Listeria monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics
Abstract
1. Introduction
2. Results
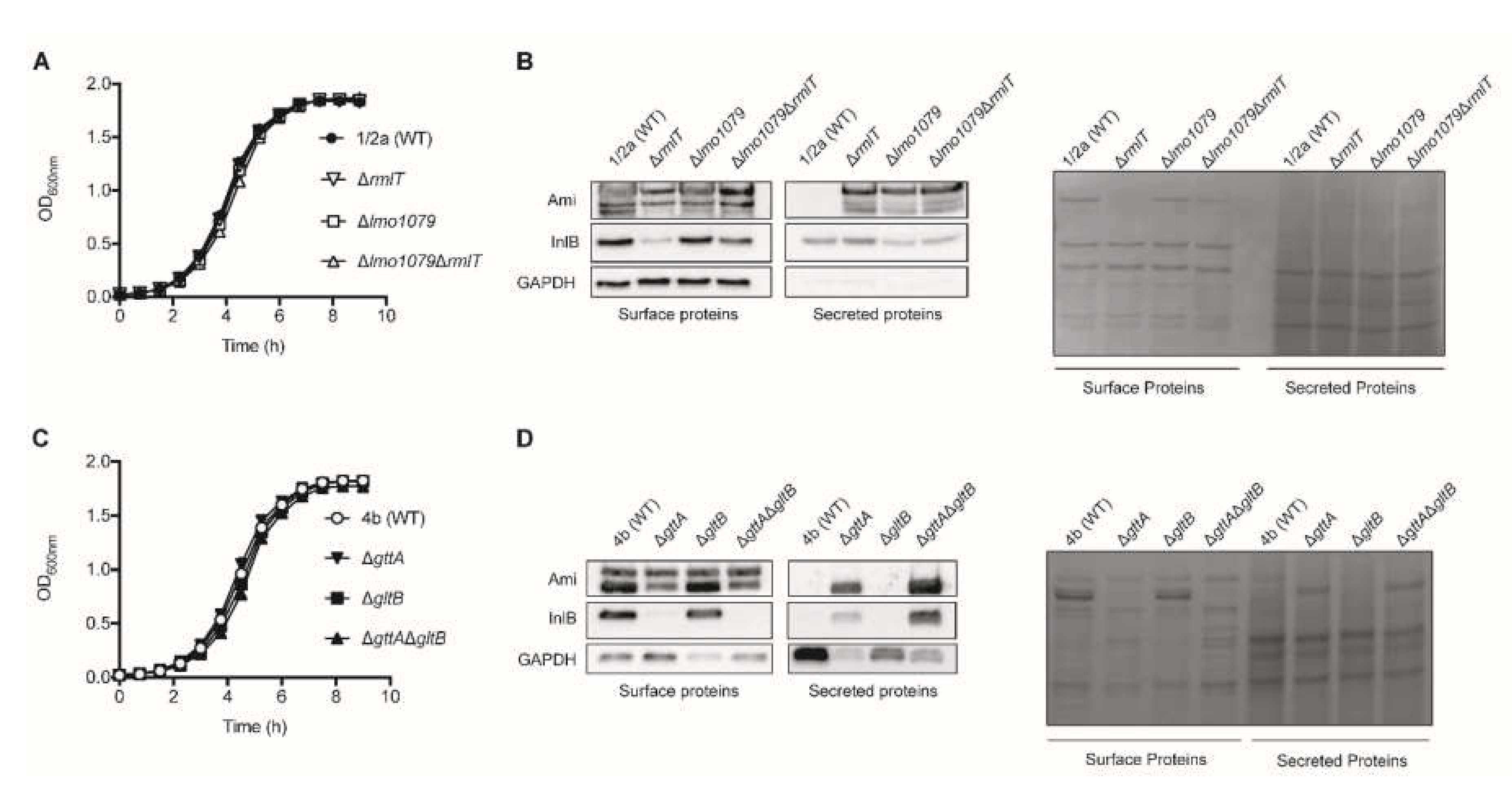
2.1. WTA-glycosylation Promotes Efficient Surface Association of Lm Virulence Factors
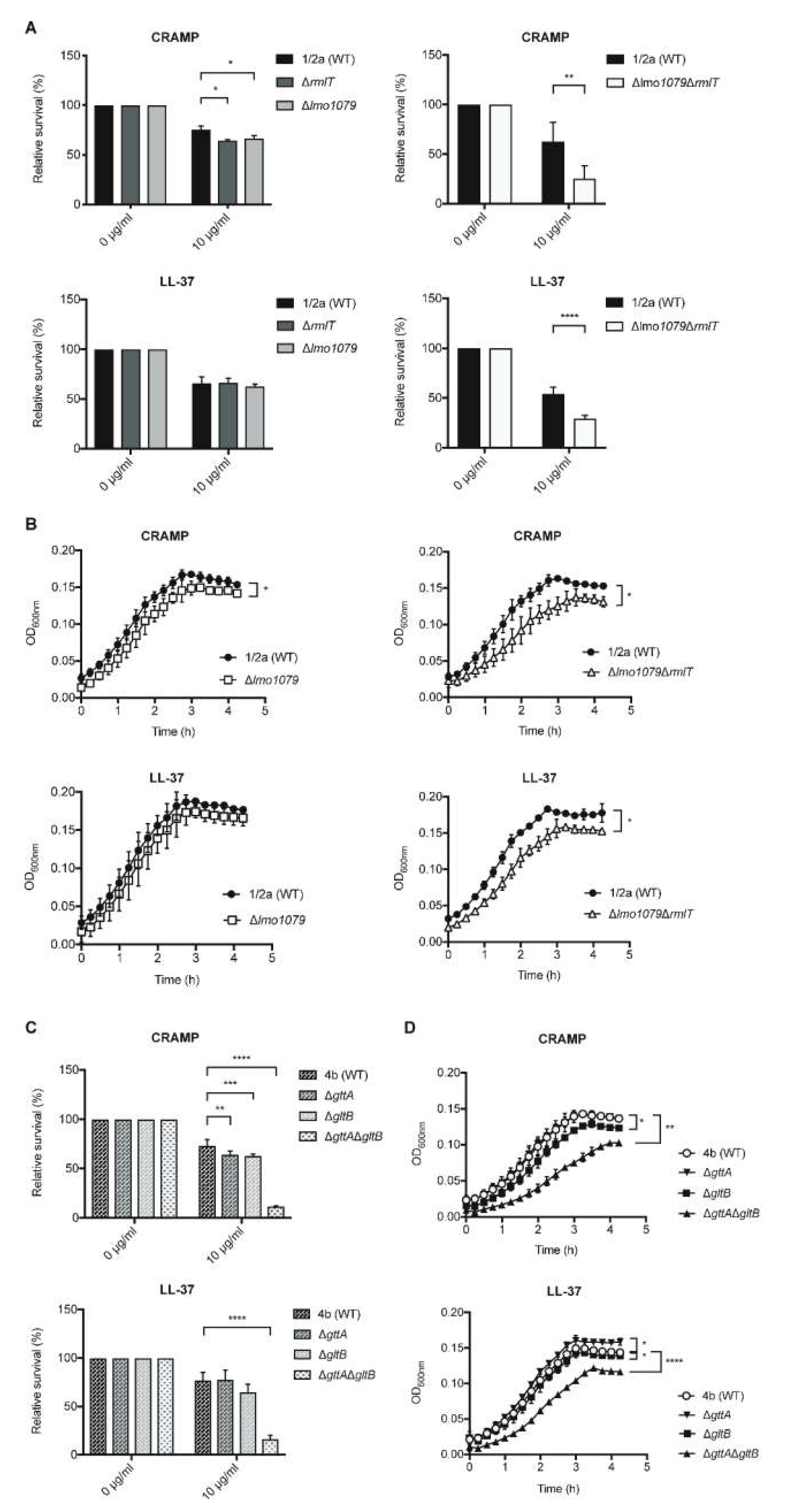
2.2. WTA-glycosylation Promotes Lm Resistance to AMPs
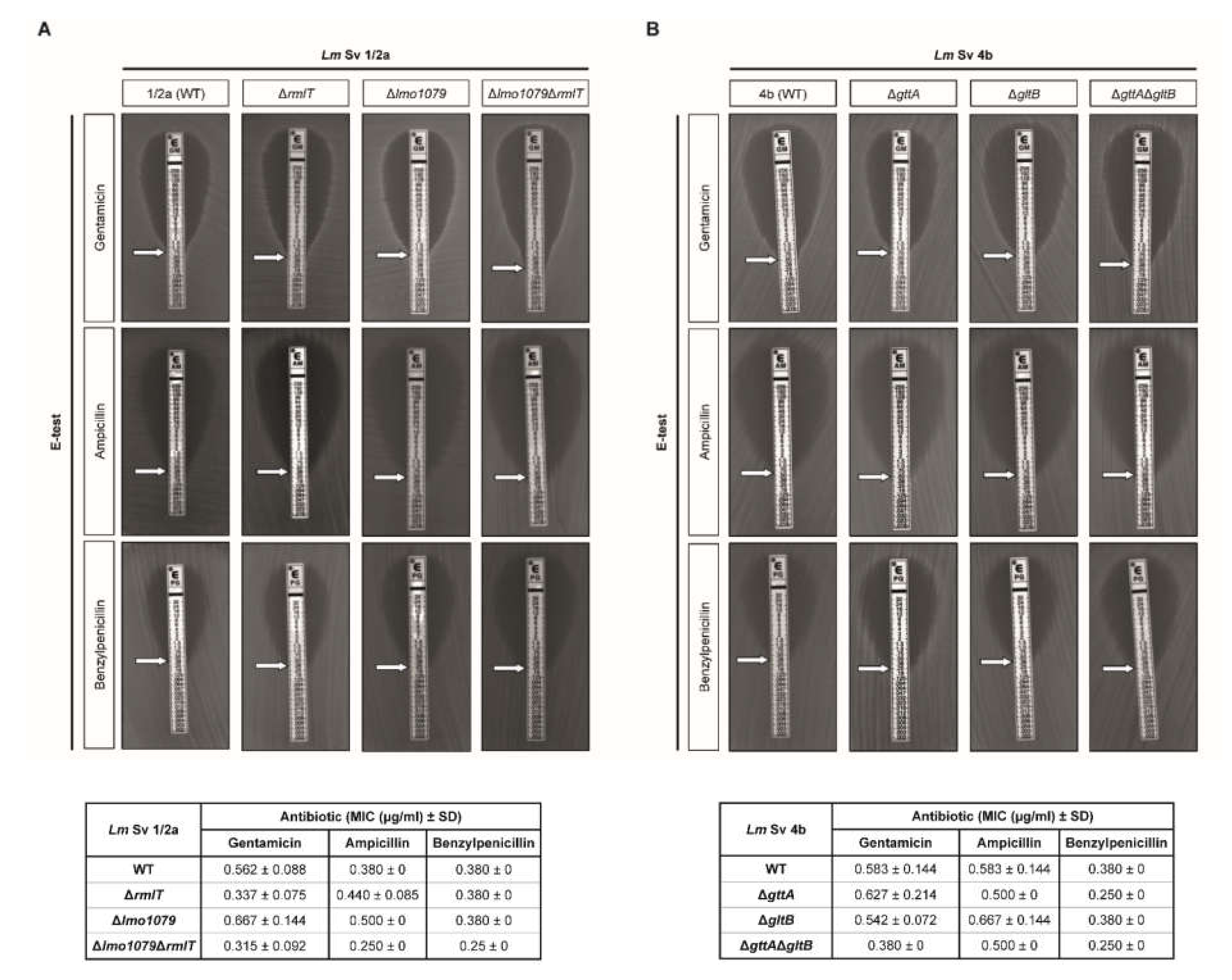
2.3. WTA-glycosylation Promotes Lm Decreased Susceptibility to Some Antibiotics
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Construction of Deletion Mutant Strains
| Bacterial Strains and Plasmid | Lab Code | Relevant Characteristics | Source |
|---|---|---|---|
| L. monocytogenes | |||
| EGD-e | DC 4 | Wild-type; Sv 1/2a | [41] |
| EGD-e ∆rmlT | DC492 | EGD-e rmlT (lmo1080) deletion mutant | [17] |
| EGD-e ∆lmo1079 | DC 858 | EGD-e lmo1079 deletion mutant | This study |
| EGD-e ∆lmo1079∆rmlT | DC 899 | EGD-e lmo1079-lmo1080 deletion mutant | This study |
| WSLC 1042 WT | DC 825 | Wild-type; Sv 4b | ATCC®23074 |
| WSLC 1042 ∆gttA | DC 826 | WSLC 1042 gttA deletion mutant | [23] |
| WSLC 1042 ∆gltB | DC 827 | WSLC 1042 gltB deletion mutant | [23] |
| WSLC 1042 ∆gttA∆gltB | DC 828 | WSLC 1042 gttAgltB deletion mutant | [23] |
| E. coli | |||
| DH5α | Competent cells | Life Technologies | |
| Plasmid | |||
| pMAD | DC 48 | Ampr and Eryr | [39] |
| Primers | Sequence (5’ → 3’) * | Restriction Enzymes | |
|---|---|---|---|
| 1 | lmo1079 UP Fw | AGTCGGATCCGGAGCATCTTCTACATTAGGC | BamHI |
| 2 | lmo1079 UP Rv | AGTCGTCGACCCATTAACTTTCTCCCTCC | SalI |
| 3 | lmo1079 DW Fw | AGTCGTCGACTAAATGAGGGAAAACGTTAGG | SalI |
| 4 | lmo1079 DW Rv | AGTCCCATGGCACCGTGAATGAACGCC | NcoI |
| 5 | lmo1080 DW Fw | CGGGTCGACTAAGAATGGAGAGAAAAGAATGAAAGG | SalI |
| 6 | lmo1080 DW Rv | CGGCCATGGGGAATGCTTTTTCATTATAGC | NcoI |
| Internal Primers | |||
| 7 | lmo1079 Fw | GCAAATTGGAATGGGAGGCG | |
