Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia insignis Leaves against Vaginal Isolates of Candida Species
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Characterization
2.2. Antifungal Assays
2.2.1. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
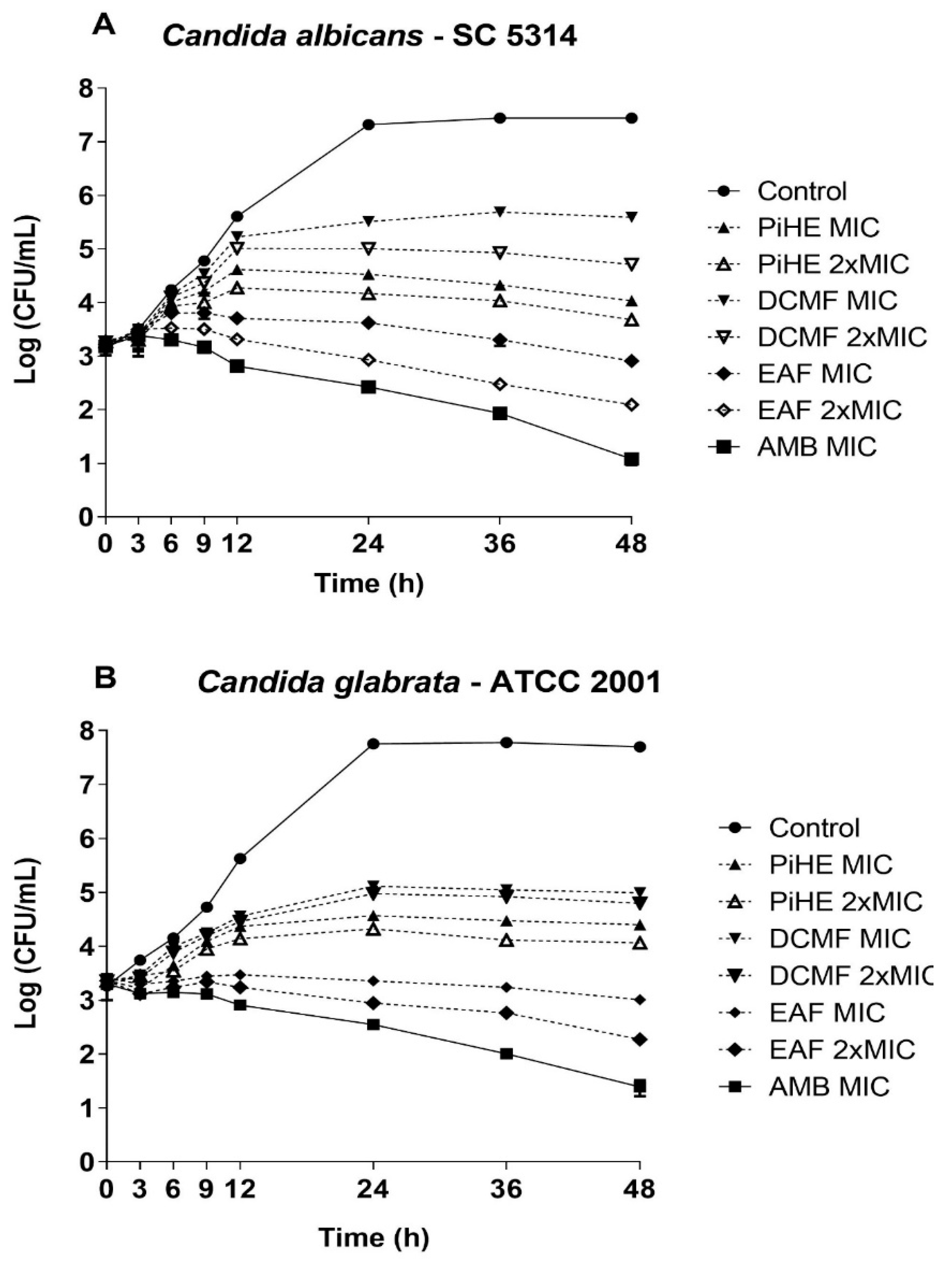
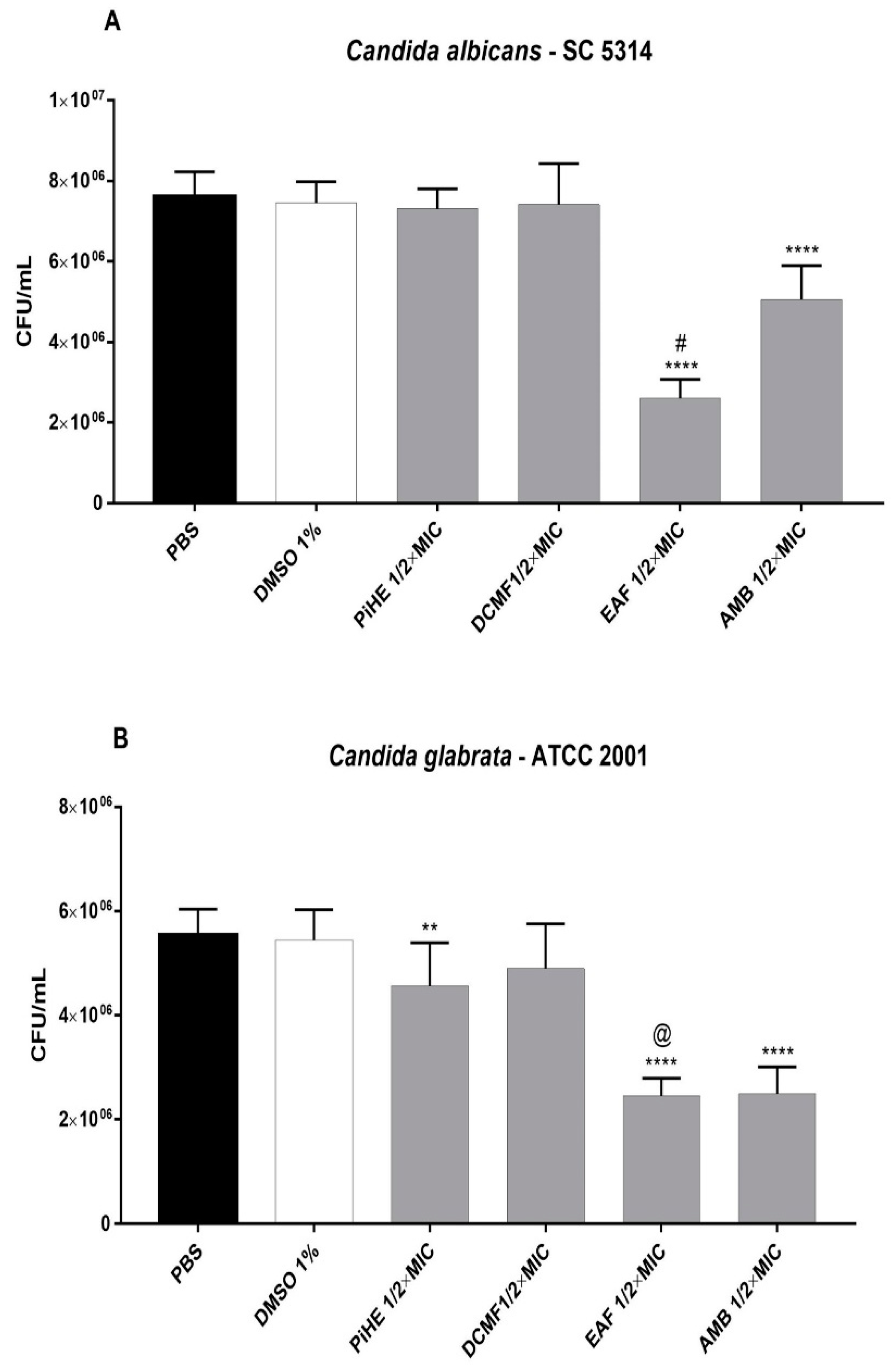
2.2.2. Killing Assay
2.3. Antivirulence Activities
2.3.1. Effect of PiHE, DCMF, and EAF on Cell Adhesion
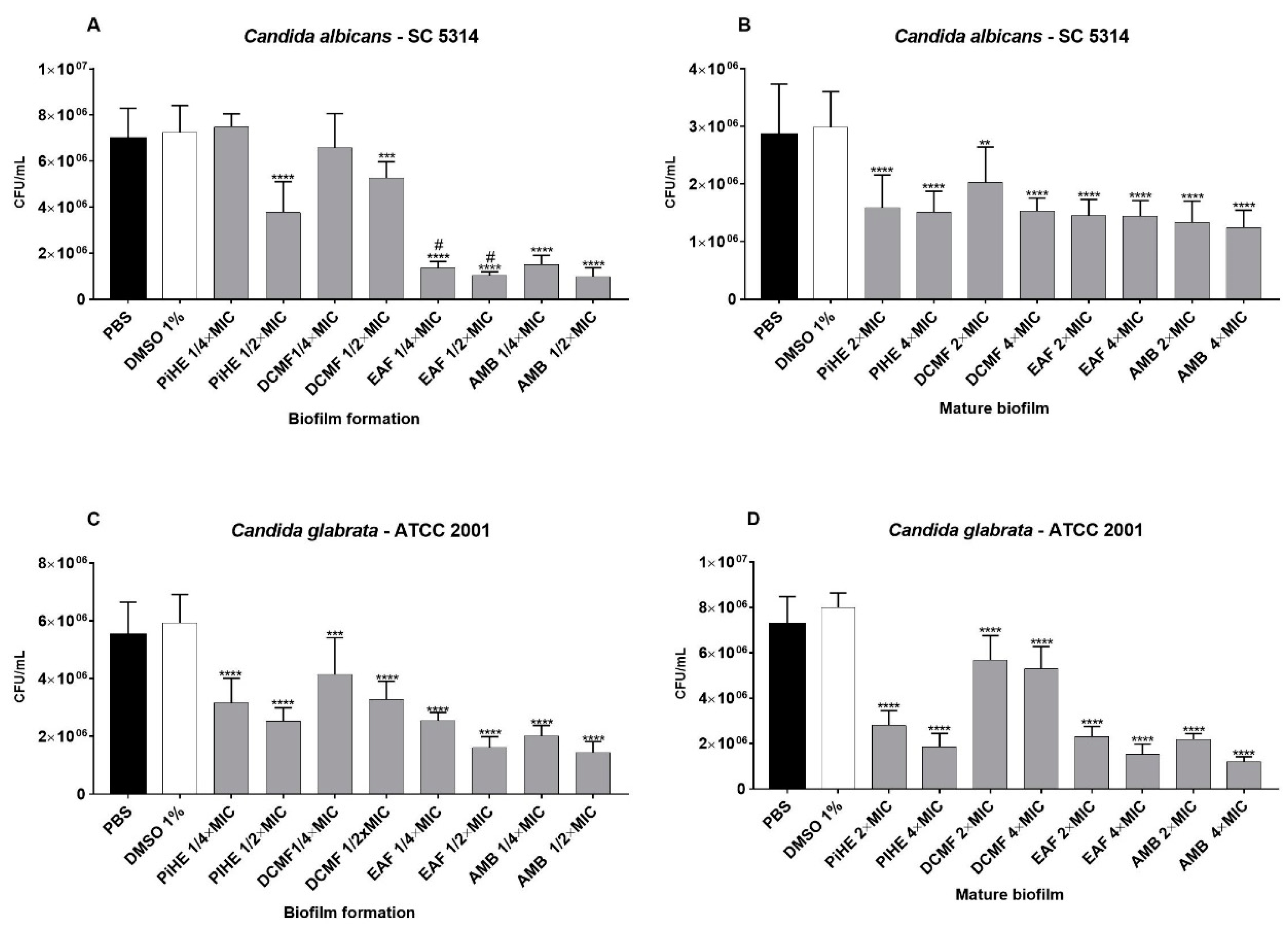
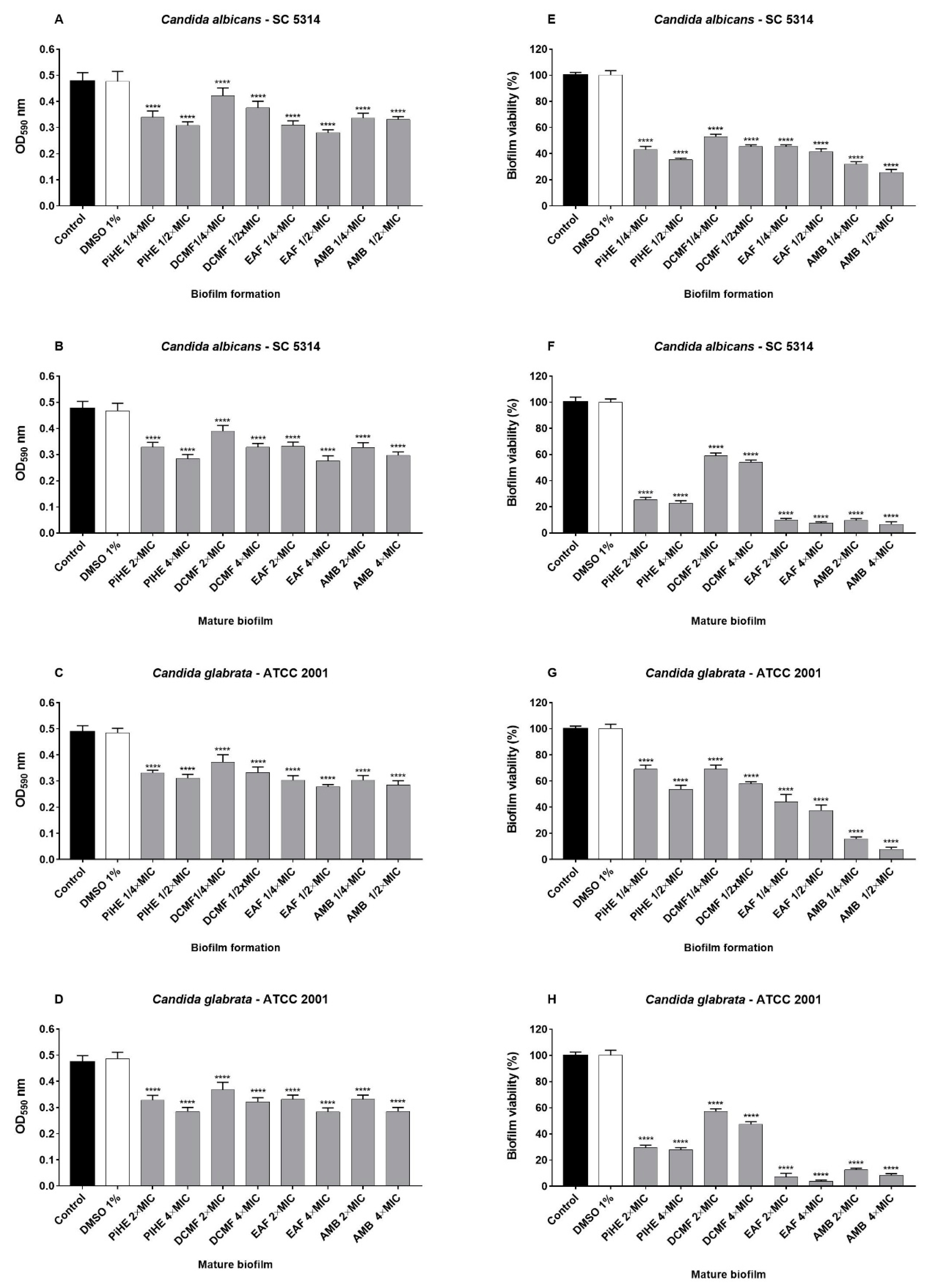
2.3.2. Effects of PiHE, DCMF, and EAF on Biofilm Formation and Pre-Formed Biofilms
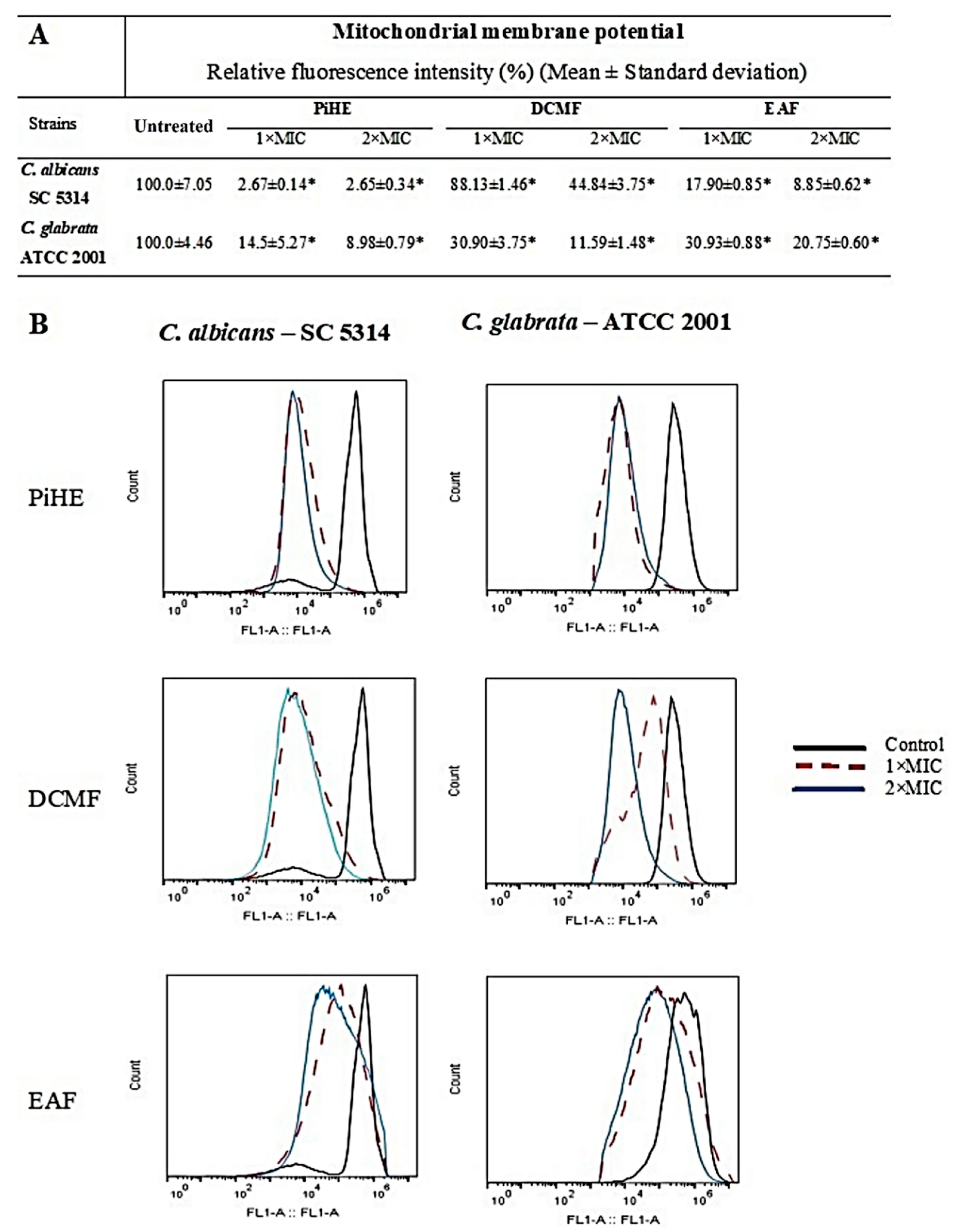
2.4. Evaluation of Mitochondrial Membrane Potential by Flow Cytometry
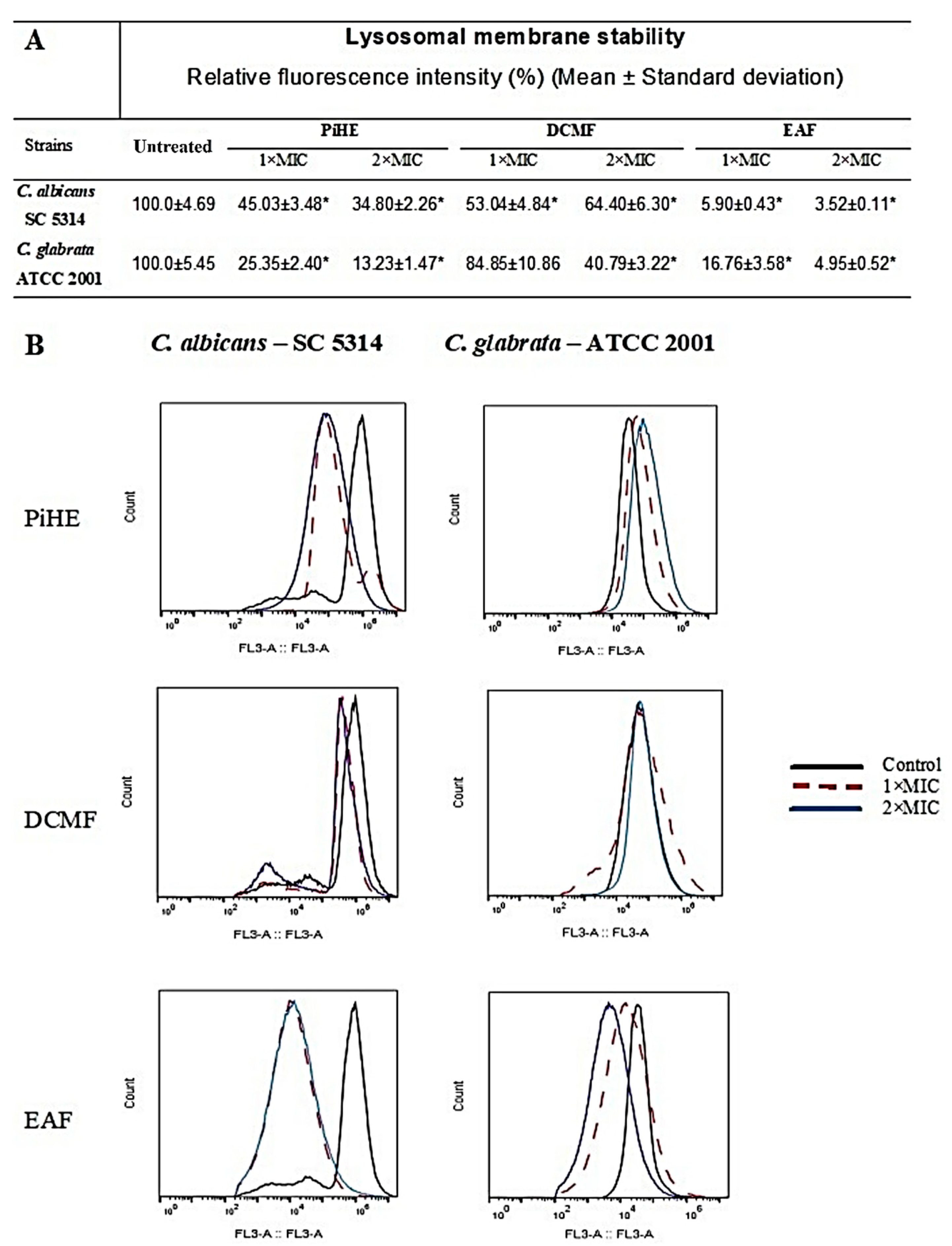
2.5. Evaluation of Lysosomal Membrane Integrity upon Treatment with PiHE, DCMF, and EAF
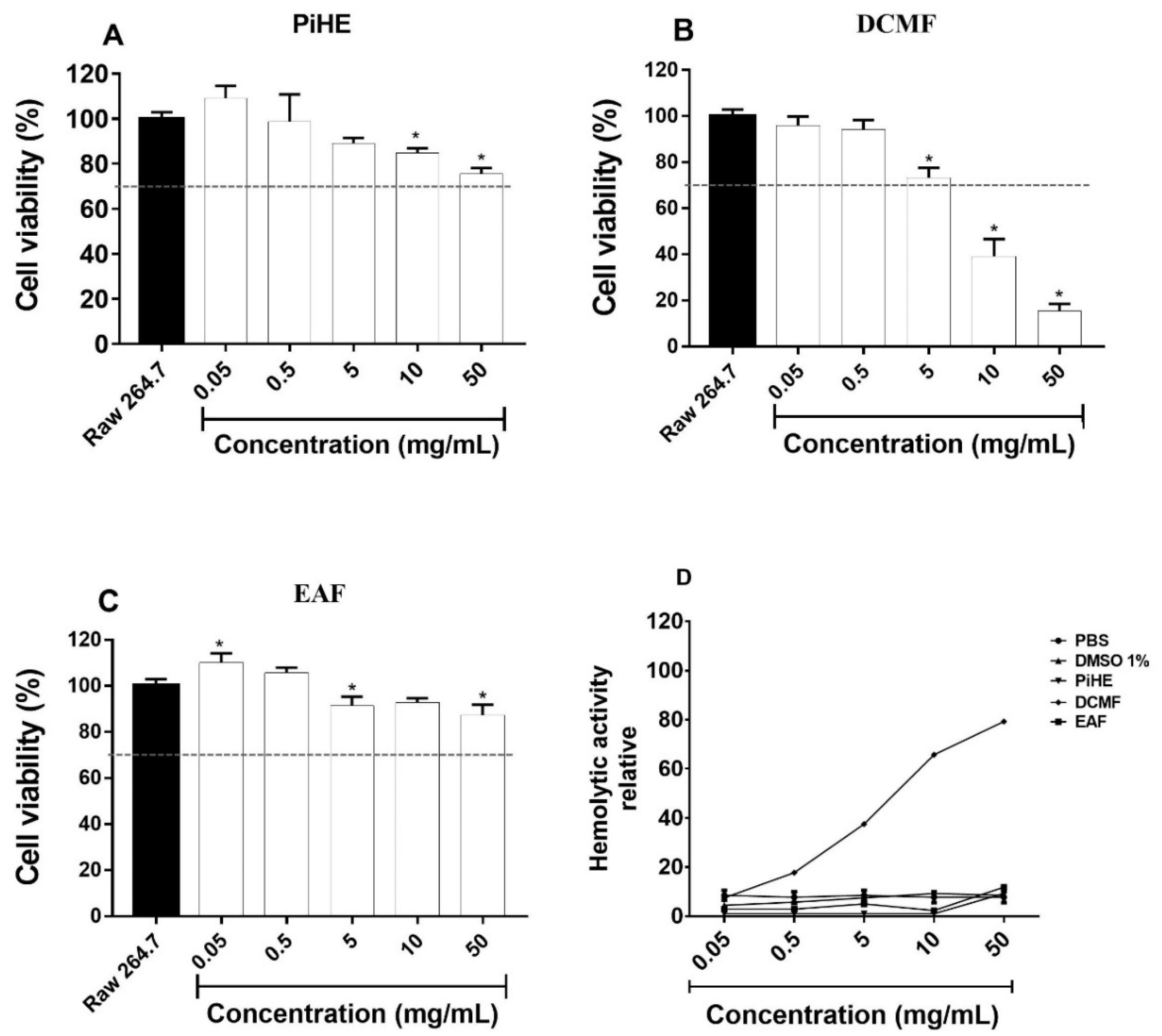
2.6. Cellular Cytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material: Collection, Identification, and Extraction
4.2. Chemical Analyses
4.2.1. HPLC Fingerprint Analysis
4.2.2. ESI-MS/MS Analysis
4.3. Antifungal Assays
4.3.1. Candida Strains, Growth Conditions, and Inoculum Preparation
4.3.2. Determination of the Minimum Inhibitory Concentration (MIC)
4.3.3. Determination of the Minimum Fungicide Concentration (MFC)
4.3.4. Time-Kill Assay
4.4. Antivirulence Activity
4.4.1. Effect of PiHE and Fractions on Candida spp. Adhesion
4.4.2. Effect of Extract and Fractions on Biofilm
Colony Count Analysis of Biofilms
Biofilm Viability Assay
Biofilm Analysis by Crystal Violet Staining
4.5. Flow Cytometry Analysis for Examining the Effect of the Extract and Fractions on the Mitochondrial Membrane Potential (ΔΨm) and Lysosomal Membrane Stability
4.6. Cytotoxicity Analysis Using Murine RAW 264.7 Cells
4.7. Hemolytic Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthamil, S.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Synergistic Effect of Quinic Acid Derived From Syzygium cumini and Undecanoic Acid Against Candida spp. Biofilm and Virulence. Front. Microbiol. 2018, 9, 2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of Recurrent Vulvovaginal Candidiasis in 5 European Countries and the United States: Results From an Internet Panel Survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef]
