan, M.; Wu, J.; Shen, X.; Gao, Q. Genetic features of Mycobacterium tuberculosis modern Beijing sublineage. Emerg. Microbes Infect. 2016, 5, e14. [Google Scholar] [CrossRef]
- Luo, T.; Comas, I.; Luo, D.; Lu, B.; Wu, J.; Wei, L.; Yang, C.; Liu, Q.; Gan, M.; Sun, G.; et al. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis Beijing family with Han Chinese. Proc. Natl. Acad. Sci. USA 2015, 112, 8136–8141. [Google Scholar] [CrossRef]
- Mokrousov, I.; Chernyaeva, E.; Vyazovaya, A.; Skiba, Y.; Solovieva, N.; Valcheva, V.; Levina, K.; Malakhova, N.; Jiao, W.W.; Gomes, L.L.; et al. Rapid assay for detection of the epidemiologically important Central Asian/Russian strain of the Mycobacterium tuberculosis Beijing genotype. J. Clin. Microbiol. 2018, 56, e01551-17. [Google Scholar] [CrossRef]
- Narvskaya, O.; Mokrousov, I.; Otten, T.; Vishnevskiy, B. Molecular markers: Application for studies of Mycobacterium tuberculosis population in Russia. In Trends in DNA Fingerprinting Research; Read, M.M., Ed.; Nova Science Publishers: New York, NY, USA, 2005. [Google Scholar]
- Mokrousov, I.; Narvskaya, O.; Vyazovaya, A.; Millet, J.; Otten, T.; Vishnevsky, B.; Rastogi, N. Mycobacterium tuberculosis Beijing genotype in Russia: In search of informative variable-number tandem-repeat loci. J. Clin. Microbiol. 2008, 46, 3576–3584. [Google Scholar] [CrossRef]
- Merker, M.; Barbier, M.; Cox, H.; Rasigade, J.P.; Feuerriegel, S.; Kohl, T.A.; Diel, R.; Borrell, S.; Gagneux, S.; Nikolayevskyy, V.; et al. Compensatory evolution drives multidrug-resistant tuberculosis in central Asia. Elife 2018, 7, e38200. [Google Scholar] [CrossRef]
- Engström, A.; Antonenka, U.; Kadyrov, A.; Kalmambetova, G.; Kranzer, K.; Merker, M.; Kabirov, O.; Parpieva, N.; Rajabov, A.; Sahalchyk, E.; et al. Population structure of drug-resistant Mycobacterium tuberculosis in Central Asia. BMC Infect. Dis. 2019, 19, 908. [Google Scholar] [CrossRef]
- Skiba, Y.; Mokrousov, I.; Ismagulova, G.; Maltseva, E.; Yurkevich, N.; Bismilda, V.; Chingissova, L.; Abildaev, T.; Aitkhozhina, N. Molecular snapshot of Mycobacterium tuberculosis population in Kazakhstan: A country-wide study. Tuberculosis 2015, 95, 538–546. [Google Scholar] [CrossRef]
- Song, T.; Park, Y.; Shamputa, I.C.; Seo, S.; Lee, S.Y.; Jeon, H.S.; Choi, H.; Lee, M.; Glynne, R.J.; Barnes, S.W.; et al. Fitness costs of rifampicin resistance in Mycobacterium tuberculosis are amplified under conditions of nutrient starvation and compensated by mutation in the β′ subunit of RNA polymerase. Mol. Microbiol. 2014, 91, 1106–1119. [Google Scholar] [CrossRef]
- Sarkar, R.; Lenders, L.; Wilkinson, K.A.; Wilkinson, R.J.; Nicol, M.P. Modern lineages of Mycobacterium tuberculosis exhibit lineage-specific patterns of growth and cytokine induction in human monocyte-derived macrophages. PLoS ONE 2012, 7, e43170. [Google Scholar] [CrossRef]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; Sabio García, J.; Morbidoni, H.R.; de la Paz Santangelo, M.; Cataldi, A.A.; Bigi, F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G. Amino acid substitution matrices from protein blocks (amino add sequence/alignment algorithms/data base srching). Proc. Natl. Acad. Sci. USA 1992, 89, 10915–10919. [Google Scholar] [CrossRef]
- Tiwari, P.; Arora, G.; Singh, M.; Kidwai, S.; Narayan, O.P.; Singh, R. MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat. Commun. 2015, 6, 6059. [Google Scholar] [CrossRef]
- Ribeiro, S.C.M.; Gomes, L.L.; Amaral, E.P.; Andrade, M.R.M.; Almeida, F.M.; Rezende, A.L.; Lanes, V.R.; Carvalho, E.C.Q.; Suffys, P.N.; Mokrousov, I.; et al. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J. Clin. Microbiol. 2014, 52, 2615–2624. [Google Scholar] [CrossRef]
- Canetti, G.; Froman, S.; Grosset, J.; Hauduroy, P.; Langerova, M.; Mahler, H.T.; Meissner, G.; Mitchison, D.A.; Sula, L. Mycobacteria: Laboratory methods for testing drug sensitivity and resistance. Bull. World Health Organ. 1963, 29, 565–578. [Google Scholar]
