Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe
Abstract
1. Introduction
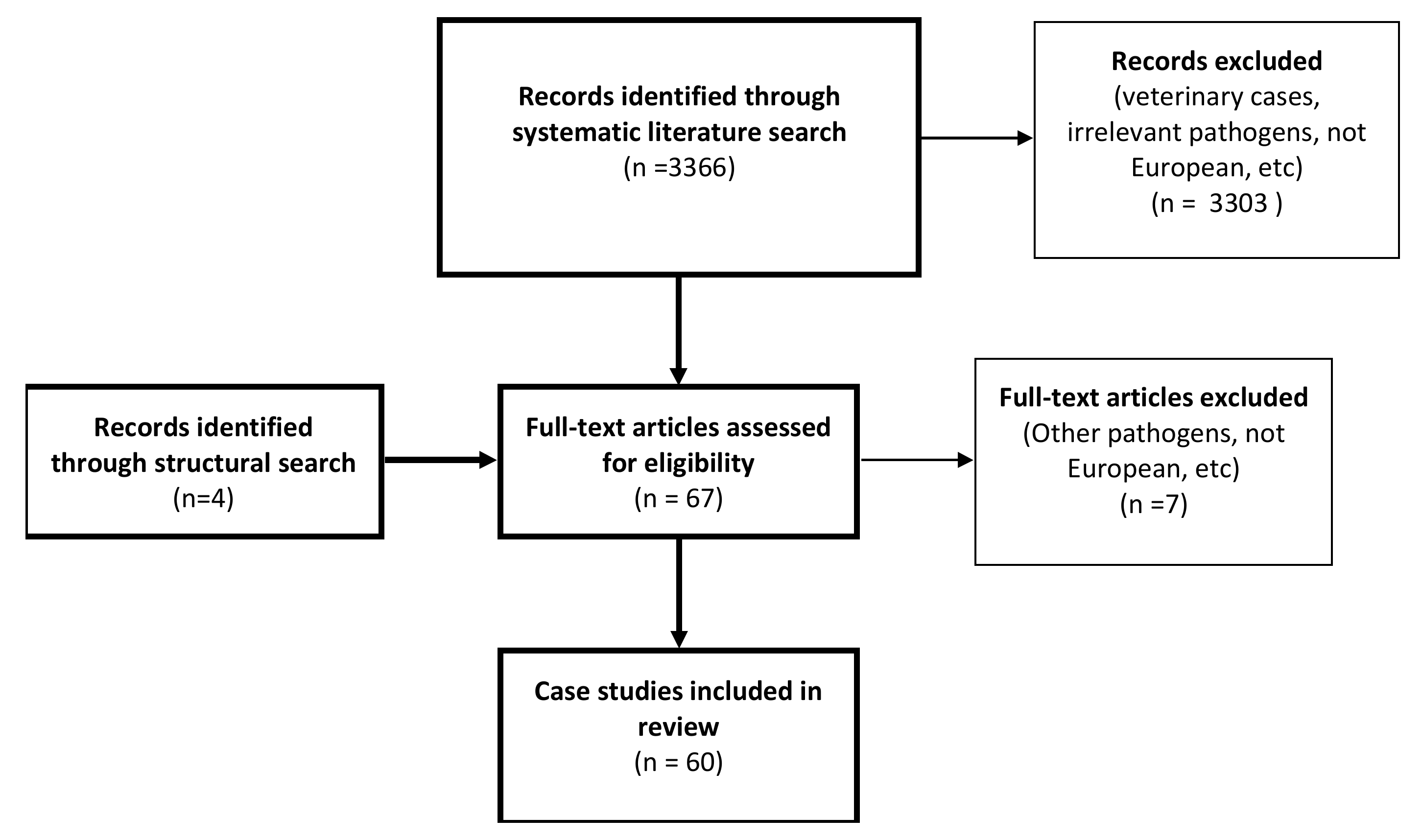
2. Results
2.1. Literature Search
2.2. Extracted Data
2.2.1. Anaplasma Phagocytophilum
Clinical Presentation
Exposure to Ticks
Culture from Ticks or Patient Materials
Pathology
Transmission Experiments
2.2.2. Babesia Species
Clinical Presentation
Exposure to Ticks
Culture from Ticks or Patient Materials
Pathology
Transmission Experiments
2.2.3. Borrelia miyamotoi
Clinical Presentation
Exposure to Ticks
Culture from Ticks or Patient Materials
Pathology
Transmission Experiments
2.2.4. Neoehrlichia mikurensis
Clinical Presentation
Exposure to Ticks
Culture from Ticks or Patient Materials
Pathology
Transmission Experiments
2.2.5. Spotted Fever Rickettsiae
Clinical Presentation
Exposure to Ticks
Culture from Ticks or Patient Materials
Pathology
Transmission Experiments
3. Discussion, Conclusions and Knowledge Gaps
4. Materials and Methods
4.1. Literature Search
4.2. Selection Criteria
4.3. Data Extraction
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hofhuis, A.; Harms, M.; van den Wijngaard, C.; Sprong, H.; van Pelt, W. Continuing increase of tick bites and Lyme disease between 1994 and 2009. Ticks Tick Borne Dis. 2015, 6, 69–74. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Hjertqvist, M.; Bergstrom, T.; Lundkvist, A. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit. Vectors 2012, 5, 184. [Google Scholar] [CrossRef]
- Kunze, U. The International Scientific Working Group on Tick-Borne Encephalitis (ISW TBE): Review of 17 years of activity and commitment. Ticks Tick Borne Dis. 2016, 7, 399–404. [Google Scholar] [CrossRef]
- Sprong, H.; Azagi, T.; Hoornstra, D.; Nijhof, A.M.; Knorr, S.; Baarsma, M.E.; Hovius, J.W. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasit. Vectors 2018, 11, 145. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubalek, Z.; Foldvari, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalska, E.; et al. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Jahfari, S.; Hofhuis, A.; Fonville, M.; van der Giessen, J.; van Pelt, W.; Sprong, H. Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS Negl. Trop. Dis. 2016, 10, e0005042. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Microbiology: Ditch the term pathogen. Nature 2014, 516, 165–166. [Google Scholar] [CrossRef]
- Pirofski, L.A.; Casadevall, A. Q and A: What is a pathogen? A question that begs the point. BMC Biol. 2012, 10, 6. [Google Scholar] [CrossRef]
- Portillo, A.; de Sousa, R.; Santibanez, S.; Duarte, A.; Edouard, S.; Fonseca, I.P.; Marques, C.; Novakova, M.; Palomar, A.M.; Santos, M.; et al. Guidelines for the Detection of Rickettsia spp. Vector Borne Zoonotic Dis. 2017, 17, 23–32. [Google Scholar] [CrossRef]
- Abdad, M.Y.; Abou Abdallah, R.; Fournier, P.E.; Stenos, J.; Vasoo, S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Wolbach, S.B.; Schlesinger, M.J. The cultivation of the micro-organisms of Rocky Mountain spotted fever (Dermocentroxenus rickettsi) and of typhus (Rickettsia prowazeki) in tissue plasma cultures. J. Med. 1923, 44, 231–256. [Google Scholar]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.B.; Chowning, W.M. Studies in Pyroplasmosis hominis (“spotted fever” or “tick fever” of the Rocky Mountains). J. Infect. Dis. 1904, 1, 31–57. [Google Scholar] [CrossRef]
- Wolbach, S.B. Studies on Rocky Mountain spotted fever. J. Med. Res. 1919, 41, 2–197. [Google Scholar]