| 8 | lmo1079 Rv | GGATGCCTTGTTGCCGAAAC | |
| 9 | lmo1080 Fw | TATTGCCACACGCTTTACCG | |
| 10 | lmo1080 Rv | CTTCCACGATTGAACGAACG | |
| 11 | lmo1492 Fw | GACGGATCCCGCAACTTCGCAAAATGGG | |
| 12 | lmo1492 Rv | AGCGTCGACGTCGCCATACCATCTGTTTG | |
| pMAD Primers | |||
| 13 | pMAD Fw | TGATGGTCGTCATCTACCTGCC | |
| 14 | pMAD Rv | CCTACGTAGGATCGATCCGACC | |
4.3. Growth Analysis in Vitro
4.4. Extracts of Lm Proteins
4.5. SDS-PAGE and Western Blot Analysis of Protein Extracts
4.6. Antimicrobial Peptides Susceptibility
4.7. Antibiotic Susceptibility
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cossart, P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- Carvalho, F.; Sousa, S.; Cabanes, D. How Listeria monocytogenes organizes its surface for virulence. Front. Cell. Infect. Microbiol. 2014, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.G.; Grundling, A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Fiedler, F. Biochemistry of the cell surface of Listeria strains: A locating general view. Infection 1988, 16 (Suppl. 2), S92–S97. [Google Scholar] [CrossRef]
- Uchikawa, K.; Sekikawa, I.; Azuma, I. Structural studies on lipoteichoic acids from four Listeria strains. J. Bacteriol. 1986, 168, 115–122. [Google Scholar] [CrossRef]
- Autret, N.; Dubail, I.; Trieu-Cuot, P.; Berche, P.; Charbit, A. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 2001, 69, 2054–2065. [Google Scholar] [CrossRef]
- Eugster, M.R.; Haug, M.C.; Huwiler, S.G.; Loessner, M.J. The cell wall binding domain of Listeria bacteriophage endolysin PlyP35 recognizes terminal GlcNAc residues in cell wall teichoic acid. Mol. Microbiol. 2011, 81, 1419–1432. [Google Scholar] [CrossRef]