- Tang, H.J.; Liu, W.L.; Lin, H.L.; Lai, C.C. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS ONE 2014, 9, e99103. [Google Scholar] [CrossRef] [Green Version]
- Tsui, C.; Kong, E.F.; Jabra–Rizk, M.A. Pathogenesis of Candida albicans Biofilm. Pathog. Dis. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Enfert, C.; Janbon, G. Biofilm formation in Candida glabrata: What have we learnt from functional genomics approaches? FEMS Yeast Res. 2016, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Zhuang, X.; Feng, X.; Zhong, L.; Ma, T. Antifungal Effects of Saponin Extract from Rhizomes of Dioscorea panthaica Prain et Burk against Candida albicans. Evid. Based Complement. Altern. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, K.; Ramos, D.F.; Carrion, L.L.; Cursino, L.M.C.; Jefreys, M.F.; Pedroza, L.S.; Osório, M.I.C.; Oliveira, J.L.; Andrade, J.I.A.; Fernandes, C.C.; et al. Antifungal activity of brazilian amazon plants extracts against some species of Candida spp. Int. J. Phytopharmacol. 2014, 5, 445–453. [Google Scholar]
- Souza, A.C.; Alves, M.M.M.; Brito, L.M.; Oliveira, L.G.C.; Sobrinho–Júnior, E.P.C.; Costa, I.C.G.; Freitas, S.D.L.; Rodrigues, K.A.F.; Chaves, M.H.; Arcanjo, D.D.R.; et al. Platonia insignis Mart., a Brazilian Amazonian Plant: The Stem Barks Extract and Its Main Constituent Lupeol Exert Antileishmanial Effects Involving Macrophages Activation. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa Júnior, J.S.; Almeida, A.A.C.; Ferraz, A.B.F.; Rossatto, R.R.; Silva, T.G.; Silva, P.B.N.; Militão, G.C.G.; Citó, A.M.G.L.; Santana, L.C.L.R.; Carvalho, F.A.A.; et al. Cytotoxic and leishmanicidal properties of garcinielliptone FC, a prenylated benzophenone from Platonia insignis. Nat. Prod. Res. 2013, 27, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.K.L.; Pereira, C.V.L.; Lima, E.S.; Veiga Junior, V.F. Química e farmacologia do bacuri (Platonia insignis). Sci. Amazon. 