- Silverman, D.J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect. Immun. 1984, 44, 545–553. [Google Scholar] [CrossRef]
- Olmer, D. Sur une infection épidémique, avec exanthème de nature indéterminée. Mars. Med. 1925, 22, 1291–1293. [Google Scholar]
- Rovery, C.; Brouqui, P.; Raoult, D. Questions on Mediterranean spotted fever a century after its discovery. Emerg. Infect. Dis. 2008, 14, 1360–1367. [Google Scholar] [CrossRef]
- Marrero, M.; Raoult, D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am. J. Trop. Med. Hyg. 1989, 40, 197–199. [Google Scholar] [CrossRef]
- Durand, P.; Conseil, E. Transmission expérimentale de la fièvre boutonneuse par Rhipicephalus sanguineus. C. R. Acad. Sci. 1930, 190, 1244. [Google Scholar]
- George, F.; Brouqui, P.; Boffa, M.C.; Mutin, M.; Drancourt, M.; Brisson, C.; Sampol, J. Demonstration of Rickettsia conorii-induced endothelial injury in vivo by measuring circulating endothelial cells, thrombomodulin, and von Willebrand factor in patients with Mediterranean spotted fever. Blood 1993, 82, 2109–2116. [Google Scholar] [CrossRef]
- Walker, D.H.; Occhino, C.; Tringali, G.R.; Di Rosa, S.; Mansueto, S. Pathogenesis of rickettsial eschars: The tache noire of boutonneuse fever. Hum. Pathol. 1988, 19, 1449–1454. [Google Scholar] [CrossRef]
- Nilsson, K.; Elfving, K.; Pahlson, C. Rickettsia helvetica in patient with meningitis, Sweden, 2006. Emerg. Infect. Dis. 2010, 16, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, C.B.; Milman, N.; Nielsen, H.W.; Krogfelt, K.A.; Larsen, K.R. A prospective study evaluating the presence of Rickettsia in Danish patients with sarcoidosis. Scand. J. Infect. Dis. 2009, 41, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Koetsveld, J.; Tijsse-Klasen, E.; Herremans, T.; Hovius, J.W.; Sprong, H. Serological and molecular evidence for spotted fever group Rickettsia and Borrelia burgdorferi sensu lato co-infections in The Netherlands. Ticks Tick Borne Dis. 2016, 7, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ocias, L.F.; Dessau, R.B.; Lebech, A.M.; Jorgensen, C.S.; Petersen, R.F.; Krogfelt, K.A. Evidence of rickettsiae in Danish patients tested for Lyme neuroborreliosis: A retrospective study of archival samples. BMC Infect. Dis. 2018, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann. N. Y. Acad. Sci. 2006, 1078, 236–247. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Dumic, I.; Patel, J.; Hart, M.; Niendorf, E.R.; Martin, S.; Ramanan, P. Splenic Rupture as the First Manifestation of Babesia Microti Infection: Report of a Case and Review of Literature. Am. J. Case Rep. 2018, 19, 335–341. [Google Scholar] [CrossRef]
- Bakken, J.S. The discovery of human granulocytotropic ehrlichiosis. J. Lab. Clin. Med. 1998, 132, 175–180. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef]
- Massung, R.F.; Levin, M.L.; Munderloh, U.G.; Silverman, D.J.; Lynch, M.J.; Kurtti, T.J. Isolation of Anaplasma phagocytophilum strain Ap-variant 1 in a tick-derived cell line. Ann. N. Y. Acad. Sci. 2006, 1078, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.L.; Nelson, C.; Vitale, B.; Madigan, J.E.; Dumler, J.S.; Kurtti, T.J.; Munderloh, U.G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 1996, 334, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R., 3rd; Dawson, J.E.; Katavolos, P.; Warner, C.K.; Kolbert, C.P.; Persing, D.H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 6209–6214. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology and Statistics. Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed on 1 January 2019).