- Faith, N.; Kathariou, S.; Cheng, Y.; Promadej, N.; Neudeck, B.L.; Zhang, Q.; Luchansky, J.; Czuprynski, C. The role of L. monocytogenes serotype 4b gtcA in gastrointestinal listeriosis in A/J mice. Foodborne Pathog. Dis. 2009, 6, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Promadej, N.; Fiedler, F.; Cossart, P.; Dramsi, S.; Kathariou, S. Cell wall teichoic acid glycosylation in Listeria monocytogenes serotype 4b requires gtcA, a novel, serogroup-specific gene. J. Bacteriol. 1999, 181, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Spears, P.A.; Havell, E.A.; Hamrick, T.S.; Goforth, J.B.; Levine, A.L.; Abraham, S.T.; Heiss, C.; Azadi, P.; Orndorff, P.E. Listeria monocytogenes wall teichoic acid decoration in virulence and cell-to-cell spread. Mol. Microbiol. 2016, 101, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Promadej, N.; Kim, J.W.; Kathariou, S. Teichoic acid glycosylation mediated by gtcA is required for phage adsorption and susceptibility of Listeria monocytogenes serotype 4b. Appl. Environ. Microbiol. 2008, 74, 1653–1655. [Google Scholar] [CrossRef]
- Eugster, M.R.; Morax, L.S.; Huls, V.J.; Huwiler, S.G.; Leclercq, A.; Lecuit, M.; Loessner, M.J. Bacteriophage predation promotes serovar diversification in Listeria monocytogenes. Mol. Microbiol. 2015, 97, 33–46. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Carvalho, F.; Atilano, M.L.; Pombinho, R.; Covas, G.; Gallo, R.L.; Filipe, S.R.; Sousa, S.; Cabanes, D. L-Rhamnosylation of Listeria monocytogenes Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane. PLoS Pathog. 2015, 11, e1004919. [Google Scholar] [CrossRef]
- Braun, L.; Dramsi, S.; Dehoux, P.; Bierne, H.; Lindahl, G.; Cossart, P. InlB: An invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 1997, 25, 285–294. [Google Scholar] [CrossRef]
- Milohanic, E.; Jonquières, R.; Cossart, P.; Berche, P.; Gaillard, J.L. The Autolysin Ami contributes to the adhesion of Listeria monocytogenes to eucaryotic cells, via its cell wall anchor. Mol. Microbiol. 2001, 39, 1212–1225. [Google Scholar] [CrossRef]