2014, 3, 39–46. [Google Scholar]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Sousa, T.O.; Silva, R.A.C.; Lima, S.G.; Feitosa, C.M.; Citó, A.M.G.L.; Cavalcante, A.A.C.M.; Freitas, R.M.; Speroto, A.R.M.; et al. Investigation of Biological Activities of Dichloromethane and Ethyl Acetate Fractions of Platonia insignis Mart. Seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef]
- Carvalho, J.E.U.; Nascimento, W.M.O. Bacuri. Platonia Insignis; Instituto Interamericano de Cooperación para la Agricultura (IICA): San José, Costa Rica, 2017. [Google Scholar]
- Nascimento, J.L.; Coêlho, A.G.; Barros, Y.S.O.; Silva, O.A.; Freitas, R.M.; Rocha, M.S.; David, J.M.; Costa Júnior, J.S.; Arcanjo, D.D.R.; Oliveira, R.C.M.; et al. Avaliação da atividade antioxidante in vitro do extrato hexânico da semente do bacuri (Platonia insignis Mart.) e de seu complexo de inclusão com β–ciclodextrina. Bol. Inf. Geum 2014, 5, 44–53. [Google Scholar]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, C.J.; Rajan, S.; Wong, S.S.W.; Tsang, D.N.C.; Lai, C.K.C.; Samaranayake, L.P.; Jin, L. Antifungal Susceptibility in Serum and Virulence Determinants of Candida Bloods tream Isolates from Hong Kong. Front. Microbiol. 2016, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single– and multi–species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, C.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 3259–3266. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Koga–Ito, C.Y. Effects of Acetone Fraction From Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Front. Microbiol. 2018, 9, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardot, M.; Imbert, C. Novel strategies against Candida biofilms: Interest of synthetic compounds. Future Microbiol. 2016, 11, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Watamoto, T.; Egusa, H.; Sawase, T.; Yatani, H. Screening of Pharmacologically Active Small Molecule Compounds Identifies Antifungal Agents Against Candida Biofilms. Front. Microbiol. 2015, 6, 1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The Anti–Adhesive Effect of Curcumin on Candida albicans Biofilms on Denture Materials. Front. Microbiol. 2017, 8, 659. [Google Scholar] [CrossRef]
