Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species
Abstract
1. Introduction
2. Results
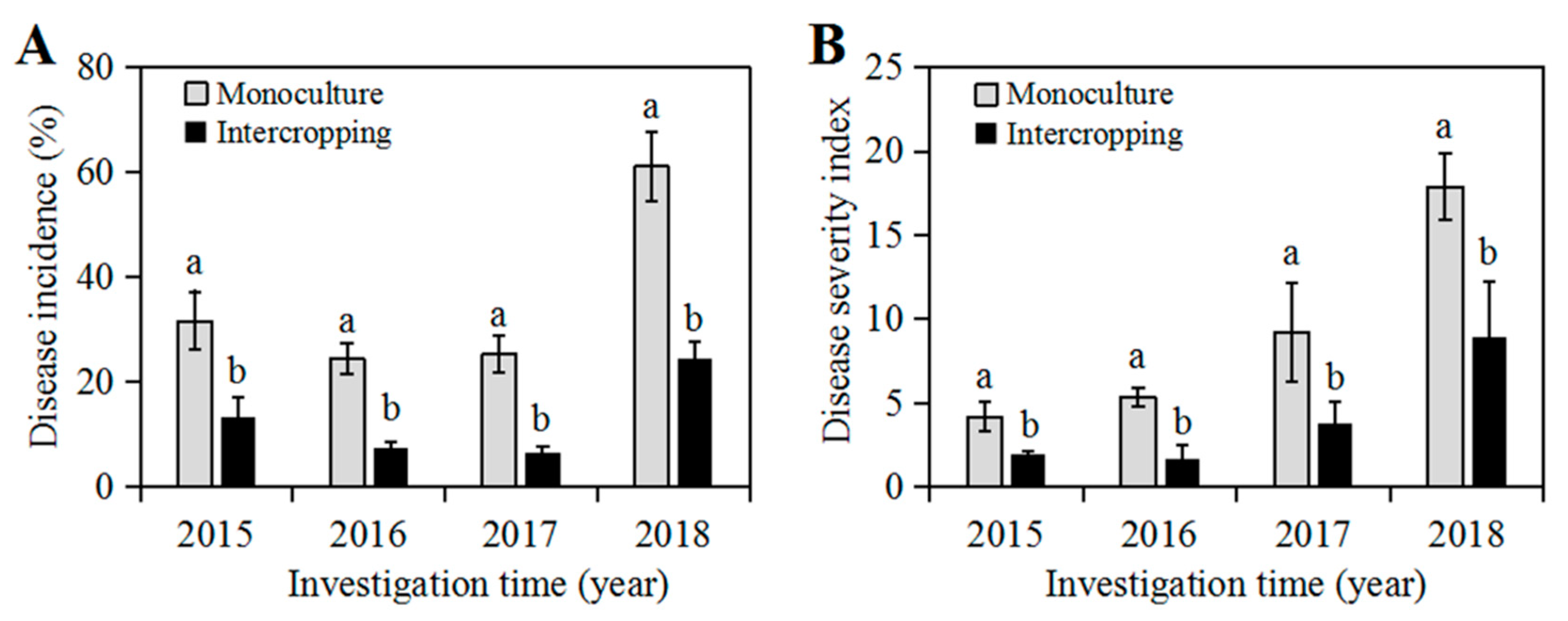
2.1. Occurrence of Soybean Root Rot in Soybean Monoculture and Maize/Soybean Relay Strip Intercropping
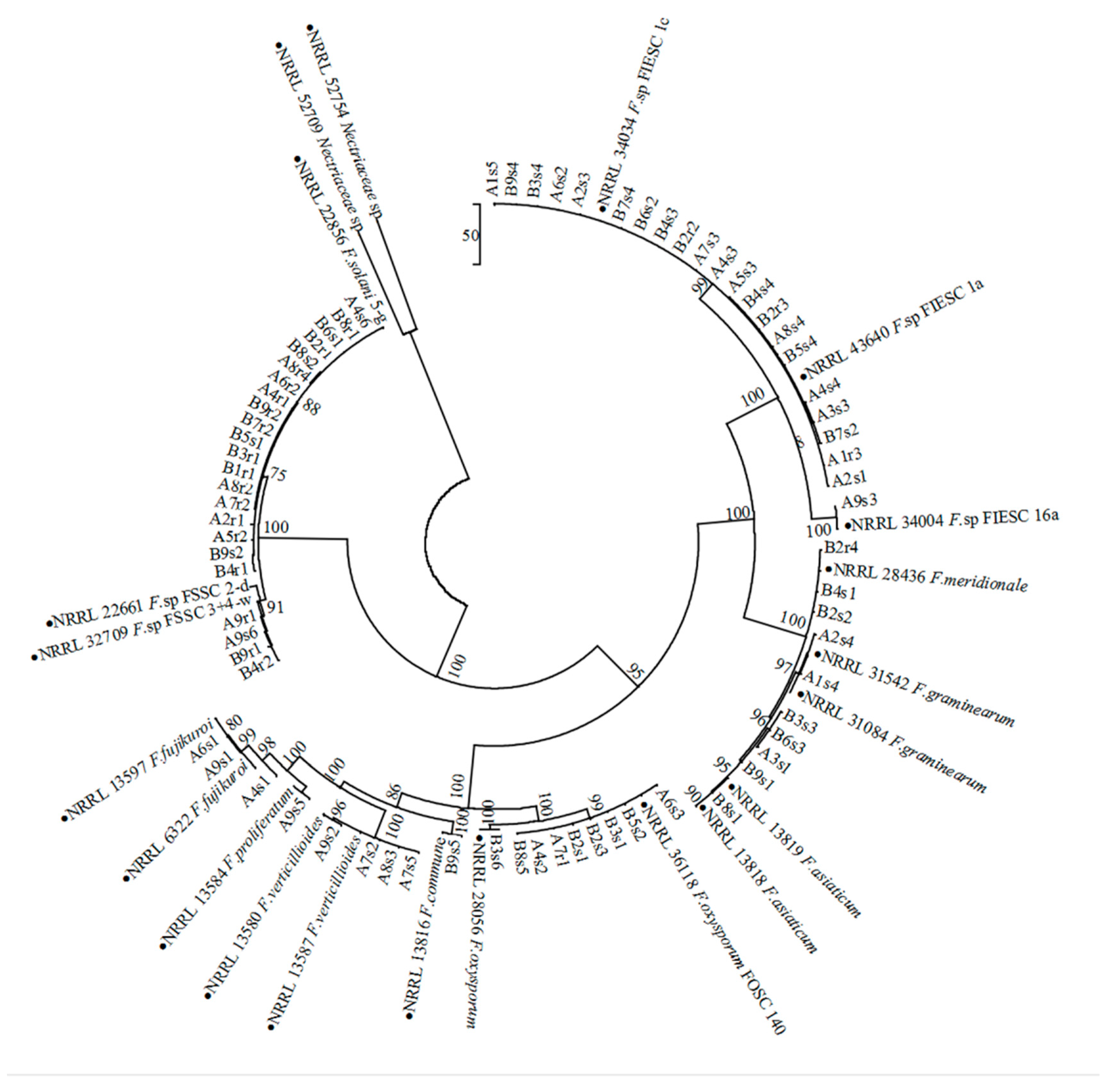
2.2. Identification of Fusarium Species from Soybean Monoculture and Maize/Soybean Relay Strip Intercropping
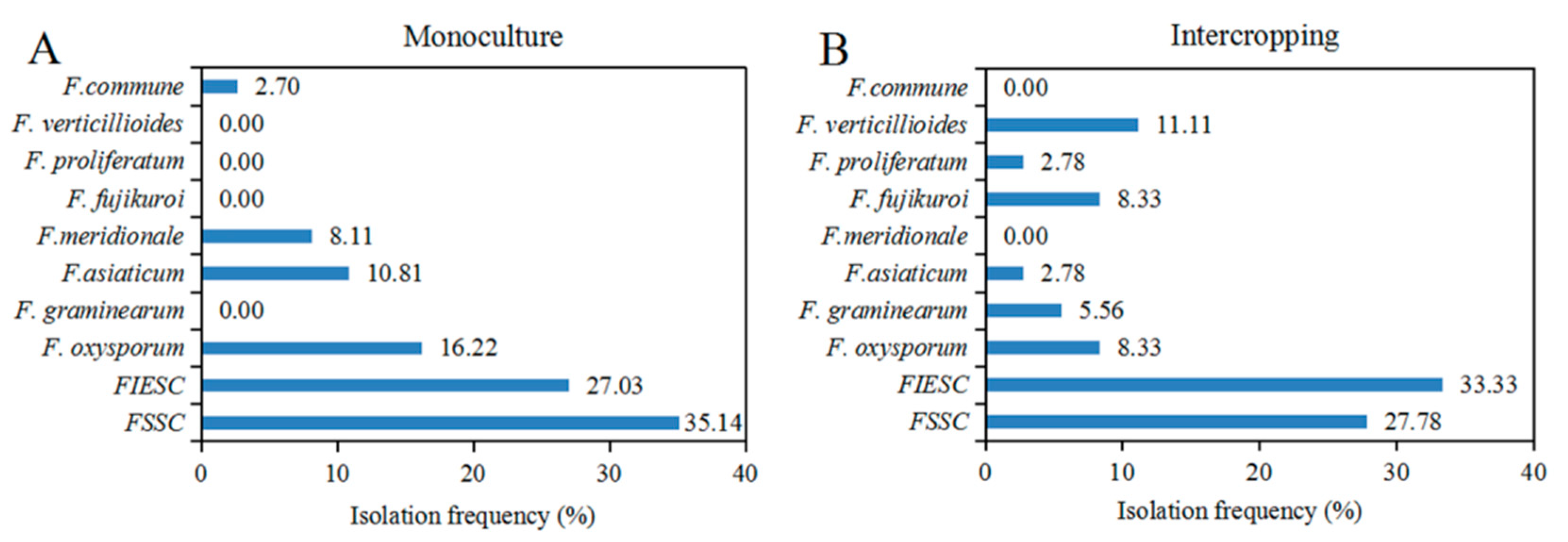
2.3. Diversity and Isolation Frequency of Fusarium Species from Monoculture and Intercropping
2.4. Pathogenicity of Fusarium Species from Monoculture and Intercropping
3. Discussion
4. Materials and Methods
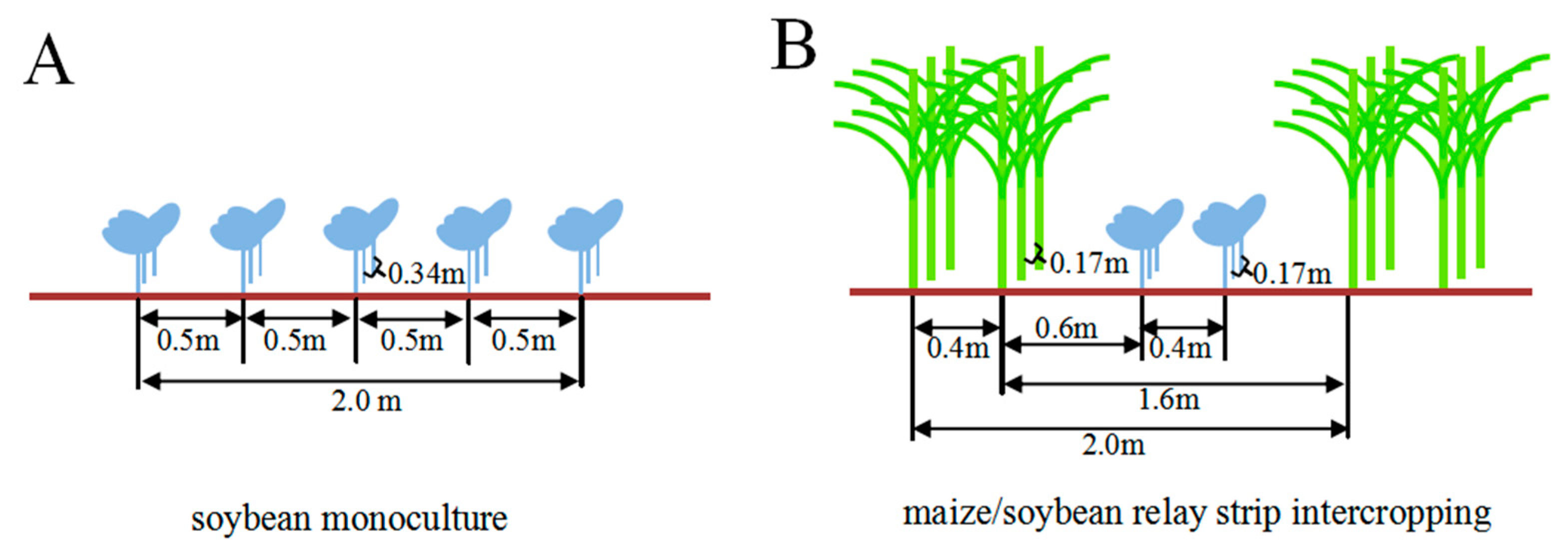
4.1. Field Experiments
4.2. Investigation and Sampling of Soybean Root Rot
4.3. Isolation of Fusarium Species
4.4. PCR Amplification and Phylogenetic Analysis
4.5. Pathogenicity Tests of Fusarium Species
4.6. Statistic Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díaz-Arias, M.M.; Leandro, L.F.; Munkvold, G.P. Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology 2013, 103, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Broders, K.D.; Lipps, P.E.; Paul, P.A.; Dorrance, A.E. Evaluation of Fusarium graminearum associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Arias, M.M.; Munkvold, G.P.; Leandro, L.F. First report of Fusarium proliferatum causing root rot on soybean (Glycine max) in the United States. Plant Dis. 2011, 95, 1316. [Google Scholar] [CrossRef] [PubMed]
- Díaz Arias, M.M.; Munkvold, G.P.; Ellis, M.L.; Leandro, L.F.S. Distribution and frequency of Fusarium species associated with soybean roots in Iowa. Plant Dis. 2013, 97, 1557–1562. [Google Scholar] [CrossRef]
- Barros, G.G.; Alaniz Zanon, M.S.; Chiotta, M.L.; Reynoso, M.M.; Scandiani, M.M.; Chulze, S.N. Pathogenicity of phylogenetic species in the Fusarium graminearum complex on soybean seedlings in Argentina. Eur. J. Plant Pathol. 2014, 138, 215–222. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Conner, R.L.; Ahmed, H.U.; Zhou, Q.; Turnbull, G.D.; Strelkov, S.E.; McLaren, D.L.; Gossen, B.D. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop Prot. 2015, 67, 52–58. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, N.; Chang, K.F.; Hwang, S.F.; Strelkov, S.E.; Conner, R.L.; McLaren, D.L.; Fu, H.; Harding, M.W.; Turnbull, G.D. Genetic diversity and aggressiveness of Fusarium species isolated from soybean in Alberta, Canada. Crop Prot. 2018, 105, 49–58. [Google Scholar] [CrossRef]