- Tijsse-Klasen, E.; Koopmans, M.P.; Sprong, H. Tick-borne pathogen—Reversed and conventional discovery of disease. Front. Public Health 2014, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Inglis, T.J. Principia aetiologica: Taking causality beyond Koch’s postulates. J. Med. Microbiol. 2007, 56, 1419–1422. [Google Scholar] [CrossRef]
- Falkow, S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev. Infect. Dis. 1988, 10, S274–S276. [Google Scholar] [CrossRef]
- Raoult, D. Uncultured candidatus neoehrlichia mikurensis. Clin. Infect. Dis. 2014, 59, 1042. [Google Scholar] [CrossRef]
- Wagemakers, A.; Oei, A.; Fikrig, M.M.; Miellet, W.R.; Hovius, J.W. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit. Vectors 2014, 7, 418. [Google Scholar] [CrossRef]
- Wenneras, C.; Bloemberg, G.; Bogdan, C. Reply to Raoult. Clin. Infect. Dis. 2014, 59, 1042–1043. [Google Scholar] [CrossRef]
- Wass, L.; Grankvist, A.; Bell-Sakyi, L.; Bergstrom, M.; Ulfhammer, E.; Lingblom, C.; Wenneras, C. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg. Microbes Infect. 2019, 8, 413–425. [Google Scholar] [CrossRef]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoersdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. Update on tick-borne bacterial diseases in Europe. Parasite 2009, 16, 259–273. [Google Scholar] [CrossRef][Green Version]
- Sanchez, E.; Vannier, E.; Wormser, G.P.; Hu, L.T. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA 2016, 315, 1767–1777. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Grankvist, A.; Sandelin, L.L.; Andersson, J.; Fryland, L.; Wilhelmsson, P.; Lindgren, P.E.; Forsberg, P.; Wenneras, C. Infections with Candidatus Neoehrlichia mikurensis and Cytokine Responses in 2 Persons Bitten by Ticks, Sweden. Emerg. Infect. Dis. 2015, 21, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Koliou, M.; Chochlakis, D.; Ioannou, I.; Mazeri, S.; Tselentis, Y. Anaplasma phagocytophilum infection in a child. Pediatr. Infect. Dis. J. 2008, 27, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Koliou, M.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Kawasaki disease and Anaplasma sp. infection of an infant in Cyprus. Int. J. Infect. Dis. 2009, 13, e71–e73. [Google Scholar] [CrossRef] [PubMed]
- Hindryckx, P.; D’Heygere, F. A 42-year-old man with persistent fever after holiday. Tijdschr. Geneeskd. 2009, 65, 495–496. [Google Scholar] [CrossRef]
- Galloo, X.; Wiels, W.; Du Four, S.; Surmont, M.; Mertens, R. Beyond Lyme: Tick-borne illness in Europe. Acta Clin. Belg. 2016, 71, 40. [Google Scholar]
- Chochlakis, D.; Psaroulaki, A.; Kokkini, S.; Kostanatis, S.; Arkalati, E.; Karagrannaki, E.; Tsiatis, K.; Tselentis, Y.; Gikas, A. First evidence of Anaplasma infection in Crete, Greece. Report of six human cases. Clin. Microbiol. Infect. 2009, 15, 8–9. [Google Scholar] [CrossRef]
- Lotric-Furlan, S.; Ruzic-Sabljic, E.; Strle, F. Concomitant human granulocytic anaplasmosis and Lyme neuroborreliosis. Clin. Microbiol. Infect. 2009, 15, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Chochlakis, D.; Ioannou, I.; Florentia, A.; Gikas, A.; Tselentis, Y. Acute anaplasmosis in humans in Cyprus. Clin. Microbiol. Infect. 2009, 15, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Haschke-Becher, E.; Bernauer, R.; Walleczek, A.M.; Apfalter, P.; Afazel-Saeedi, S.; Kraus, J.; Ladurner, G.; Strasser, P. First detection of the Anaplasma phagocytophilum groEL-A genotype in man. J. Infect. 2010, 60, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Novakova, M.; Vichova, B.; Majlathova, V.; Lesnakova, A.; Pochybova, M.; Petko, B. First case of human granulocytic anaplasmosis from Slovakia. Ann. Agric. Environ. Med. 2010, 17, 173–175. [Google Scholar]
- Vogl, U.M.; Presterl, E.; Stanek, G.; Ramharter, M.; Gattringer, K.B.; Graninger, W. First described case of human granulocytic anaplasmosis in a patient in Eastern Austria. Wien. Med. Wochenschr. 2010, 160, 91–93. [Google Scholar] [CrossRef]
- Jereb, M.; Pecaver, B.; Tomazic, J.; Muzlovic, I.; Avsic-Zupanc, T.; Premru-Srsen, T.; Levicnik-Stezinar, S.; Karner, P.; Strle, F. Severe human granulocytic anaplasmosis transmitted by blood transfusion. Emerg. Infect. Dis. 2012, 18, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Koebel, C.; Kern, A.; Edouard, S.; Hoang, A.T.; Celestin, N.; Hansmann, Y.; Jaulhac, B.; Brouqui, P.; De Martino, S.J. Human granulocytic anaplasmosis in eastern France: Clinical presentation and laboratory diagnosis. Diagn. Microbiol. Infect. Dis. 2012, 72, 214–218. [Google Scholar] [CrossRef]
- Vanicek, J.; Stastnik, M.; Kianicka, B.; Bares, M.; Bulik, M. Rare neurological presentation of human granulocytic anaplasmosis. Eur. J. Neurol. 2013, 20, e70–e72. [Google Scholar] [CrossRef]
- Hagedorn, P.; Imhoff, M.; Fischer, C.; Domingo, C.; Niedrig, M. Human granulocytic anaplasmosis acquired in Scotland, 2013. Emerg. Infect. Dis. 2014, 20, 1079–1081. [Google Scholar] [CrossRef]
- Hing, M.; Woestyn, S.; Van Bosterhaut, B.; Desbonnet, Y.; Heyman, P.; Cochez, C.; Silaghi, C.; Sprong, H.; Fournier, P.E.; Raoult, D.; et al. Diagnosis of human granulocytic anaplasmosis in Belgium by combining molecular and serological methods. New Microbes New Infect. 2014, 2, 177–178. [Google Scholar] [CrossRef][Green Version]
- Picco, P.; Naselli, A.; Pala, G.; Rizzo, F.; Damasio, B.; Buoncompagni, A.; Martini, A. Whole-body MRI as an unconventional diagnostic tool in a pediatric patient with systemic infection. Acta Radiol. Short Rep. 2014, 3, 2047981614549571. [Google Scholar] [CrossRef] [PubMed]
