Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed
Abstract
1. Introduction
2. Results
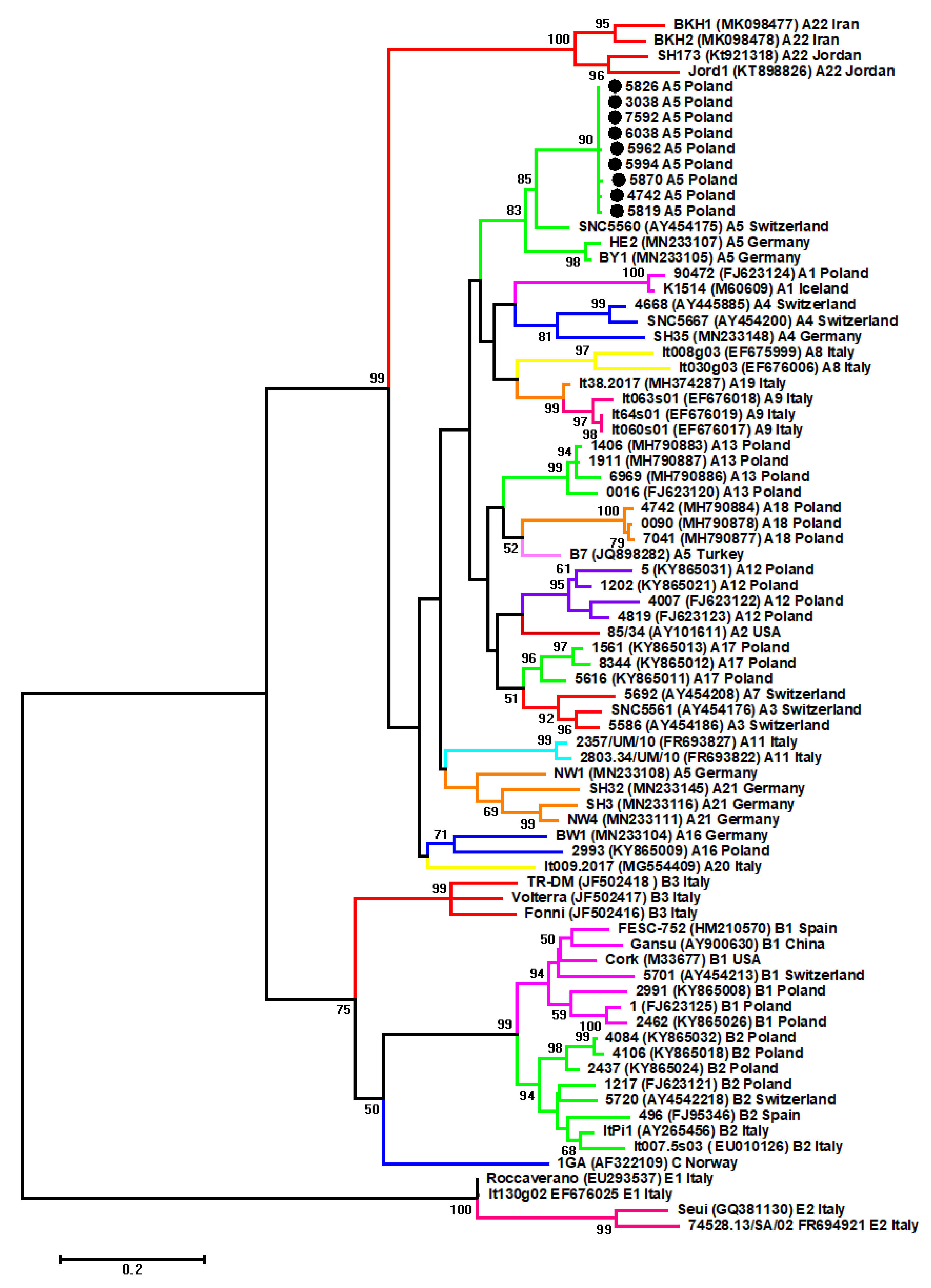
2.1. Phylogenetic Analysis of SRLV Based on Gag Fragment
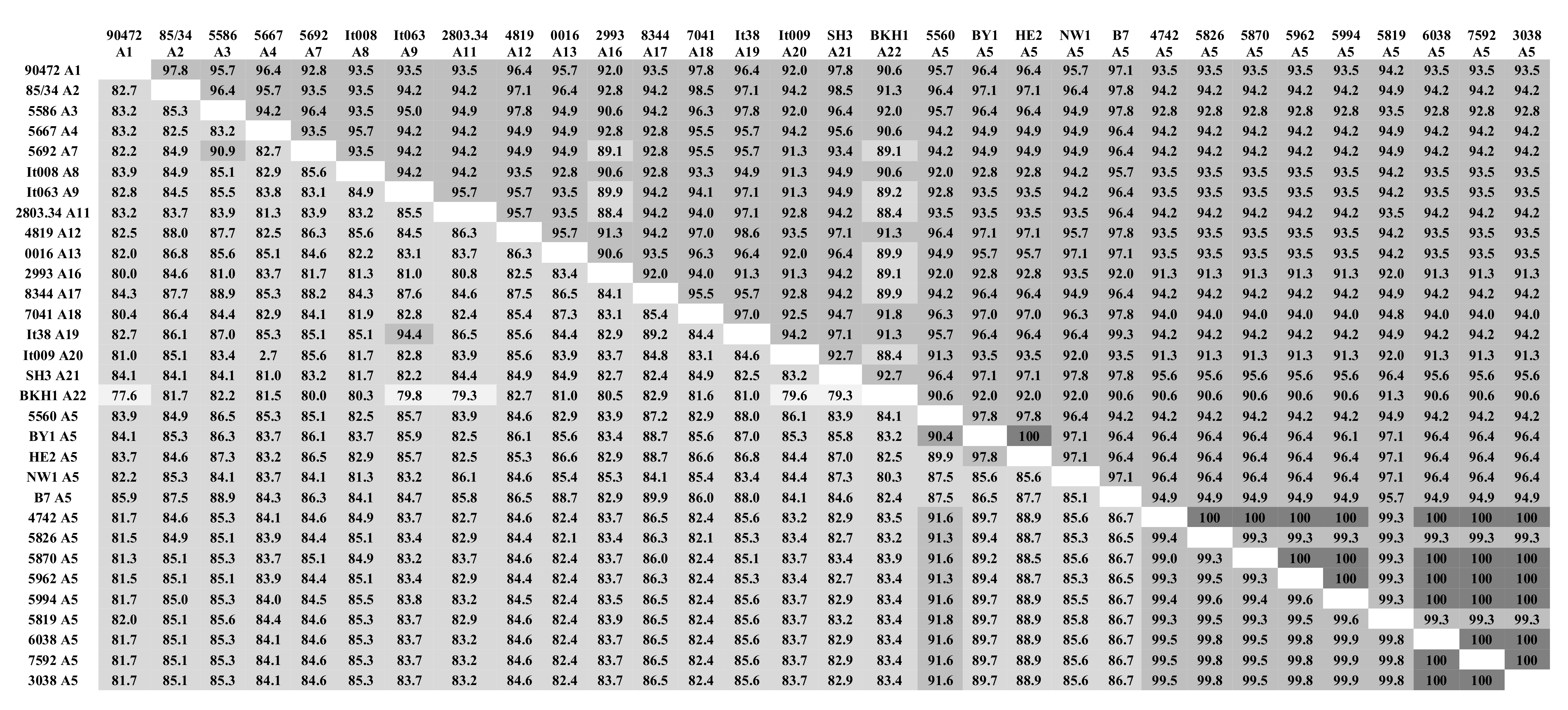
2.2. Genetic Distance and Pairwise Comparison
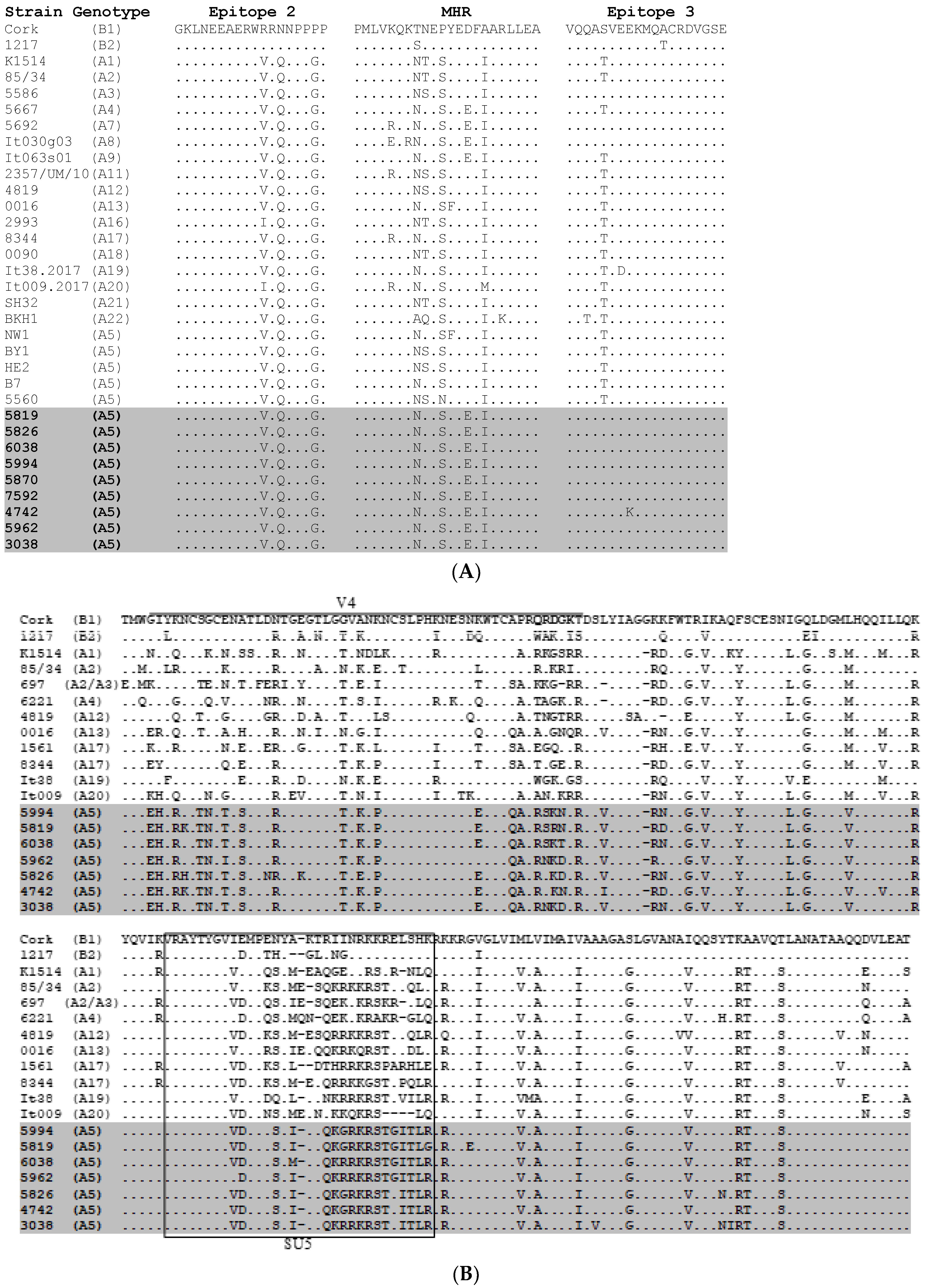
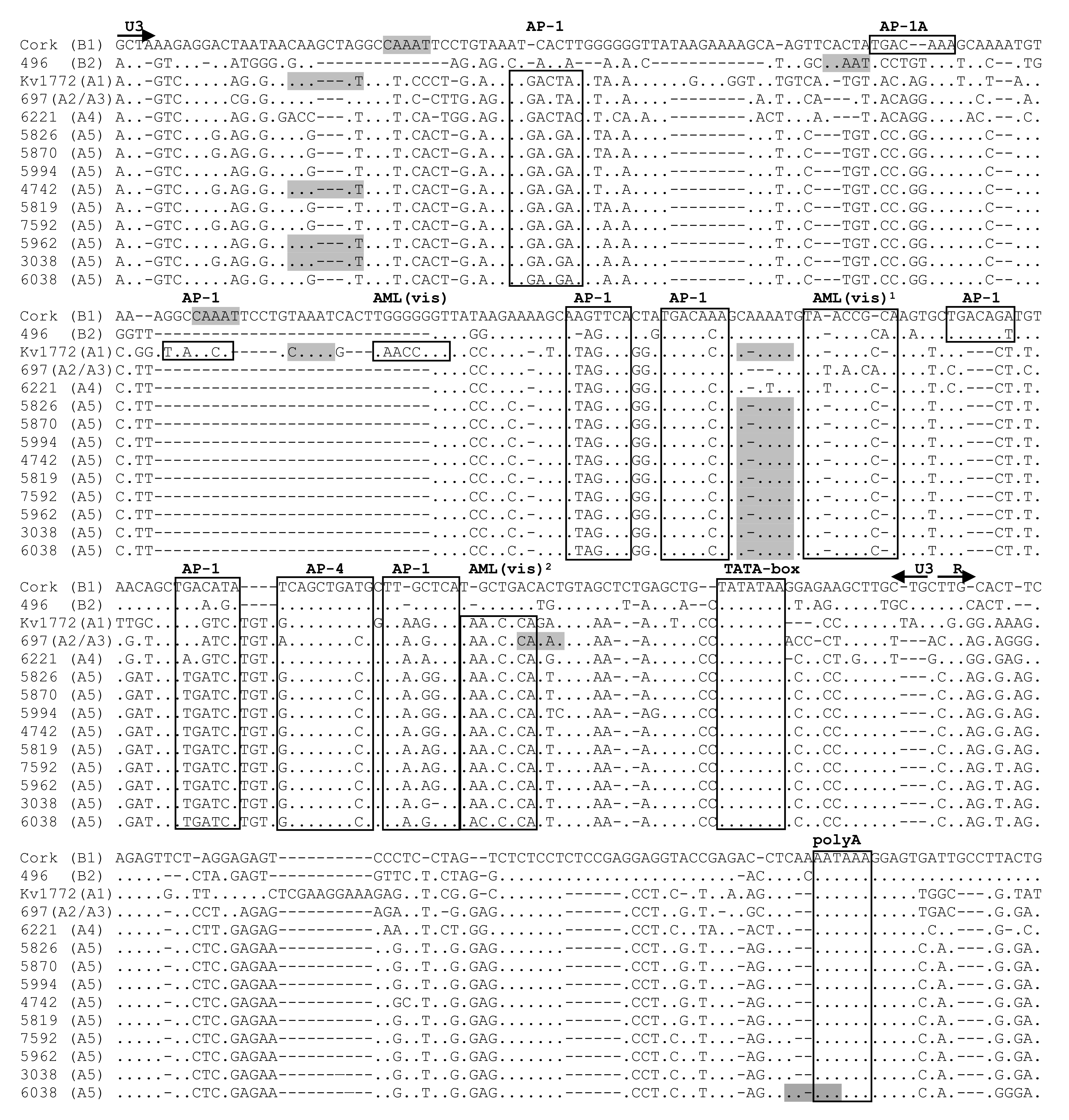
2.3. Comparative Analysis of Immunodominant and Genetic Regions
3. Discussion
4. Materials and Methods
4.1. Animals and Samples
4.2. Serological Study
4.3. PCR Amplification and Sanger Sequencing
4.4. Bioinformatic Analysis
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Minardi da Cruz, J.C.; Singh, D.K.; Lamara, A.; Chebloune, Y. Small ruminant lentiviruses (SRLVs) break the species barrier to acquire new host range. Viruses 2013, 5, 1867–1884. [Google Scholar] [CrossRef] [PubMed]
- Peterhans, E.; Greenland, T.; Badiola, J.; Harkiss, G.; Bertoni, G.; Amorena, B.; Eliaszewicz, M.; Juste, R.A.; Krassnig, R.; Lafont, J.-P.; et al. Routes of transmission and consequences of small ruminant lentiviruses (SRLVs) infection and eradication schemes. Vet. Res. 2004, 35, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.; Wolinsky, J.S.; Clements, J.E.; Strandberg, J.D.; Griffin, D.E.; Cork, L.C. Slow virus replication: The role of macrophages in the persistence and expression of visna viruses of sheep and goats. J. Gen. Virol. 1982, 59, 345–356. [Google Scholar] [CrossRef]
- Ryan, S.; Tiley, L.; McConnell, I.; Blacklaws, B. Infection of dendritic cells by the Maedi-Visna lentivirus. J. Virol. 2000, 74, 10096–10103. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; Fuertes, M.; García-Pariente, C.; Otaola, J.; Delgado, L.; Giraldez, J.; García Marín, J.F.; Carmen Ferreras, M.; Pérez, V. Impact of maedi-visna in intensively managed dairy sheep. Vet. J. 2013, 197, 607–612. [Google Scholar] [CrossRef]