- Nelson, B.D.; Hansen, J.M.; Windels, C.E.; Helms, T.C. Reaction of soybean cultivars to isolates of Fusarium solani from the Red River Valley. Plant Dis. 1997, 81, 664–668. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Scandiani, M.M.; Luque, A.; Colletto, A.; Biasoli, M.; Lenzi, M.L.; Salas, G.; González, V.; Ploper, L.D.; et al. Soybean sudden death syndrome species diversity within North and South America revealed by multilocus genotyping. Phytopathology 2010, 100, 58–71. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xue, A.G.; Cober, E.R.; Morrison, M.J.; Zhang, H.J.; Zhang, S.Z.; Gregorich, E. Prevalence, pathogenicity and cultivar resistance of Fusarium and Rhizoctonia species causing soybean root rot. Can. J. Plant Sci. 2013, 93, 221–236. [Google Scholar] [CrossRef]
- Chang, K.F.; Conner, R.L.; Hwang, S.F.; Ahmed, H.U.; McLaren, D.L.; Gossen, B.D.; Turnbull, G.D. Effects of seed treatments and inoculum density of Fusarium avenaceum and Rhizoctonia solani on seedling blight and root rot of faba bean. Can. J. Plant Sci. 2014, 94, 693–700. [Google Scholar] [CrossRef]
- Liu, L.; Kloepper, J.W.; Tuzun, S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting Rhizobacteria. Phytopathology 1995, 85, 695–698. [Google Scholar] [CrossRef]
- Wang, L.Y.; Xie, Y.S.; Cui, Y.Y.; Xu, J.; He, W.; Chen, H.G.; Guo, J.H. Conjunctively screening of biocontrol agents (BCAs) against fusarium root rot and fusarium head blight caused by Fusarium graminearum. Microbiol. Res. 2015, 177, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, M.; Xu, R.; Wang, X.; Pan, R.; Kim, H.J.; Liao, H. Root interactions in a maize/soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS ONE 2014, 9, e95031. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Cao, H.; Nawaz, M.A.; Sohail, H.; Huang, Y.; Cheng, F.; Kong, Q.; Bie, Z. Wheat intercropping enhances the resistance of watermelon to Fusarium wilt. Front. Plant Sci. 2018, 9, 696. [Google Scholar] [CrossRef]
- Wei, W.; Xu, Y.L.; Zhu, L.; Zhang, S.L.; Li, S. Impact of long-term continuous cropping on the Fusarium population in soybean rhizosphere. Chin. J. Appl. Ecol. 2014, 25, 497–504. [Google Scholar]
- Boudreau, M.A. Disease in intercropping systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Tanveer, M.; Anjum, S.A.; Hussain, S.; Cerdà, A.; Ashraf, U. Relay cropping as a sustainable approach: Problems and opportunities for sustainable crop production. Environ. Sci. Pollut. Res. 2017, 24, 6973–6988. [Google Scholar] [CrossRef]