- Ludovico, P.; Sansonetty, F.; Côrte–Real, M. Assessment of mitochondrial membrane potential in yeast cell populations by flow cytometry. Microbiology 2001, 147, 3335–3343. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.R.; Andrade Neto, J.B.; Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhaes, H.I.F.; Cavalcante, B.C.; Gaspar, D.M.; Andrade, G.M.; Lima, I.S.P.; et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.B.A.; da Silva, C.R.; Neta, M.A.S.; Campos, R.S.; Siebra, J.T.; Silva, R.A.C.; Gaspar, D.M.; Magalhães, H.I.F.; de Moraes, M.O.; Lobo, M.D.P.; et al. Antifungal Activity of Naphthoquinoidal Compounds In Vitro against Fluconazole Resistant Strains of Different Candida Species: A Special Emphasis on Mechanisms of Action on Candida tropicalis. PLoS ONE 2014, 9, e93698. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, D.W.; Hyon, S.H.; Park, J.C. Apoptosis of human fibrosarcoma HT–1080 cells by epigallocatechin–3–O–gallate via induction of p53 and caspases as well as suppression of Bcl–2 and phosphorylated nuclear factor– B. Apoptosis 2011, 16, 75–85. [Google Scholar] [CrossRef]
- Hwang, B.; Hwang, J.S.; Lee, J.; Kim, J.K.; Kim, S.R.; Kim, Y.; Lee, D.G. Induction of yeast apoptosis by an antimicrobial peptide, papiliocin. Biochem. Biophys. Res. Commun. 2011, 408, 89–93. [Google Scholar] [CrossRef]
- Neto, W.R.N.; Farah, E.I.; Santos, Á.R.C.; Mendes, B.S.; Sousa, L.V.N.F.; Rodrigues, J.F.S.; Alves, J.C.O.; Ferreira, G.F.; Santos, J.R.; Santos, L.C.N.; et al. Eugenol Induces Phenotypic Alterations and Increases the Oxidative Burst in Cryptococcus. Front. Microbiol. 2017, 8, 2419. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth informational supplement; M27–S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Terças, A.G.; Monteiro, A.S.; Moffa, E.B.; Santos, J.R.A.; Sousa, E.M.; Pinto, A.R.B.; Costa, P.C.S.