- Keen, J.; Hungerford, L.; Wittum, T.; Kwang, J.; Littledike, E.T. Rick factors for seroprevalence of ovine lentivirus in breeding ewe flocks in Nebraska USA. Prev. Vet. Met. 1997, 30, 81–94. [Google Scholar] [CrossRef]
- Larruskain, A.; Jugo, B.M. Retroviral infections in sheep and goats: Small ruminant lentiviruses and host interaction. Viruses 2013, 5, 2043–2061. [Google Scholar] [CrossRef]
- Oskarsson, T.; Hreggvidsdottir, H.S.; Agnarsdottir, G.; Matthiasdottir, S.; Ogmundsdottir, M.H.; Jónsson, S.R.; Georgsson, G.; Ingvarsson, S.; Andrésson, O.S.; Andrésdóttir, V. Duplicated sequence motif in the long terminal repeat of maedi-visna virus extends cell tropism and is associated with neurovirulence. J. Virol. 2007, 81, 4052–4057. [Google Scholar] [CrossRef]
- Angelopoulou, K.; Poutahidis, T.; Brellou, G.D.; Greenland, T.; Vlemmas, I. A deletion in the R region of long terminal repeats in small ruminant lentiviruses is associated with decreased pathology in the lung. Vet. J. 2008, 175, 346–355. [Google Scholar] [CrossRef]
- Barros, S.C.; Ramos, F.; Duarte, M.; Fagulha, T.; Cruz, B.; Fevereiro, M. Genomic characterization of a slow/low maedi visna virus. Virus Genes 2004, 29, 199–210. [Google Scholar] [CrossRef]
- Glaria, I.; Reina, R.; Ramírez, H.; de Andrés, X.; Crespo, H.; Jauregui, P.; Salazar, E.; Luján, L.; Pérez, M.M.; Benavides, J.; et al. Visna/Maedi virus genetic characterization and serological diagnosis of infection in sheep from a neurological outbreak. Vet. Microbiol. 2012, 155, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.; Vitu, C.; Russo, P.; Mornex, J.F.; Peterhans, E. Meadi-Visna virus infection in sheep: A review. Vet. Res. 1998, 29, 341–367. [Google Scholar] [PubMed]