- Van Embden, J.D.A.; Cave, M.D.; Crawford, J.T.; Dale, J.W.; Eisenach, K.D.; Gicquel, B.; Hermans, P.; Martin, C.; McAdam, R.; Shinnick, T.M.; et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: Recommendations for a standardized methodology. J. Clin. Microbiol. 1993, 31, 406–409. [Google Scholar] [CrossRef]
- Kamerbeek, J.; Schouls, L.; Kolk, A.; Van Agterveld, M.; Van Soolingen, D.; Kuijper, S.; Bunschoten, A.; Molhuizen, H.; Shaw, R.; Goyal, M.; et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 1997, 35, 907–914. [Google Scholar] [CrossRef]
- Supply, P.; Allix, C.; Lesjean, S.; Cardoso-Oelemann, M.; Rüsch-Gerdes, S.; Willery, E.; Savine, E.; De Haas, P.; Van Deutekom, H.; Roring, S.; et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2006, 44, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Shitikov, E.; Vyazovaya, A.; Malakhova, M.; Guliaev, A.; Bespyatykh, J.; Proshina, E.; Pasechnik, O.; Mokrousov, I. Simple assay for detection of the central Asia outbreak clade of the mycobacterium tuberculosis Beijing genotype. J. Clin. Microbiol. 2019, 57, e00215-19. [Google Scholar] [CrossRef] [PubMed]
- Schleusener, V.; Köser, C.U.; Beckert, P.; Niemann, S.; Feuerriegel, S. Mycobacterium tuberculosis resistance prediction and lineage classification from genome sequencing: Comparison of automated analysis tools. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Antibiotic | MIC, mg/L | Interpretation | Drug Resistance Marker |
|---|---|---|---|
| Isoniazid (INH) | >1 | R | KatG (S315T) |
| Rifampicin (RIF) | >40 | R | RpoB (S450L) |
| Streptomycin (STR) | >10 | R | RpsL (K43R) |
| Ethambutol (EMB) | >5 | R | EmbB (M306V) |
| Amikacin (AMK) | >30 | R | rrs (a1401g) |
| Kanamycin (KAN) | >30 | R | rrs (a1401g) |
| Capreomycin (CAP) | >30 | R | rrs (a1401g) |
| Ofloxacin (OFX) | ≤3 | S | - |
| Strain | The Mortality Rate on 30th p.i. Day, % | Animal Appearance | Lungs | Liver |
|---|---|---|---|---|
| H37Rv | 0 | Mild depletion, smooth fur | 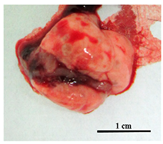 Pale pink colored with pale mass inclusions | 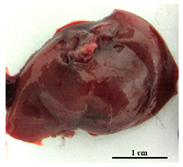 Smooth, intense brown, normal volume |
| Rostov | ~50% | Extreme emaciated, hunched posture, “ruffled” fur, reduced movement | 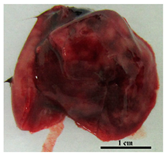 Intensively hyperemic, no visible nodules |  Dark brown with multiple nodules, fatty degeneration |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fursov, M.V.; Shitikov, E.A.; Bespyatykh, J.A.; Bogun, A.G.; Kislichkina, A.A.; Kombarova, T.I.; Rudnitskaya, T.I.; Grishenko, N.S.; Ganina, E.A.; Domotenko, L.V.; et al. Genotyping, Assessment of Virulence and Antibacterial Resistance of the Rostov Strain of Mycobacterium tuberculosis Attributed to the Central Asia Outbreak Clade. Pathogens 2020, 9, 335. https://doi.org/10.3390/pathogens9050335
Fursov MV, Shitikov EA, Bespyatykh JA, Bogun AG, Kislichkina AA, Kombarova TI, Rudnitskaya TI, Grishenko NS, Ganina EA, Domotenko LV, et al. Genotyping, Assessment of Virulence and Antibacterial Resistance of the Rostov Strain of Mycobacterium tuberculosis Attributed to the Central Asia Outbreak Clade. Pathogens. 2020; 9(5):335. https://doi.org/10.3390/pathogens9050335
Chicago/Turabian StyleFursov, Mikhail V., Egor A. Shitikov, Julia A. Bespyatykh, Alexander G. Bogun, Angelina A. Kislichkina, Tatiana I. Kombarova, Tatiana I. Rudnitskaya, Natalia S. Grishenko, Elena A. Ganina, Lubov V. Domotenko, and et al. 2020. "Genotyping, Assessment of Virulence and Antibacterial Resistance of the Rostov Strain of Mycobacterium tuberculosis Attributed to the Central Asia Outbreak Clade" Pathogens 9, no. 5: 335. https://doi.org/10.3390/pathogens9050335
APA StyleFursov, M. V., Shitikov, E. A., Bespyatykh, J. A., Bogun, A. G., Kislichkina, A. A., Kombarova, T. I., Rudnitskaya, T. I., Grishenko, N. S., Ganina, E. A., Domotenko, L. V., Fursova, N. K., Potapov, V. D., & Dyatlov, I. A. (2020). Genotyping, Assessment of Virulence and Antibacterial Resistance of the Rostov Strain of Mycobacterium tuberculosis Attributed to the Central Asia Outbreak Clade. Pathogens, 9(5), 335. https://doi.org/10.3390/pathogens9050335