- Jonquieres, R.; Bierne, H.; Fiedler, F.; Gounon, P.; Cossart, P. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: A novel mechanism of protein association at the surface of gram-positive bacteria. Mol. Microbiol. 1999, 34, 902–914. [Google Scholar] [CrossRef]
- Percy, M.G.; Karinou, E.; Webb, A.J.; Grundling, A. Identification of a Lipoteichoic Acid Glycosyltransferase Enzyme Reveals that GW-Domain-Containing Proteins Can Be Retained in the Cell Wall of Listeria monocytogenes in the Absence of Lipoteichoic Acid or Its Modifications. J. Bacteriol. 2016, 198, 2029–2042. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Sousa, S.; Cabanes, D. l-Rhamnosylation of wall teichoic acids promotes efficient surface association of Listeria monocytogenes virulence factors InlB and Ami through interaction with GW domains. Environ. Microbiol. 2018, 20, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Sumrall, E.T.; Shen, Y.; Keller, A.P.; Rismondo, J.; Pavlou, M.; Eugster, M.R.; Boulos, S.; Disson, O.; Thouvenot, P.; Kilcher, S.; et al. Phage resistance at the cost of virulence: Listeria monocytogenes serovar 4b requires galactosylated teichoic acids for InlB-mediated invasion. PLoS Pathog. 2019, 15, e1008032. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Xia, G.; Luhachack, L.G.; Campbell, J.; Meredith, T.C.; Chen, C.; Winstel, V.; Gekeler, C.; Irazoqui, J.E.; Peschel, A.; et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 18909–18914. [Google Scholar] [CrossRef]
- Dortet, L.; Veiga-Chacon, E.; Cossart, P. Listeria monocytogenes. In Encyclopedia of Microbiolog; Schaechter, M., Schaechter, M., Eds.; Institut Pasteur: Paris, France, 2009; pp. 182–198. [Google Scholar]