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their antifungal Activities against Candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (Coriander) Essential Oil: Antifungal Activity and Mode of Action on Candida spp., and Molecular Targets Affected in Human Whole–Genome Expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In Vitro and In Vivo Antifungal Activity of Lichochalcone–against Candida albicans Biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Valle Arevalo, A.; Rodriguez, D.L.; Nobile, C.J. In vitro culturing and screening of Candida albicans biofilms. Curr. Protoc. Microbiol. 2018, e60. [Google Scholar] [CrossRef]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.A.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of Biofilm Formation and the Interaction between Candida albicans and Methicillin–Susceptible (MSSA) and –Resistant Staphylococcus aureus (MRSA). PLoS ONE 2015, 10, e0123206. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; Campos, R.D.S.; Costa Silva, R.A.; Freitas, D.D.; Nascimento, F.B.S.A.; de Andrade, L.N.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in fluconazole–resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [Green Version]
- Ronot, X.; Benel, L.; Adolphe, M.; Mounolou, J.C. Mitochondrial analysis in living cells: The use of rhodamine 123 and flowcytometry. Biol. Cell 1986, 57, 1–7. [Google Scholar] [CrossRef]
- Olsson, M.; Rundquist, I.; Brunk, U. Flow cytofluorometry of lysosomal acridine orange uptake by living cultured cells. Effect of trypsinization and starvation. Acta Pathol. Microbiol. Immunol. Scand. 1987, 95, 159–165. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Liu, J.; Wei, L.; Song, B.; Shao, L. Exposure of the murine RAW 264.7 macrophage cell line to dicalcium silicate coating: Assessment of cytotoxicity and pro–inflammatory effects. J. Mater. Sci. Mater. Med. 2016, 27, 59. [Google Scholar] [CrossRef]
- Silva, A.P.S.; Nascimento, S.L.C.; Martins, F.C.S.; Araújo, J.M.; Correia, M.T.S.; Cavalcanti, M.S.; Lima, V.L.M. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front. Microbiol. 2016, 7, 963. [Google Scholar] [CrossRef] [Green Version]