- Saltarelli, M.; Querat, G.; Konings, D.A.M.; Vigne, R.; Clements, J.E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology 1990, 179, 347–364. [Google Scholar] [CrossRef]
- Gomez-Lucia, E.; Barquero, N.; Domenech, A. Maedi-Visna virus: Current perspectives. Vet. Med. 2018, 21, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.; Pittau, M.; Tolari, F.; Erre, G.; Kwang, J. Genetic and antigenic characterization of caev (caprine arthritis-encephalitis virus) recombinant transmembrane protein. Vet. Microbiol. 1995, 45, 363–370. [Google Scholar] [CrossRef]
- Molaee, V.; Bazzucchi, M.; De Mia, G.M.; Otarod, V.; Abdollahi, D.; Rosati, S.; Lühken, G. Phylogenetic analysis of small ruminant lentiviruses in Germany and Iran suggests their expansion with domestic sheep. Sci. Rep. 2020, 10, 2243. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, G.; Quasso, A.; Moroni, P. Phylogenetic analysis of small-ruminant lentivirus subtype B1 in mixed flocks: Evidence for natural transmission from goats to sheep. Virology 2005, 339, 147–152. [Google Scholar] [CrossRef]
- Leroux, C.; Cruz, J.C.; Mornex, J.F. SRLVs: A genetic continuum of lentiviral species in sheep and goats with cumulative evidence of cross species transmission. Curr. HIV Res. 2010, 8, 94–100. [Google Scholar]
- Glaria, I.; Reina, R.; Crespo, H.; de Andrés, X.; Ramírez, H.; Biescas, E.; Pérez, M.M.; Badiola, J.; Luján, L.; Amorena, B.; et al. Phylogenetic analysis of SRLV sequences from an arthritic sheep outbreak demonstrates the introduction of CAEV-like viruses among Spanish sheep. Vet. Microbiol. 2009, 138, 156–162. [Google Scholar] [CrossRef]