- Du, J.; Han, T.; Gai, J.; Yong, T.; Sun, X.; Wang, X.; Yang, F.; Liu, J.; Shu, K.; Liu, W.; et al. Maize-soybean strip intercropping: Achieved a balance between high productivity and sustainability. J. Integr. Agric. 2018, 17, 747–754. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Li, X.; Yang, S.; Rengei, Z. Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecifc interactions on nutrients. Field Crop. Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]
- Dong, N.; Tang, M.M.; Zhang, W.P.; Bao, X.G.; Wang, Y.; Christie, P.; Li, L. Temporal differentiation of crop growth as one of the drivers of intercropping yield advantage. Sci. Rep. 2018, 8, 3110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107. [Google Scholar] [CrossRef]
- Qin, A.Z.; Huang, G.B.; Chai, Q.; Yu, A.Z.; Huang, P. Grain yield and soil respiratory response to intercropping systems on arid land. Field Crop. Res. 2013, 144. [Google Scholar] [CrossRef]
- Mao, L.L.; Zhang, L.Z.; Li, W.Q.; Van der Werf, W.; Sun, J.H.; Spiertz, H.; Li, L. Yield advantage and water saving in maize/pea intercrop. Field Crop. Res. 2012, 138, 11–20. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Qi, L.; Mei, X.; Liao, J.; Ding, X.; Deng, W.; Fan, L.; He, X.; Vivanco, J.M.; et al. Plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS ONE 2014, 9, e115052. [Google Scholar] [CrossRef]
- Ren, L.X.; Su, S.M.; Yang, X.M.; Xu, Y.C.; Huang, Q.W.; Shen, Q.R. Intercropping with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biol. Biochem. 2008, 40, 834–844. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Gómez-Rodrıguez, O.; Zavaleta-Mejıa, E.; Gonzalez-Hernandez, V.; LiveraMunoz, M.; Cárdenas-Soriano, E. Allelopathy and microclimatic modification of intercropping with marigold on tomato early blight disease development. Field Crop. Res. 2003, 83, 27–34. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Li, X.; Peter, C.; Sun, J.; Yang, S.; Tang, C. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosyst. 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.C.; Liao, D.P.; Lu, F.Z.; Gao, R.C.; Liu, W.G.; Yong, T.W.; Wu, X.L.; Du, J.B.; Liu, J.; et al. Yield response to different planting geometries in maize-soybean relay strip intercropping systems. Agron. J. 2015, 107, 296–304. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crop. Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Yong, T.W.; Chen, P.; Dong, Q.; Du, Q.; Yang, F.; Wang, X.C.; Liu, W.G.; Yang, W.Y. Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system. J. Integr. Agric. 2018, 17, 664–676. [Google Scholar] [CrossRef]