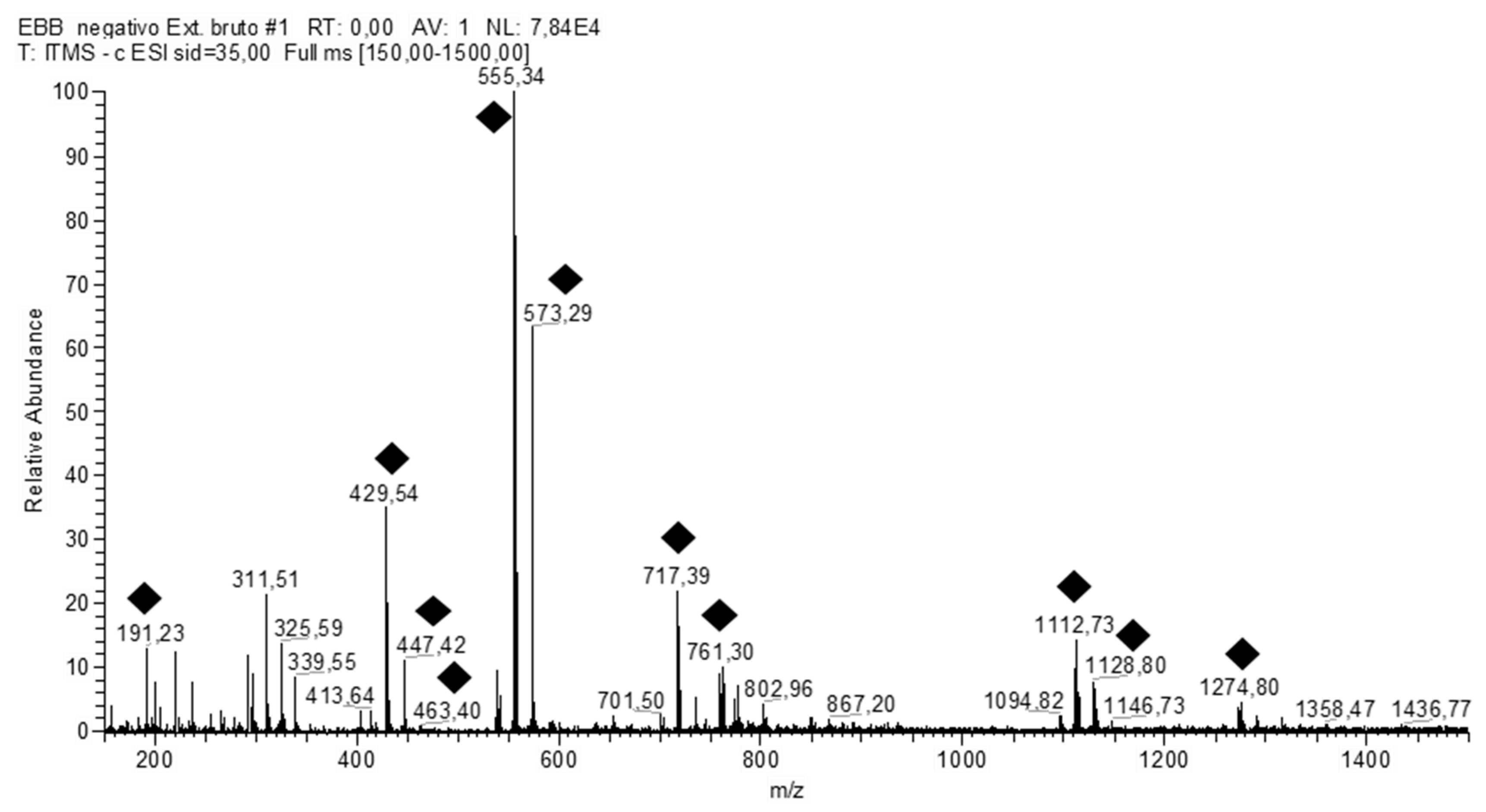
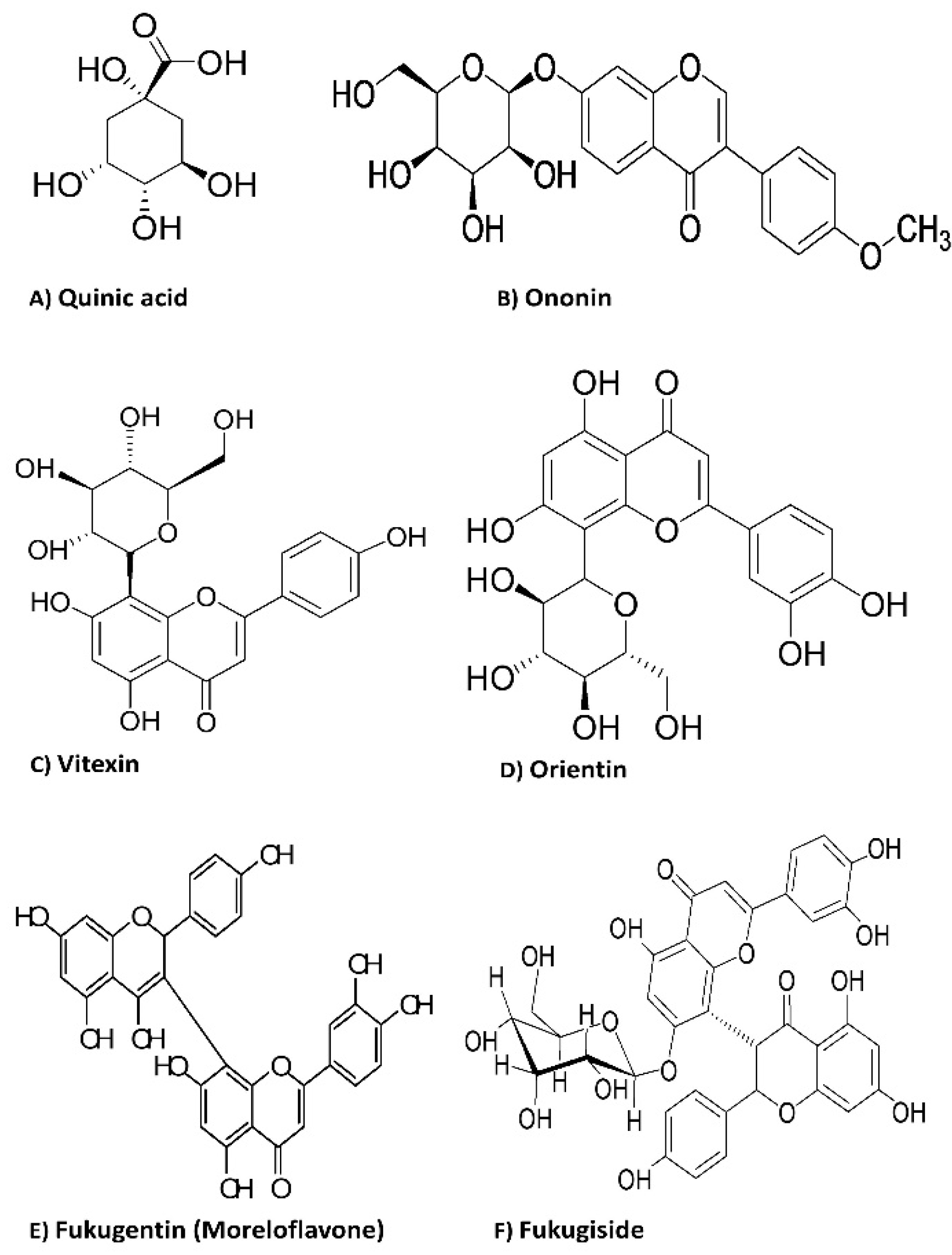
| Sample | Number | [M − H]− | RT | MSn Fragments | Names of the Suggested Structures |
|---|---|---|---|---|---|
| PiHE | 1 | 191 | 18.80 | 173, 111, 85 | Quinic acid |
| 2 | 429 | 18.61 | 267 | Ononin | |
| 3 | 431 | 18.43 | 295, 269 | Vitexin | |
| 4 | 447 | 18.24 | 429, 419, 285, 151 | Orientin | |
| 5 | 555 | 17.42 | 429, 401, 295, 267 | Fukugentin | |
| 6 | 573 | 16.33 | 447, 420, 419, 403, 296, 269,268 | NI | |
| 7 | 717 | 14.31 | 591, 565, 555, 429, 403, 401, 295, 267 | Fukugiside | |
| 8 | 761 | 12.49 | 634, 608, 431 | NI | |
| 9 | 777 | 10.35 | 447 | NI | |
| 10 | 1111 | 9.53 | 571, 537, 509, 429 | Fukugentin dimer | |
| 11 | 1113 | 8.04 | 555, 429 | Fukugentin dimer | |
| 12 | 1129 | 6.14 | 1111, 1003, 877, 555, 429 | Fukugentin dimer | |
| 13 | 1131 | 5.40 | 555, 429 | NI | |
| 14 | 1275 | 1.39 | 718, 429 | NI | |
| DCMF | 15 | 573 | 8.34 | 557, 431, 295, 269 | NI |
| 16 | 555 | 9.12 | 431, 429, 295, 269 | Fukugentin | |
| 17 | 429 | 9.56 | 429, 403, 295 | Ononin | |
| 18 | 561 | 10.10 | 539, 429, 413, 387 | NI | |
| EAF | 19 | 573 | 6.40 | 447, 419, 269 | NI |
| 20 | 717 | 6.62 | 657, 635, 555, 429 | Fukugiside | |
| 21 | 429 | 6.69 | 295, 269 | Ononin | |
| 22 | 555 | 7.90 | 429 | Fukugentin | |
| 23 | 1134 | 13.34 | 623, 555, 429, 299, 225 | NI |
| Platonia insignis | Antifungals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STRAINS | PiHE | DCMF | EAF | AMB | FLZ | ||||||
| Candida albicans | MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | MIC | MIC |
| A1 Ca | 2.60 | 4.20 | 1.60 | 1.00 | 5.20 | 5.00 | 0.70 | 0.80 | 1.20 | 0.001 | 0.008 |
| A2 Ca | 2.60 | 5.20 | 2.00 | 1.00 | 3.60 | 3.50 | 1.00 | 1.00 | 1.00 | 0.0005 | 0.008 |
| A3 Ca | 1.80 | 3.60 | 2.00 | 3.10 | 10.40 | 3.30 | 0.50 | 0.50 | 1.00 | 0.00025 | 0.004 |
| A4 Ca | 2.60 | 8.30 | 3.20 | 1.30 | 4.20 | 3.20 | 1.30 | 2.60 | 2.00 | 0.0005 | 0.016 |
| A5 Ca | 4.20 | 8.30 | 2.00 | 1.00 | 4.20 | 4.00 | 1.30 | 2.60 | 2.00 | 0.0005 | 0.016 |