- Deubelbeiss, M.; Blatti-Cardinaux, L.; Zahno, M.L.; Zanoni, R.; Vogt, H.R.; Posthaus, H.; Bertoni, G. Characterization of small ruminant lentivirus A4 subtype isolates and assessment of their pathogenic potential in naturally infected goats. Virol. J. 2014, 11, 65. [Google Scholar] [CrossRef]
- Olech, M.; Rachid, A.; Croisé, B.; Kuźmak, J.; Valas, S. Genetic and antigenic characterization of small ruminant lentiviruses circulating in Poland. Virus Res. 2012, 163, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Valas, S.; Kuźmak, J. Epidemiological survey in single-species flocks from Poland reveals expanded genetic and antigenic diversity of small ruminant lentiviruses. PLoS ONE 2018, 13, e193892. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Murawski, M.; Kuźmak, J. Molecular analysis of small-ruminant lentiviruses in Polish flocks reveals the existence of a novel subtype in sheep. Arch. Virol. 2019, 164, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Muz, D.; Oğuzoğlu, T.C.; Rosati, S.; Reina, R.; Bertolotti, L.; Burgu, I. First molecular characterization of visna/maedi viruses from naturally infected sheep in Turkey. Arch. Virol. 2013, 158, 559–570. [Google Scholar] [CrossRef]
- Hötzel, I.; Kumpula-McWhirter, N.; Cheevers, W.P. Rapid evolution of two discrete regions of the caprine arthritis-encephalitis virus envelope surface glycoprotein during persistent infection. Virus Res. 2002, 84, 17–25. [Google Scholar] [CrossRef]
- Hess, J.L.; Pyper, J.M.; Clements, J.E. Nucleotide sequence and transcriptional activity of the caprine arthritis-encephalitis virus long terminal repeat. J. Virol. 1986, 60, 385–393. [Google Scholar] [CrossRef]