- Yin, Y.; Yao, H.; Doijad, S.; Kong, S.; Shen, Y.; Cai, X.; Tan, W.; Wang, Y.; Feng, Y.; Ling, Z.; et al. A hybrid sub-lineage of Listeria monocytogenes comprising hypervirulent isolates. Nat. Commun. 2019, 10, 4283. [Google Scholar] [CrossRef]
- Cederlund, A.; Gudmundsson, G.H.; Agerberth, B. Antimicrobial peptides important in innate immunity. FEBS J. 2011, 278, 3942–3951. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Farha, M.A.; Leung, A.; Sewell, E.W.; D’Elia, M.A.; Allison, S.E.; Ejim, L.; Pereira, P.M.; Pinho, M.G.; Wright, G.D.; Brown, E.D. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to beta-lactams. ACS Chem. Biol. 2013, 8, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Golemi-Kotra, D. Dual roles of FmtA in Staphylococcus aureus cell wall biosynthesis and autolysis. Antimicrob. Agents Chemother. 2012, 56, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Bielmann, R.; Habann, M.; Eugster, M.R.; Lurz, R.; Calendar, R.; Klumpp, J.; Loessner, M.J. Receptor binding proteins of Listeria monocytogenes bacteriophages A118 and P35 recognize serovar-specific teichoic acids. Virology 2015, 477, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Hupfeld, M.; Klumpp, J.; Loessner, M.J. Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses 2018, 10, 397. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr Rev Food Sci Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Lipsitch, M.; Samore, M.H. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 2002, 8, 347–354. [Google Scholar] [CrossRef]
- Allen, R.C.; Popat, R.; Diggle, S.P.; Brown, S.P. Targeting virulence: Can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014, 12, 300–308. [Google Scholar] [CrossRef]
- Arnaud, M.; Chastanet, A.; Debarbouille, M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 2004, 70, 6887–6891. [Google Scholar] [CrossRef]