| A6 Ca | 4.20 | 7.30 | 1.80 | 1.00 | 4.20 | 4.00 | 1.30 | 2.60 | 2.00 | 0.0005 | 0.016 |
| A7 Ca | 2.60 | 9.40 | 3.60 | 0.70 | 4.20 | 6.40 | 1.30 | 1.90 | 1.50 | 0.001 | 0.016 |
| A8 Ca | 2.60 | 5.20 | 2.00 | 0.90 | 2.60 | 2.90 | 1.00 | 2.10 | 2.00 | 0.0005 | 0.008 |
| A9 Ca | 6.30 | 8.30 | 1.30 | 2.30 | 10.40 | 4.40 | 1.00 | 1.80 | 0.80 | 0.00025 | 0.008 |
| A10 Ca | 6.30 | 14.60 | 2.30 | 1.80 | 5.20 | 2.90 | 1.30 | 1.30 | 1.00 | 0.0005 | 0.016 |
| Candida glabrata | |||||||||||
| B1 Cg | 2.60 | 3.10 | 1.20 | 1.80 | 3.60 | 2.00 | 0.30 | 0.70 | 2.50 | 0.0005 | 0.004 |
| B2 Cg | 3.10 | 3.10 | 1.00 | 2.10 | 3.60 | 1.80 | 0.30 | 0.40 | 1.50 | 0.002 | 0.004 |
| B3 Cg | 1.60 | 5.20 | 3.30 | 2.60 | 10.40 | 4.00 | 0.20 | 0.30 | 1.70 | 0.002 | 0.008 |
| B4 Cg | 3.60 | 5.20 | 1.40 | 4.20 | 10.40 | 2.50 | 1.00 | 1.60 | 1.50 | 0.001 | 0.008 |
| B5 Cg | 8.30 | 16.70 | 2.00 | 4.20 | 12.50 | 3.00 | 0.30 | 0.70 | 2.00 | 0.0005 | 0.008 |
| B6 Cg | 5.20 | 10.40 | 2.00 | 20.80 | 41.70 | 2.00 | 0.20 | 0.40 | 2.10 | 0.002 | 0.008 |
| SC 5314-Ca | 2.080 | 6.30 | 3.00 | 1.00 | 1.80 | 1.80 | 1.00 | 1.30 | 1.30 | 0.0005 | 0.016 |
| ATCC 2001-Cg | 5.20 | 8.30 | 1.60 | 8.30 | 20.80 | 2.50 | 0.50 | 1.30 | 2.50 | 0.00025 | 0.016 |
| ATCC 90028-Ca | 5.20 | 10.40 | 2.00 | 10.40 | 20.80 | 2.00 | 0.40 | 1.60 | 4.00 | 0.001 | 0.008 |
| Species (n of Isolates) | Antifungal Agent | MIC mg/mL | MFC mg/mL | ||||
|---|---|---|---|---|---|---|---|
| Range | Geometric Means | MIC90 | Range | Geometric Means | MFC | ||
| Candida albicans (10) | PiHE | 1.8–6.30 | 3.18 | 3.58 | 3.60–14.60 | 6.45 | 7.44 |
| DCMF | 0.7–3.10 | 1.20 | 1.41 | 2.60–10.40 | 4.78 | 5.42 | |
| EAF | 0.50–1.30 | 0.98 | 1.07 | 0.50–2.60 | 1.46 | 1.72 | |
| Candida glabrata (6) | PiHE | 1.60–8.30 | 3.45 | 4.07 | 2.30–25.00 | 5.69 | 7.28 |
| DCMF | 1.8–20.8 | 3.74 | 5.95 | 3.60–41.70 | 9.09 | 13.70 | |
| EAF | 0.2–1.00 | 0.30 | 0.38 | 0.30–2.60 | 0.54 | 0.68 | |
| SC 5314-Ca | PiHE | 0.40–3.12 | 1.71 | 2.08 | 4.80–8.6 | 6.25 | 6.30 |
| DCMF | 0.70–1.80 | 0.96 | 1.00 | 1.56–3.12 | 1.63 | 1.80 | |
| EAF | 0.78–1.56 | 0.96 | 1.00 | 0.78–2.00 | 1.21 | 1.30 | |