- Welc-Faleciak, R.; Kowalec, M.; Zajkowska, J.; Pancewicz, S.A.; Sinski, E. Clinical and molecular features of one case of human infection with Anaplasma phagocytophilum from Podlaskie Province in eastern Poland. Ann. Agric. Environ. Med. 2015, 22, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.C.; Nunez, M.J.; Portillo, A.; Oteo, J.A. Human anaplasmosis: Two case-reports. Enferm. Infecc. Microbiol. Clin. 2015, 33, 68–69. [Google Scholar] [CrossRef]
- Markowicz, M.; Schotta, A.M.; Wijnveld, M.; Stanek, G. Human granulocytic anaplasmosis acquired in Connecticut, USA, diagnosed in Vienna, Austria, 2015. Diagn. Microbiol. Infect. Dis. 2016, 84, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Pokorn, M.; Zupanc, T.A.; Strle, F. Pediatric Human Granulocytic Anaplasmosis is Rare in Europe. Pediatr. Infect. Dis. J. 2016, 35, 358–359. [Google Scholar] [CrossRef]
- Tsiodras, S.; Spanakis, N.; Spanakos, G.; Pervanidou, D.; Georgakopoulou, T.; Campos, E.; Petra, T.; Kanellopoulos, P.; Georgiadis, G.; Antalis, E.; et al. Fatal human anaplasmosis associated with macrophage activation syndrome in Greece and the Public Health response. J. Infect. Public Health 2017, 10, 819–823. [Google Scholar] [CrossRef]
- Lagler, H.; Harrison, N.; Kussmann, M.; Obermuller, M.; Burgmann, H.; Makristathis, A.; Ramharter, M. Direct detection of Anaplasma phagocytophilum by polymerase chain reaction followed by electrospray ionization mass spectrometry from human blood. Int. J. Infect. Dis. 2017, 60, 61–63. [Google Scholar] [CrossRef]
- Vikse, J.; Klos, J.; Berg, A. A travelling camper with a spiking fever, headache, myalgia, hepatitis, and intracellular inclusions. Lancet Infect. Dis. 2017, 17, 1318. [Google Scholar] [CrossRef]
- Hulinska, D.; Votypka, J.; Plch, J.; Vlcek, E.; Valesova, M.; Bojar, M.; Hulinsky, V.; Smetana, K. Molecular and microscopical evidence of Ehrlichia spp. and Borrelia burgdorferi sensu lato in patients, animals and ticks in the Czech Republic. New Microbiol. 2002, 25, 437–448. [Google Scholar]
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef]
- Granquist, E.G.; Aleksandersen, M.; Bergstrom, K.; Dumler, S.J.; Torsteinbo, W.O.; Stuen, S. A morphological and molecular study of Anaplasma phagocytophilum transmission events at the time of Ixodes ricinus tick bite. Acta Vet. Scand. 2010, 52, 43. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S. Anaplasma phagocytophilum—The most widespread tick-borne infection in animals in Europe. Vet. Res. Commun. 2007, 31, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Fourie, J.J.; Evans, A.; Labuschagne, M.; Crafford, D.; Madder, M.; Pollmeier, M.; Schunack, B. Transmission of Anaplasma phagocytophilum (Foggie, 1949) by Ixodes ricinus (Linnaeus, 1758) ticks feeding on dogs and artificial membranes. Parasit. Vectors 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Casey, A.N.; French, N.P.; Woldehiwet, Z. A review of studies on the transmission of Anaplasma phagocytophilum from sheep: Implications for the force of infection in endemic cycles. Exp. Appl. Acarol. 2002, 28, 195–202. [Google Scholar] [CrossRef]
- Tomassone, L.; Berriatua, E.; De Sousa, R.; Duscher, G.G.; Mihalca, A.D.; Silaghi, C.; Sprong, H.; Zintl, A. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 2018, 251, 17–26. [Google Scholar] [CrossRef]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Foldvari, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit. Vectors 2014, 7, 365. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Foldvari, G.; Jahfari, S.; Rigo, K.; Jablonszky, M.; Szekeres, S.; Majoros, G.; Toth, M.; Molnar, V.; Coipan, E.C.; Sprong, H. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in urban hedgehogs. Emerg. Infect. Dis. 2014, 20, 496–498. [Google Scholar] [CrossRef]
- Dunaj, J.; Moniuszko-Malinowska, A.; Swiecicka, I.; Andersson, M.; Czupryna, P.; Rutkowski, K.; Zambrowski, G.; Zajkowska, J.; Grygorczuk, S.; Kondrusik, M.; et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv. Med. Sci. 2018, 63, 167–172. [Google Scholar] [CrossRef]
- Ramharter, M.; Walochnik, J.; Lagler, H.; Winkler, S.; Wernsdorfer, W.H.; Stoiser, B.; Graninger, W. Clinical and molecular characterization of a near fatal case of human babesiosis in Austria. J. Travel Med. 2010, 17, 416–418. [Google Scholar] [CrossRef][Green Version]
- Holler, J.G.; Roser, D.; Nielsen, H.V.; Eickhardt, S.; Chen, M.; Lester, A.; Bang, D.; Frandsen, C.; David, K.P. A case of human babesiosis in Denmark. Travel Med. Infect. Dis. 2013, 11, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Poisnel, E.; Ebbo, M.; Berda-Haddad, Y.; Faucher, B.; Bernit, E.; Carcy, B.; Piarroux, R.; Harle, J.R.; Schleinitz, N. Babesia microti: An unusual travel-related disease. BMC Infect. Dis. 2013, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Surgers, L.; Belkadi, G.; Foucard, A.; Lalande, V.; Girard, P.M.; Hennequin, C. Babesiosis and Lyme disease co-infection in a female patient returning from the United States. Med. Mal. Infect. 2015, 45, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, J.; Zarnowska-Prymek, H.; Stanczak, J.; Kozlowska, J.; Wiercinska-Drapalo, A. Symptomatic co-infection with Babesia microti and Borrelia burgdorferi in patient after international exposure; a challenging case in Poland. Ann. Agric. Environ. Med. 2016, 23, 387–389. [Google Scholar] [CrossRef]
- Arsuaga, M.; Gonzalez, L.M.; Lobo, C.A.; de la Calle, F.; Bautista, J.M.; Azcarate, I.G.; Puente, S.; Montero, E. First Report of Babesia microti-Caused Babesiosis in Spain. Vector Borne Zoonotic Dis. 2016, 16, 677–679. [Google Scholar] [CrossRef]
- De Ramon, C.; Cid, J.; Rodriguez-Tajes, S.; Alvarez-Martinez, M.J.; Valls, M.E.; Fernandez, J.; Lozano, M. Severe Babesia microti infection in an American immunocompetent patient diagnosed in Spain. Transfus. Apher. Sci. 2016, 55, 243–244. [Google Scholar] [CrossRef]