- Chen, P.; Du, Q.; Liu, X.; Zhou, L.; Hussain, S.; Lei, L.; Song, C.; Wang, X.; Liu, W.; Yang, F.; et al. Effects of reduced nitrogen inputs on crop yield and nitrogen use efficiency in a long-term maize-soybean relay strip intercropping system. PLoS ONE 2017, 12, e0184503. [Google Scholar] [CrossRef]
- Su, B.Y.; Liu, X.; Cui, L.; Xiang, B.; Yang, W.Y. Suppression of weeds and increases in food production in higher crop diversity planting arrangements: A case study of relay intercropping. Crop Sci. 2018, 58, 1729–1739. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Shang, J.; Zhang, L.; Chen, Y.K.; Chang, X.L.; Du, J.B.; Yong, T.W.; Wu, X.L.; Yu, L.; Zeng, S.H.; et al. Effects of different field configuration modes on population distribution of soybean major insect pests. J. Sichuan Agric. Univ. 2018, 36, 297–308. [Google Scholar]
- Aoki, T.; Ward, T.J.; Kistler, C.; O’Donnell, K. Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium Head blight cereal pathogens. JSM Mycotoxins 2012, 62, 91–102. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.R.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying Fusarium from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar]
- O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Rober, V.A.R.; Crous, P.W.; Johnston, P.R.; Aoki, T.; Rooney, A.P.; et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Kvas, M.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Steekamp, E.T. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 2009, 34, 1–21. [Google Scholar]
- O’ Donnelll, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysөe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Horberg, H.M. Patterns of splash dispersed conidia of Fusarium poae and Fusarium culmorum. Eur. J. Plant Pathol. 2002, 108, 73–80. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J.; et al. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crop. Res. 2017, 2, 16–23. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Mallon, C.A.; van Elsas, J.D.; Salles, J.F. Microbial invasions: The process, patterns, and mechanisms. Trends Microbiol. 2015, 23, 719–729. [Google Scholar] [CrossRef]
- Velluti, A.; Marín, S.; Gonzalez, R.; Ramos, A.J.; Sanchis, V. Fumonisin B1, zearalenone and deoxynivalenol production by Fusarium moniliforme, F. proliferatum and F. graminearum in mixed cultures on irradiated maize kernels. J. Sci. Food Agric. 2001, 81, 88–94. [Google Scholar] [CrossRef]