- Cardinaux, L.; Zahno, M.L.; Deubelbeiss, M.; Zanoni, R.; Vogt, H.R.; Bertoni, G. Virological and phylogenetic characterization of attenuated small ruminant lentivirus isolates eluding efficient serological detection. Vet. Microbiol. 2013, 162, 572–581. [Google Scholar] [CrossRef]
- Mazzei, M.; Abi-Said, M.R.; Hué, S.; Singer, J.B.; Hughes, J.; Gifford, R.J. Global genetic diversity of small ruminant lentiviruses, and a hypothesis regarding their pandemic spread. bioRxiv 2018. [Google Scholar] [CrossRef]
- Kuhar, U.; Barlic-Maganja, D.; Grom, J. Phylogenetic analysis of small ruminant lentiviruses detected in Slovenia. Vet. Microbiol. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Shah, C.; Böni, J.; Huder, J.B.; Vogt, H.R.; Mühlherr, J.; Zanoni, R.; Miserez, R.; Lutz, H.; Schüpbach, J. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: Evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade. Virology 2004, 319, 12–26. [Google Scholar] [CrossRef]
- Grego, E.; Profiti, M.; Giammarioli, M.; Giannino, L.; Rutili, D.; Woodall, C.; Rosati, S. Genetic heterogeneity of small ruminant lentiviruses involves immunodominant epitope of capsid antigen and affects sensitivity of single-strain-based immunoassay. Clin. Diagn. Lab. Immunol. 2002, 9, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Valas, S.; Benoit, C.; Baudry, C.; Perrin, G.; Mamoun, R.Z. Variability and immunogenicity of caprine arthritis encephalitis virus surface glycoprotein. J. Virol. 2000, 74, 6178–6185. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Robinson, B.A.; Chutiraka, K.; Geary, C.D.; Reed, J.C.; Lingappa, J.R. Mutations of Conserved Residues in the Major Homology Region Arrest Assembling HIV-1 Gag as a Membrane-Targeted Intermediate Containing Genomic RNA and Cellular Proteins. J. Virol. 2015, 90, 1944–1963. [Google Scholar] [CrossRef]