- Mcnamara, R.; van der Velde, J.; Meike, R. Babesiosis-an unusual cause of acute confusion in an elderly man. Eur. Geriatr. Med. 2010, 1, S158. [Google Scholar]
- Browne, S.; Ryan, Y.; Goodyer, M.; Gilligan, O. Fatal babesiosis in an asplenic patient. Br. J. Haematol. 2010, 148, 494. [Google Scholar] [CrossRef]
- Haapasalo, K.; Suomalainen, P.; Sukura, A.; Siikamaki, H.; Jokiranta, T.S. Fatal babesiosis in man, Finland, 2004. Emerg. Infect. Dis. 2010, 16, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Martinot, M.; Zadeh, M.M.; Hansmann, Y.; Grawey, I.; Christmann, D.; Aguillon, S.; Jouglin, M.; Chauvin, A.; De Briel, D. Babesiosis in immunocompetent patients, Europe. Emerg. Infect. Dis. 2011, 17, 114–116. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Rojo, S.; Gonzalez-Camacho, F.; Luque, D.; Lobo, C.A.; Montero, E. Severe babesiosis in immunocompetent man, Spain, 2011. Emerg. Infect. Dis. 2014, 20, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Castro, E.; Lobo, C.A.; Richart, A.; Ramiro, R.; Gonzalez-Camacho, F.; Luque, D.; Velasco, A.C.; Montero, E. First report of Babesia divergens infection in an HIV patient. Int. J. Infect. Dis. 2015, 33, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Morch, K.; Holmaas, G.; Frolander, P.S.; Kristoffersen, E.K. Severe human Babesia divergens infection in Norway. Int. J. Infect. Dis. 2015, 33, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tanyel, E.; Guler, N.; Hokelek, M.; Ulger, F.; Sunbul, M. A case of severe babesiosis treated successfully with exchange transfusion. Int. J. Infect. Dis. 2015, 38, 83–85. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.; Lyons, C.; Abdou, M.; Patowary, R.; Aslam, S.; Kinsella, N.; Zintl, A.; Hunfeld, K.P.; Wormser, G.P.; Gray, J.; et al. Splenic dysfunction from celiac disease resulting in severe babesiosis. Ticks Tick Borne Dis. 2017, 8, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Asensi, V.; Gonzalez, L.M.; Fernandez-Suarez, J.; Sevilla, E.; Navascues, R.A.; Suarez, M.L.; Lauret, M.E.; Bernardo, A.; Carton, J.A.; Montero, E. A fatal case of Babesia divergens infection in Northwestern Spain. Ticks Tick Borne Dis. 2018, 9, 730–734. [Google Scholar] [CrossRef]
- Haselbarth, K.; Kurz, M.; Hunfeld, K.P.; Krieger, G. Babesiosis in an immunocompromised German patient. Med. Klin. 2008, 103, 104–107. [Google Scholar] [CrossRef]
- Blum, S.; Gattringer, R.; Haschke, E.; Walochnik, J.; Tschurtschenthaler, G.; Lang, F.; Oberbauer, R. The case: Hemolysis and acute renal failure. Babesiosis. Kidney Int. 2011, 80, 681–683. [Google Scholar] [CrossRef]
- Blackberg, J.; Lazarevic, V.L.; Hunfeld, K.P.; Persson, K.E.M. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann. Hematol. 2018, 97, 731–733. [Google Scholar] [CrossRef]
- Malandrin, L.; L’Hostis, M.; Chauvin, A. Isolation of Babesia divergens from carrier cattle blood using in vitro culture. Vet. Res. 2004, 35, 131–139. [Google Scholar] [CrossRef]
- Gorenflot, A.; Brasseur, P.; Precigout, E.; L’Hostis, M.; Marchand, A.; Schrevel, J. Cytological and immunological responses to Babesia divergens in different hosts: Ox, gerbil, man. Parasitol. Res. 1991, 77, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, I.; Gonzalez, L.M.; Estrada, K.; Grande, R.; Zaballos, A.; Lobo, C.A.; Barrera, J.; Sanchez-Flores, A.; Montero, E. High-Quality Draft Genome Sequence of Babesia divergens, the Etiological Agent of Cattle and Human Babesiosis. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Krampitz, H.E. Babesia microti: Morphology, distribution and host relationship in Germany. Zentralbl. Bakteriol. Orig. A 1979, 244, 411–415. [Google Scholar] [PubMed]
- Bonnet, S.; Jouglin, M.; L’Hostis, M.; Chauvin, A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 2007, 13, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Zintl, A.; Hildebrandt, A.; Hunfeld, K.P.; Weiss, L. Zoonotic babesiosis: Overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; von Stedingk, L.V.; Gurtelschmid, M.; Granstrom, M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 2002, 40, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Young, E.R. The transmission of a human strain of Babesia divergens by Ixodes ricinus ticks. J. Parasitol. 1980, 66, 359–360. [Google Scholar] [CrossRef]
- Bonnet, S.; Brisseau, N.; Hermouet, A.; Jouglin, M.; Chauvin, A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus. Vet. Res. 2009, 40, 21. [Google Scholar] [CrossRef]
- Hovius, J.W.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef]
- Boden, K.; Lobenstein, S.; Hermann, B.; Margos, G.; Fingerle, V. Borrelia miyamotoi-Associated Neuroborreliosis in Immunocompromised Person. Emerg. Infect. Dis. 2016, 22, 1617–1620. [Google Scholar] [CrossRef]
- Hoornstra, D.; Koetsveld, J.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Borrelia miyamotoi Disease in an Immunocompetent Patient, Western Europe. Emerg. Infect. Dis. 2018, 24, 1770–1772. [Google Scholar] [CrossRef]
- Kuleshov, K.V.; Hoornstra, D.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Draft Whole-Genome Sequences of Two Western European Borrelia miyamotoi Isolates. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef]
- Wagemakers, A.; Staarink, P.J.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015, 31, 260–269. [Google Scholar] [CrossRef]
- Henningsson, A.J.; Asgeirsson, H.; Hammas, B.; Karlsson, E.; Parke, A.; Hoornstra, D.; Wilhelmsson, P.; Hovius, J.W. Two Cases of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerg. Infect. Dis. 2019, 25, 1965–1968. [Google Scholar] [CrossRef]
- Narasimhan, S.; Hoornstra, D. Ixodes scapularis and Ixodes ricinus murine transmission of an American, European and Japanese Borrelia miyamotoi tick strain. Unpublished.