- Chang, X.L.; Dai, H.; Wang, D.P.; Zhou, H.H.; He, W.Q.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.S.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Machado, J.D.C.; Machado, A.Q.; Pozza, E.A.; Machado, C.F.; Zancan, W.L.A. Inoculum potential of Fusarium verticillioides and performance of maize seeds. Trop. Plant Pathol. 2013, 38, 213–217. [Google Scholar] [CrossRef]
- Pedrozo, R.; Little, C.R. Fusarium verticillioides inoculum potential influences soybean seed quality. Eur. J. Plant Pathol. 2017, 148, 749–754. [Google Scholar] [CrossRef]
- Miedaner, T.; Bolduan, C.; Melchinger, A.E. Aggressiveness and mycotoxin production of eight isolates each of Fusarium graminearum and Fusarium verticillioides for ear rot on susceptible and resistant early maize inbred lines. Eur. J. Plant Pathol. 2010, 127, 113–123. [Google Scholar] [CrossRef]
- Zhou, H.H.; Yan, L.; Wang, D.P.; Yong, T.W.; Gong, G.S.; Shang, J.; Yang, W.Y.; Chang, X.L. Identification of Fusarium species causing maize stalk rot in maize soybean relay intercropping pattern in Sichuan Province. J. Sichuan Agric. Univ. 2018, 36, 598–604. [Google Scholar]
- Naeem, M.; Li, H.; Yan, L.; Raza, M.A.; Gong, G.; Chen, H.; Yang, C.; Zhang, M.; Shang, J.; Liu, T.; et al. Characterization and pathogenicity of Fusarium species associated with soybean pods in maize/soybean strip intercropping. Pathogens 2019, 8, 245. [Google Scholar] [CrossRef]
- Beyer, M.; Klix, M.B.; Klink, H.; Verreet, J.A. Quantifying the effects of previous crop, tillage, cultivar and triazole fungicides on the deoxynivalenol content of wheat grain—A review. J. Plant Dis. Prot. 2006, 113, 241–246. [Google Scholar] [CrossRef]
- Sella, L.; Gazzetti, K.; Castiglioni, C.; Schafer, W.; Favaron, F. Fusarium graminearum possesses virulence factors common to Fusarium head blight of wheat and seedling rot of soybean but differing in their impact on disease severity. Mycology 2014, 104, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, H.; der Lee, T.V.; Waalwijk, C.; Chen, W.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Ficoseco, M.E.; Jimenez, C.M.; Vattuone, M.A.; Catalan, C.A. Trichothecene genotypes and chemotypes in Fusarium graminearum complex strains isolated from maize fields of northwest Argentina. Int. J. Food Microbiol. 2012, 153, 229–233. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Jeon, J.J.; Kim, H.S.; Zeller, K.A.; Carter, L.L.; Leslie, J.F.; Lee, Y.W. Population structure of and mycotoxin production by Fusarium graminearum from maize in South Korea. Appl. Environ. Microbiol. 2012, 78, 2161–2167. [Google Scholar] [CrossRef]