- Chu, H.H.; Chang, Y.F.; Wang, C.T. Mutations in the alpha-helix directly C-terminal to the major homology region of human immunodeficiency virus type 1 capsid protein disrupt Gag multimerization and markedly impair virus particle production. J. Biomed. Sci. 2006, 13, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.; Profiti, M.; Grego, E.; Carrozza, M.L.; Mazzei, M.; Bandecchi, P. Antigenic variability of ovine lentivirus isolated in Italy. Vet. Res. Commun. 2004, 1, 319–322. [Google Scholar] [CrossRef]
- Mordasini, F.; Vogt, H.R.; Zahno, M.L.; Maeschli, A.; Nenci, C.; Zanoni, R.; Peterhans, E.; Bertoni, G. Analysis of the antibody response to an immunodominant epitope of the envelope glycoprotein of a lentivirus and its diagnostic potential. J. Clin. Microbiol. 2006, 44, 981–991. [Google Scholar] [CrossRef]
- Ramírez, H.; Reina, R.; Amorena, B.; de Andrés, D.; Martínez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef]
- Skraban, R.; Matthíasdóttir, S.; Torsteinsdóttir, S.; Agnarsdóttir, G.; Gudmundsson, B.; Georgsson, G.; Meloen, R.H.; Andrésson, O.S.; Staskus, K.A.; Thormar, H.; et al. Naturally occurring mutations within 39 amino acids in the envelope glycoprotein of maedi-visna virus alter the neutralization phenotype. J. Virol. 1999, 73, 8064–8072. [Google Scholar] [CrossRef]
- Verschoor, E.J.; Boven, L.A.; Blaak, H.; van Vliet, A.L.; Horzinek, M.C.; de Ronde, A. A Single Mutation within the V3 Envelope Neutralization Domain of Feline Immunodeficiency Virus Determines Its Tropism for CRFK Cells. J. Virol. 1995, 69, 4752–4757. [Google Scholar] [CrossRef]
- De Jong, J.J.; De Ronde, A.; Keulen, W.; Tersmette, M.; Goudsmit, J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: Analysis by single amino acid substitution. J. Virol. 1992, 66, 6777–6780. [Google Scholar] [CrossRef]