- Van Duijvendijk, G.; Coipan, C.; Wagemakers, A.; Fonville, M.; Ersoz, J.; Oei, A.; Foldvari, G.; Hovius, J.; Takken, W.; Sprong, H. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit. Vectors 2016, 9, 97. [Google Scholar] [CrossRef]
- Fehr, J.S.; Bloemberg, G.V.; Ritter, C.; Hombach, M.; Luscher, T.F.; Weber, R.; Keller, P.M. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg. Infect. Dis. 2010, 16, 1127–1129. [Google Scholar] [CrossRef]
- Von Loewenich, F.D.; Geissdorfer, W.; Disque, C.; Matten, J.; Schett, G.; Sakka, S.G.; Bogdan, C. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: Evidence for a European sequence variant. J. Clin. Microbiol. 2010, 48, 2630–2635. [Google Scholar] [CrossRef]
- Welinder-Olsson, C.; Kjellin, E.; Vaht, K.; Jacobsson, S.; Wenneras, C. First case of human "Candidatus Neoehrlichia mikurensis" infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 2010, 48, 1956–1959. [Google Scholar] [CrossRef]
- Pekova, S.; Vydra, J.; Kabickova, H.; Frankova, S.; Haugvicova, R.; Mazal, O.; Cmejla, R.; Hardekopf, D.W.; Jancuskova, T.; Kozak, T. Candidatus Neoehrlichia mikurensis infection identified in 2 hematooncologic patients: Benefit of molecular techniques for rare pathogen detection. Diagn. Microbiol. Infect. Dis. 2011, 69, 266–270. [Google Scholar] [CrossRef]
- Grankvist, A.; Andersson, P.O.; Mattsson, M.; Sender, M.; Vaht, K.; Hoper, L.; Sakiniene, E.; Trysberg, E.; Stenson, M.; Fehr, J.; et al. Infections with the tick-borne bacterium "Candidatus Neoehrlichia mikurensis" mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin. Infect. Dis. 2014, 58, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, K.; Jonsson, G.; Lindell, P.; Gulfe, A.; Ingvarsson, R.; Lindqvist, E.; Saxne, T.; Grankvist, A.; Wenneras, C.; Marsal, J. Recurrent fever caused by Candidatus Neoehrlichia mikurensis in a rheumatoid arthritis patient treated with rituximab. Rheumatology 2015, 54, 369–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Portillo, A.; Santibanez, P.; Palomar, A.M.; Santibanez, S.; Oteo, J.A. ‘Candidatus Neoehrlichia mikurensis’ in Europe. New Microbes New Infect. 2018, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; Fonville, M.; van Leeuwen, A.D.; Sprong, H. Co-infections and transmission dynamics in a tick-borne bacterium community exposed to songbirds. Environ. Microbiol. 2016, 18, 988–996. [Google Scholar] [CrossRef]
- Richter, D.; Matuschka, F.R. “Candidatus Neoehrlichia mikurensis,” Anaplasma phagocytophilum, and lyme disease spirochetes in questing european vector ticks and in feeding ticks removed from people. J. Clin. Microbiol. 2012, 50, 943–947. [Google Scholar] [CrossRef]
- Jahfari, S.; Fonville, M.; Hengeveld, P.; Reusken, C.; Scholte, E.J.; Takken, W.; Heyman, P.; Medlock, J.M.; Heylen, D.; Kleve, J.; et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasit. Vectors 2012, 5, 74. [Google Scholar] [CrossRef]
- Andersson, M.; Bartkova, S.; Lindestad, O.; Raberg, L. Co-infection with ‘Candidatus Neoehrlichia Mikurensis’ and Borrelia afzelii in Ixodes ricinus ticks in southern Sweden. Vector Borne Zoonotic Dis. 2013, 13, 438–442. [Google Scholar] [CrossRef]
- Burri, C.; Schumann, O.; Schumann, C.; Gern, L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2014, 5, 245–251. [Google Scholar] [CrossRef]
- Nilsson, K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J. Infect. 2009, 58, 79–82. [Google Scholar] [CrossRef]
- Nilsson, K.; Wallmenius, K.; Pahlson, C. Coinfection with Rickettsia helvetica and Herpes Simplex Virus 2 in a Young Woman with Meningoencephalitis. Case Rep. Infect. Dis. 2011, 2011, 469194. [Google Scholar] [CrossRef]
- Jado, I.; Oteo, J.A.; Aldamiz, M.; Gil, H.; Escudero, R.; Ibarra, V.; Portu, J.; Portillo, A.; Lezaun, M.J.; Garcia-Amil, C.; et al. Rickettsia monacensis and human disease, Spain. Emerg. Infect. Dis. 2007, 13, 1405–1407. [Google Scholar] [CrossRef]
- Madeddu, G.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Maida, I.; Fiori, M.L.; Rezza, G.; Mura, M.S. Rickettsia monacensis as cause of Mediterranean spotted fever-like illness, Italy. Emerg. Infect. Dis. 2012, 18, 702–704. [Google Scholar] [CrossRef]
- Tijsse-Klasen, E.; Sprong, H.; Pandak, N. Co-infection of Borrelia burgdorferi sensu lato and Rickettsia species in ticks and in an erythema migrans patient. Parasit. Vectors 2013, 6, 347. [Google Scholar] [CrossRef]
- Elfving, K.; Lukinius, A.; Nilsson, K. Life cycle, growth characteristics and host cell response of Rickettsia helvetica in a Vero cell line. Exp. Appl. Acarol. 2012, 56, 179–187. [Google Scholar] [CrossRef]
- Dong, X.; El Karkouri, K.; Robert, C.; Gavory, F.; Raoult, D.; Fournier, P.E. Genomic comparison of Rickettsia helvetica and other Rickettsia species. J. Bacteriol. 2012, 194, 2751. [Google Scholar] [CrossRef]