- Zhang, H.; Brankovics, B.; Luo, W.; Xu, J.; Xu, J.S.; Guo, C.; Guo, J.G.; Jin, S.L.; Chen, W.Q.; Feng, J. Crops are a main driver for species diversity and the toxigenic potential of Fusarium isolates in maize ears in China. World Mycotoxin J. 2016, 9, 701–715. [Google Scholar] [CrossRef]
- Lee, J.; Chang, I.Y.; Kim, H.; Yun, S.H.; Leslie, J.F.; Lee, Y.W. Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Appl. Environ. Microbiol. 2009, 75, 3289–3295. [Google Scholar] [CrossRef]
- Parikh, L.; Kodati, S.; Eskelson, M.J.; Adesemoye, A.O. Identification and pathogenicity of Fusarium spp. in row crops in Nebraska. Crop Prot. 2018, 108, 120–127. [Google Scholar] [CrossRef]
- Meisner, A.; de Boer, W. Strategies to maintain natural biocontrol of soil-borne crop diseases during severe drought and rainfall events. Front. Microbiol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Q.; Zhang, X.; Sarpong, C.K.; Wang, W.; Yong, T.; Wang, X.C.; Wang, Y.; Yang, W. Crop productivity and nutrients recovery in maize-soybean additive relay intercropping systems under subtropical regions in Southwest China. Int. J. Plant Prod. 2020. [Google Scholar] [CrossRef]
- Killebrew, J.F.; Roy, K.W.; Lawrence, G.W.; Mclean, K.S.; Hodges, H.H. Greenhouse and field evaluation of Fusarium solani pathogenicity to soybean seedlings. Plant Dis. 1998, 72, 1067–1070. [Google Scholar] [CrossRef]


| Isolate Code | Planting Pattern | GenBank Accession Number | Fusarium Species a | Fusarium Species Complex | |
|---|---|---|---|---|---|
| EF-1α | RPB2 | ||||
| A1s4 | Intercropping | MK560306 | MN892318 | F. graminearum | Fusarium graminearum species complex, FGSC [37] |
| A1s5 | Intercropping | MK560320 | MN892327 | FIESC | Fusarium incarnatum-equiseti species complex, FIESC [38,39] |
| A1r3 | Intercropping | MK560319 | MN892328 | FIESC | FIESC |
| A2s1 | Intercropping | MK560321 | MN892326 | FIESC | FIESC |
| A2s3 | Intercropping | MK560322 | MN892325 | FIESC | FIESC |
| A2s4 | Intercropping | MK560307 | MN892317 | F. graminearum | FGSC |
| A2r1 | Intercropping | MK560280 | MN892289 | FSSC | Fusarium solani species complex, FSSC [40] |
| A3s1 | Intercropping | MK560335 | MN892344 | F. asiaticum | FGSC |
| A3s3 | Intercropping | MN892351 | MN892338 | FSSC | FSSC |
| A4s1 | Intercropping | MK560308 | MN892321 | F. fujikuroi | Fusarium fujikuroi species complex, FFSC [41] |
| A4s2 | Intercropping | MK560300 | MN892307 | F. oxysporum | Fusarium oxysporum species complex, FOSC [8,39,40] |