- Rachid, A.; Croisé, B.; Russo, P.; Vignoni, M.; Lacerenza, D.; Rosati, S.; Kuźmak, J.; Valas, S. Diverse host-virus interactions following caprine arthritis-encephalitis virus infection in sheep and goats. J. Gen. Virol. 2013, 94, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, K.; Brellou, G.D.; Greenland, T.; Vlemmas, I. A novel deletion in the LTR region of a Greek small ruminant lentivirus may be associated with low pathogenicity. Virus Res. 2006, 118, 178–184. [Google Scholar] [CrossRef]
- Agnarsdóttir, G.; Thorsteinsdóttir, H.; Óskarsson, T.; Matthíasdóttir, S.; St Haflidadóttir, B.; Andrésson, Ó.S.; Andrésdóttir, V. The long terminal repeat is a determinant of cell tropism of maedi-visna virus. J. Gen. Virol. 2000, 81, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; Andrésdóttir, V.; Fevereiro, M. Cellular specificity and replication rate of Maedi Visna virus in vitro can be controlled by LTR sequences. Arch. Virol. 2005, 150, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; McElliott, V.; Vapniarsky, N.; Oliver, A.; Rowe, J. Tissue tropism and promoter sequence variation in caprine arthritis encephalitis virus infected goats. Virus Res. 2010, 151, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Hillman, C.; Castillo, D.; Vapniarsky, N.; Rowe, J. The presence or absence of the gamma-activated site determines IFN gamma-mediated transcriptional activation in CAEV promoters cloned from the mammary gland and joint synovium of a single CAEV-infected goat. Virus Res. 2012, 163, 537–545. [Google Scholar] [CrossRef]
- Blatti-Cardinaux, L.; Pisoni, G.; Stoffel, M.H.; Zanoni, R.; Zahno, M.L.; Bertoni, G. Generation of a molecular clone of an attenuated lentivirus, a first step in understanding cytopathogenicity and virulence. Virology 2016, 487, 50–58. [Google Scholar] [CrossRef]