- Simser, J.A.; Palmer, A.T.; Fingerle, V.; Wilske, B.; Kurtti, T.J.; Munderloh, U.G. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl. Environ. Microbiol. 2002, 68, 4559–4566. [Google Scholar] [CrossRef]
- Nilsson, K.; Lindquist, O.; Pahlson, C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet 1999, 354, 1169–1173. [Google Scholar] [CrossRef]
- Nilsson, K.; Pahlson, C.; Lukinius, A.; Eriksson, L.; Nilsson, L.; Lindquist, O. Presence of Rickettsia helvetica in granulomatous tissue from patients with sarcoidosis. J. Infect. Dis. 2002, 185, 1128–1138. [Google Scholar] [CrossRef]
- Nielsen, H.; Fournier, P.E.; Pedersen, I.S.; Krarup, H.; Ejlertsen, T.; Raoult, D. Serological and molecular evidence of Rickettsia helvetica in Denmark. Scand. J. Infect. Dis. 2004, 36, 559–563. [Google Scholar] [CrossRef]
- Heylen, D.; Fonville, M.; Docters van Leeuwen, A.; Stroo, A.; Duisterwinkel, M.; van Wieren, S.; Diuk-Wasser, M.; de Bruin, A.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasit. Vectors 2017, 10, 497. [Google Scholar] [CrossRef]
- Akl, T.; Bourgoin, G.; Souq, M.L.; Appolinaire, J.; Poirel, M.T.; Gibert, P.; Abi Rizk, G.; Garel, M.; Zenner, L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite 2019, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Gajda, E.; Hildebrand, J.; Sprong, H.; Bunkowska-Gawlik, K.; Perec-Matysiak, A.; Coipan, E.C. Spotted fever rickettsiae in wild-living rodents from south-western Poland. Parasit. Vectors 2017, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Elfving, K.; Malmsten, J.; Dalin, A.M.; Nilsson, K. Serologic and Molecular Prevalence of Rickettsia helvetica and Anaplasma phagocytophilum in Wild Cervids and Domestic Mammals in the Central Parts of Sweden. Vector Borne Zoonotic Dis. 2015, 15, 529–534. [Google Scholar] [CrossRef]
- Laaksonen, M.; Klemola, T.; Feuth, E.; Sormunen, J.J.; Puisto, A.; Makela, S.; Penttinen, R.; Ruohomaki, K.; Hanninen, J.; Saaksjarvi, I.E.; et al. Tick-borne pathogens in Finland: Comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasit. Vectors 2018, 11, 556. [Google Scholar] [CrossRef]
- Minichova, L.; Hamsikova, Z.; Mahrikova, L.; Slovak, M.; Kocianova, E.; Kazimirova, M.; Skultety, L.; Stefanidesova, K.; Spitalska, E. Molecular evidence of Rickettsia spp. in ixodid ticks and rodents in suburban, natural and rural habitats in Slovakia. Parasit. Vectors 2017, 10, 158. [Google Scholar] [CrossRef]
- Biernat, B.; Stanczak, J.; Michalik, J.; Sikora, B.; Cieniuch, S. Rickettsia helvetica and R. monacensis infections in immature Ixodes ricinus ticks derived from sylvatic passerine birds in west-central Poland. Parasitol. Res. 2016, 115, 3469–3477. [Google Scholar] [CrossRef]
- Honig, V.; Carolan, H.E.; Vavruskova, Z.; Massire, C.; Mosel, M.R.; Crowder, C.D.; Rounds, M.A.; Ecker, D.J.; Ruzek, D.; Grubhoffer, L.; et al. Broad-range survey of vector-borne pathogens and tick host identification of Ixodes ricinus from Southern Czech Republic. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Piesman, J.; Gern, L. Lyme borreliosis in Europe and North America. Parasitology 2004, 129, S191–S220. [Google Scholar] [CrossRef]
- Valarcher, J.F.; Hagglund, S.; Juremalm, M.; Blomqvist, G.; Renstrom, L.; Zohari, S.; Leijon, M.; Chirico, J. Tick-borne encephalitis. Rev. Sci. Tech. 2015, 34, 453–466. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne encephalitis virus—A review of an emerging zoonosis. J. Gen. Virol. 2009, 90, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Hulinska, D.; Votypka, J.; Vanousova, D.; Hercogova, J.; Hulinsky, V.; Drevova, H.; Kurzova, Z.; Uherkova, L. Identification of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in patients with erythema migrans. Folia Microbiol. 2009, 54, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova Heroldova, M.; Dvorackova, M. Seroprevalence of Anaplasma phagocytophilum in patients with suspected Lyme borreliosis. Epidemiol. Mikrobiol. Imunol. 2014, 63, 297–302. [Google Scholar] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Gray, J.S.; Hunfeld, K.P. Human babesiosis in Europe: What clinicians need to know. Infection 2013, 41, 1057–1072. [Google Scholar] [CrossRef]
- Swanson, S.J.; Neitzel, D.; Reed, K.D.; Belongia, E.A. Coinfections acquired from ixodes ticks. Clin. Microbiol. Rev. 2006, 19, 708–727. [Google Scholar] [CrossRef]
- Krause, P.J.; Telford, S.R., 3rd; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef]
- Belongia, E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002, 2, 265–273. [Google Scholar] [CrossRef]
- Parasites—Babesiosis. Available online: https://www.cdc.gov/parasites/babesiosis/data-statistics/index.html (accessed on 16 December 2019).