| A4s3 | Intercropping | MK560323 | MN892336 | FIESC | FIESC |
| A4s4 | Intercropping | MK560324 | MN892341 | FIESC | FIESC |
| A4s6 | Intercropping | MK560283 | MN892295 | F. solani | FSSC |
| A4r1 | Intercropping | MK560282 | MN892296 | FSSC | FSSC |
| A5s3 | Intercropping | MK560325 | MN892342 | FIESC | FIESC |
| A5r2 | Intercropping | MK560284 | MN892284 | FSSC | FSSC |
| A6s1 | Intercropping | MK560309 | MN892320 | F. fujikuroi | FFSC |
| A6s2 | Intercropping | MK560326 | MN892324 | FIESC | FIESC |
| A6s3 | Intercropping | MK560301 | MN892306 | F. oxysporum | FOSC |
| A6r2 | Intercropping | MK560285 | MN892294 | F. solani | FSSC |
| A7s2 | Intercropping | MK560261 | MN892281 | F. verticillioides | FFSC |
| A7s3 | Intercropping | MK560327 | MN892323 | FIESC | FIESC |
| A7s5 | Intercropping | MK560262 | MN892280 | F. verticillioides | FFSC |
| A7r1 | Intercropping | MK560302 | MN892305 | F. oxysporum | FOSC |
| A7r2 | Intercropping | MK560286 | MN892293 | F. solani | FSSC |
| A8s3 | Intercropping | MK560263 | MN892279 | F. verticillioides | FFSC |
| A8s4 | Intercropping | MK560328 | MN892343 | FIESC | FIESC |
| A8r2 | Intercropping | MK560288 | MN892291 | FSSC | FSSC |
| A8r4 | Intercropping | MK560287 | MN892292 | F. solani | FSSC |
| A9s1 | Intercropping | MK560310 | MN892319 | F. fujikuroi | FFSC |
| A9s2 | Intercropping | MK560264 | MN892278 | F. verticillioides | FFSC |
| A9s3 | Intercropping | MK560329 | MN892322 | FIESC | FIESC |
| A9s5 | Intercropping | MK560292 | MN892349 | F. proliferatum | FFSC |
| A9s6 | Intercropping | MK560290 | MN892285 | FSSC | FSSC |
| A9r1 | Intercropping | MK560291 | MN892290 | FSSC | FSSC |
| B1r1 | Monoculture | MK560265 | MN892304 | FSSC | FSSC |
| B2s1 | Monoculture | MK560293 | MN892313 | F. oxysporum | FOSC |
| B2s2 | Monoculture | MK560303 | MN892316 | F. meridionale | FGSC |
| B2s3 | Monoculture | MK560294 | MN892312 | F. oxysporum | FOSC |
| B2r1 | Monoculture | MK560266 | MN892288 | FSSC | FSSC |
| B2r2 | Monoculture | MK560311 | MN892335 | FIESC | FIESC |
| B2r3 | Monoculture | MK560312 | MN892337 | FIESC | FIESC |
| B2r4 | Monoculture | MK560304 | MN892315 | F. meridionale | FGSC |
| B3s1 | Monoculture | MK560295 | MN892311 | FIESC | FOSC |
| B3s3 | Monoculture | MK560331 | MN892348 | F. asiaticum | FGSC |
| B3s4 | Monoculture | MK560313 | MN892334 | FIESC | FIESC |
| B3s6 | Monoculture | MK560296 | MN892310 | F. oxysporum | FOSC |
| B3r1 | Monoculture | MK560267 | MN892303 | FSSC | FSSC |
| B4s1 | Monoculture | MK560305 | MN892314 | F. meridionale | FGSC |