- Pombinho, R.; Camejo, A.; Vieira, A.; Reis, O.; Carvalho, F.; Almeida, M.T.; Pinheiro, J.C.; Sousa, S.; Cabanes, D. Listeria monocytogenes CadC regulates cadmium efflux and fine-tunes lipoprotein localization to escape the host immune response and promote infection. J. Infect. Dis. 2017, 215, 1468–1479. [Google Scholar] [CrossRef]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative genomics of Listeria species. Science 2001, 294, 849–852. [Google Scholar] [CrossRef]
- Braun, L.; Nato, F.; Payrastre, B.; Mazie, J.C.; Cossart, P. The 213-amino-acid leucine-rich repeat region of the Listeria monocytogenes InlB protein is sufficient for entry into mammalian cells, stimulation of PI 3-kinase and membrane ruffling. Mol. Microbiol. 1999, 34, 10–23. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | Strip Concentration (μg/mL) |
|---|---|
| Gentamicin Ampicillin Benzylpenicillin | 0.016-256 0.016-256 0.002-32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meireles, D.; Pombinho, R.; Carvalho, F.; Sousa, S.; Cabanes, D. Listeria monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics. Pathogens 2020, 9, 290. https://doi.org/10.3390/pathogens9040290
Meireles D, Pombinho R, Carvalho F, Sousa S, Cabanes D. Listeria monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics. Pathogens. 2020; 9(4):290. https://doi.org/10.3390/pathogens9040290
Chicago/Turabian StyleMeireles, Diana, Rita Pombinho, Filipe Carvalho, Sandra Sousa, and Didier Cabanes. 2020. "Listeria monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics" Pathogens 9, no. 4: 290. https://doi.org/10.3390/pathogens9040290
APA StyleMeireles, D., Pombinho, R., Carvalho, F., Sousa, S., & Cabanes, D. (2020). Listeria monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics. Pathogens, 9(4), 290. https://doi.org/10.3390/pathogens9040290