| ATCC 2001-Cg | PiHE | 3.12–6.30 | 5.11 | 5.20 | 6.20–12.50 | 7.93 | 8.30 |
| DCMF | 6.30–12.50 | 8.01 | 8.30 | 12.10–25.00 | 19.79 | 20.8 | |
| EAF | 0.39–0.78 | 0.49 | 0.50 | 0.78–2.20 | 1.19 | 1.30 | |
| ATCC 90028-Ca | PiHE | 3.10–6.30 | 5.08 | 5.20 | 6.20–25.00 | 9.49 | 10.40 |
| DCMF | 6.30–12.50 | 10.17 | 10.40 | 12.10–25.00 | 19.79 | 20.8 | |
| EAF | 0.10–1.00 | 0.37 | 0.40 | 0.90–2.30 | 1.52 | 1.60 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.F.; da Rocha, C.Q.; da Silva, L.C.N.; Carvalho Júnior, A.R.; Mendes, I.N.F.V.; de Araruna, A.B.; Motta, E.P.; Silva, R.d.S.; Campos, C.D.L.; Farias, J.R.; et al. Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia insignis Leaves against Vaginal Isolates of Candida Species. Pathogens 2020, 9, 84. https://doi.org/10.3390/pathogens9020084
da Silva AF, da Rocha CQ, da Silva LCN, Carvalho Júnior AR, Mendes INFV, de Araruna AB, Motta EP, Silva RdS, Campos CDL, Farias JR, et al. Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia insignis Leaves against Vaginal Isolates of Candida Species. Pathogens. 2020; 9(2):84. https://doi.org/10.3390/pathogens9020084
Chicago/Turabian Styleda Silva, Anderson França, Cláudia Quintino da Rocha, Luís Cláudio Nascimento da Silva, Alexsander Rodrigues Carvalho Júnior, Iven Neylla Farias Vale Mendes, Andrea Borges de Araruna, Elizangela Pestana Motta, Rayssa de Sousa Silva, Carmem Duarte Lima Campos, Josivan Regis Farias, and et al. 2020. "Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia insignis Leaves against Vaginal Isolates of Candida Species" Pathogens 9, no. 2: 84. https://doi.org/10.3390/pathogens9020084
APA Styleda Silva, A. F., da Rocha, C. Q., da Silva, L. C. N., Carvalho Júnior, A. R., Mendes, I. N. F. V., de Araruna, A. B., Motta, E. P., Silva, R. d. S., Campos, C. D. L., Farias, J. R., Oliveira, A. d. S., Silva, D. H. d. S., Nascimento, F. R. F., Guerra, R. N. M., & Monteiro, C. A. (2020). Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia insignis Leaves against Vaginal Isolates of Candida Species. Pathogens, 9(2), 84. https://doi.org/10.3390/pathogens9020084