| B4s3 | Monoculture | MK560314 | MN892333 | FIESC | FIESC |
| B4s4 | Monoculture | MK560315 | MN892339 | FIESC | FIESC |
| B4r1 | Monoculture | MK560268 | MN892283 | FSSC | FSSC |
| B4r2 | Monoculture | MK560269 | MN892286 | FSSC | FSSC |
| B5s1 | Monoculture | MK560270 | MN892302 | FSSC | FSSC |
| B5s2 | Monoculture | MK560297 | MN892309 | F. oxysporum | FOSC |
| B5s4 | Monoculture | MN892352 | MN892332 | FSSC | FSSC |
| B6s1 | Monoculture | MK560272 | MN892301 | FSSC | FSSC |
| B6s2 | Monoculture | MK560316 | MN892331 | FIESC | FIESC |
| B6s3 | Monoculture | MK560332 | MN892347 | F. asiaticum | FGSC |
| B7s2 | Monoculture | MK560317 | MN892340 | FIESC | FIESC |
| B7s4 | Monoculture | MK560318 | MN892330 | FIESC | FIESC |
| B7r2 | Monoculture | MK560273 | MN892300 | FSSC | FSSC |
| B8s1 | Monoculture | MK560333 | MN892346 | F. asiaticum | FGSC |
| B8s2 | Monoculture | MK560274 | MN892299 | FSSC | FSSC |
| B8s4 | Monoculture | MN892354 | MN892308 | FSSC | FSSC |
| B8r1 | Monoculture | MK560276 | MN892298 | FSSC | FSSC |
| B9s1 | Monoculture | MK560334 | MN892345 | F. asiaticum | FGSC |
| B9s2 | Monoculture | MK560277 | MN892282 | FSSC | FSSC |
| B9s4 | Monoculture | MN892353 | MN892329 | FIESC | FIESC |
| B9s5 | Monoculture | MK560330 | MN892350 | F. commune | Fusarium nisikadoi species complex, FNSC [42] |
| B9r1 | Monoculture | MK560278 | MN892287 | FSSC | FSSC |
| B9r2 | Monoculture | MK560279 | MN892297 | FSSC | FSSC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Yan, L.; Naeem, M.; Khaskheli, M.I.; Zhang, H.; Gong, G.; Zhang, M.; Song, C.; Yang, W.; Liu, T.; et al. Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species. Pathogens 2020, 9, 211. https://doi.org/10.3390/pathogens9030211
Chang X, Yan L, Naeem M, Khaskheli MI, Zhang H, Gong G, Zhang M, Song C, Yang W, Liu T, et al. Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species. Pathogens. 2020; 9(3):211. https://doi.org/10.3390/pathogens9030211
Chicago/Turabian StyleChang, Xiaoli, Li Yan, Muhammd Naeem, Muhammad Ibrahim Khaskheli, Hao Zhang, Guoshu Gong, Min Zhang, Chun Song, Wenyu Yang, Taiguo Liu, and et al. 2020. "Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species" Pathogens 9, no. 3: 211. https://doi.org/10.3390/pathogens9030211
APA StyleChang, X., Yan, L., Naeem, M., Khaskheli, M. I., Zhang, H., Gong, G., Zhang, M., Song, C., Yang, W., Liu, T., & Chen, W. (2020). Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species. Pathogens, 9(3), 211. https://doi.org/10.3390/pathogens9030211