- Hildebrandt, A.; Hunfeld, K.P.; Baier, M.; Krumbholz, A.; Sachse, S.; Lorenzen, T.; Kiehntopf, M.; Fricke, H.J.; Straube, E. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 595–601. [Google Scholar] [CrossRef]
- Koetsveld, J.; Kolyasnikova, N.M.; Wagemakers, A.; Toporkova, M.G.; Sarksyan, D.S.; Oei, A.; Platonov, A.E.; Hovius, J.W. Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin. Microbiol. Infect. 2017, 23, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, K.V.; Margos, G.; Fingerle, V.; Koetsveld, J.; Goptar, I.A.; Markelov, M.L.; Kolyasnikova, N.M.; Sarksyan, D.S.; Kirdyashkina, N.P.; Shipulin, G.A.; et al. Whole genome sequencing of Borrelia miyamotoi isolate Izh-4: Reference for a complex bacterial genome. BMC Genom. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Quarsten, H.; Grankvist, A.; Hoyvoll, L.; Myre, I.B.; Skarpaas, T.; Kjelland, V.; Wenneras, C.; Noraas, S. Candidatus Neoehrlichia mikurensis and Borrelia burgdorferi sensu lato detected in the blood of Norwegian patients with erythema migrans. Ticks Tick Borne Dis. 2017, 8, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for lyme disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Del Bene, V.E. Temperature. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
| Microorganism | Number of Cases | PCR (*Sequenced) | Serology | Blood Smear | Bone Marrow Smear | Other Microscopy | Cultivation |
|---|---|---|---|---|---|---|---|
| A. phagocytophilum | 32 | 23 (*17) | 26 | 7 | 2 | - | - |
| B. microti | 7 | 7 (*5) | 2 | 6 | - | - | - |
| B. divergens | 11 | 7 (*7) | 5 | 10 | - | - | - |
| B. venatorum/EU1 | 3 | 3 (*3) | 1 | 2 | - | - | - |
| B. miyamotoi | 3 | 2 (*2) | 1 | - | - | 2 | - |
| N. mikurensis | 14 | 14 (*13) | - | - | - | 2 | - |
| R. helvetica | 3 | 3 (*3) | 2 | - | - | - | 2 |
| R. monacensis | 4 | 4 (*4) | 3 | - | - | - | 1 |
| Microorganism | Number of Cases * | Clinical Manifestations * | Exposure to Ticks * | Culture from Ticks or Patients ** | Pathology * | Transmission Experiments ** |
|---|---|---|---|---|---|---|
| A. phagocytophilum | 32 [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] | Fever (88%), headache (56%), malaise (41%), myalgia (41%), arthralgia (25%), erythematous skin manifestations such as morbilliform and maculopapular rashes (22%), lymphadenopathy (19%), abdominal complaints (16%),anemia (13%), chills (13%), splenomegaly (9%), rigors (6%), dark urine (3%) | History of tick-bite (34%), exposure to ticks (38%) | [70] (Cultivated from patient blood) | Leucocytopenia (50%), positive blood/bone marrow smear (25%). | [74](infection of I. ricinus from sheep and to naïve dogs), [75] (infection of I. ricinus from sheep under natural conditions) |
| B. microti | 7 [79,80,81,82,83,84,85] | Fever (100%), anemia (86%), malaise (57%), chills (57%), headache (43%), abdominal complaints (43%), skin manifestation (erythematous rash) (14%) | History of tick-bite (14%), exposure to ticks outside Europe (86%) | None | anemia (86%), thrombocytopenia (86%), elevated liver enzymes (86%), elevated kidney function (14%), | [107] (infection of I. ricinus from and to gerbils) |
| B. divergens | 11 [86,87,88,89,90,91,92,93,94,95] | Fever (82%), anemia (45%), malaise (36%), abdominal complaints (36%), jaundice (36%), dark urine (27%), headache (18%), myalgia (18%), arthralgia (18%) | History of tick-bite (45%), exposure/risk (55%) | [102] (Cultivated from patient blood) | Thrombocytopenia (73%), elevated kidney values (55%), elevated liver enzymes (45%), anemia (45%) | [108] (infection of I. ricinus to cattle and gerbils) |
| B. venatorum/EU1 | 3 [96,97,98] | Anemia (100%), fever (67%), dark urine (67%), myalgia (33%), rigors (33%) | Exposure/risk (33%) | None | Anemia (100%), elevated kidney values (100%) and thrombocytopenia (33%) | [109] (infection of I. ricinus from and to sheep blood) |
| B. miyamotoi | 3 [107,108,109] | Neurological complaints (67%), headache (67%), abdominal complaints (33%), myalgia (33%), arthalgia (33%), skin manifestations (33%), lymphadenopathy (33%), weight loss (33%) | History of tick-bite (100%) | [113] (Cultivated from I. ricinus) | Unknown | [117] (infection from wild I. ricinus to naive mice), [116] (infection of I. ricinus from and to CD1 mice) |
| N. mikurensis | 14 [43,112,113,114,115,116,117] | Fever (86%), skin manifestation (erythematous rash, erysipelas-like rash and EM; 36%), anemia (29%), malaise (14%), arthralgia (14%), chills (14%), abdominal complaints (14%), oedema (14%), weight loss (7%), myalgia (7%) | History of tick-bite (36%), exposure/risk (14%) | [41] ( Cultivated from patient blood) | Unknown | [129] (infection from wild rodents to I. ricinus) |
| R. helvetica | 3 [19,126,127] | Fever (100%), headache (100%), photophobia (67%), skin manifestation (macular rash) (33%), myalgia (33%), arthralgia (33%) | Exposure/risk (100%) | [22,131] (Cultivated from patient CSF), [135](Cultivated from I. ricinus) | Unknown | None |
| R. monacensis | 4 [128,129,130], | Skin manifestation (100%) (erythematous rash, maculopapular rash and EM), fever (75%), headache (75%), anemia (25%) | History of tick-bite (50%), exposure/risk (25%) | [132] (Cultivated from patient blood), [137](Cultivated from I. ricinus) | Unknown | None |
| Tick-Borne Microorganism | European Derived Cases | Cultivated from | Pathology | Transmission Experiments | ||
|---|---|---|---|---|---|---|
| Ticks | Patients | |||||
| B. burgdorferi s.l. | + | + | + | + | + | |
| TBE virus | + | + | + | + | + | |
| A. phagocytophilum | + | - | + | + | +/& | |
| B. divergens | + | - | + | + | + | |
| B. microti | - | - | - | + | + | |
| B. venatorum | + | - | - | + | + | |
| B. miyamotoi | + | + | - | - | +/# | |
| N. mikurensis | + | - | + | - | - | |
| R. helvetica | + | + | + | - | - | |
| R. monacensis | + | + | + | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azagi, T.; Hoornstra, D.; Kremer, K.; Hovius, J.W.R.; Sprong, H. Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe. Pathogens 2020, 9, 150. https://doi.org/10.3390/pathogens9020150
Azagi T, Hoornstra D, Kremer K, Hovius JWR, Sprong H. Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe. Pathogens. 2020; 9(2):150. https://doi.org/10.3390/pathogens9020150
Chicago/Turabian StyleAzagi, Tal, Dieuwertje Hoornstra, Kristin Kremer, Joppe W. R. Hovius, and Hein Sprong. 2020. "Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe" Pathogens 9, no. 2: 150. https://doi.org/10.3390/pathogens9020150
APA StyleAzagi, T., Hoornstra, D., Kremer, K., Hovius, J. W. R., & Sprong, H. (2020). Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe. Pathogens, 9(2), 150. https://doi.org/10.3390/pathogens9020150