- Murphy, B.G.; Hötzel, I.; Jasmer, D.P.; Davis, W.C.; Knowles, D. TNF alpha and GM-CSF-induced activation of the CAEV promoter is independent of AP-1. Virology 2006, 352, 188–199. [Google Scholar] [CrossRef]
- Mendiola, W.P.S.; Tórtora, J.L.; Martínez, H.A.; García, M.M.; Cuevas-Romero, S.; Cerriteño, J.L.; Ramírez, H. Genotyping Based on the LTR Region of Small Ruminant Lentiviruses from Naturally Infected Sheep and Goats from Mexico. Biomed. Res. Int. 2019, 12, 4279573. [Google Scholar] [CrossRef]
- Colitti, B.; Coradduzza, E.; Puggioni, G.; Capucchio, M.T.; Reina, R.; Bertolotti, L.; Rosati, S. A new approach for Small Ruminant Lentivirus full genome characterization revealed the circulation of divergent strains. PLoS ONE 2019, 14, e0212585. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
| A1 | A2 | A3 | A4 | A7 | A8 | A9 | A11 | A12 | A13 | A16 | A17 | A18 | A19 | A20 | A21 | A22 | 5560 | HE2 | BY1 | NW1 | B7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A2 | 17.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A3 | 16.1 | 13.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A4 | 17.1 | 17.0 | 16.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A7 | 17.9 | 15.1 | 9.1 | 17.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A8 | 17.5 | 17.1 | 16.3 | 17.3 | 16.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A9 | 16.6 | 15.5 | 13.6 | 16.2 | 15.4 | 15.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A11 | 17.1 | 16.1 | 15.5 | 18.3 | 16.2 | 16.9 | 14.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A12 | 17.4 | 12.2 | 13.1 | 17.0 | 13,2 | 16.9 | 16.7 | 14.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A13 | 16.2 | 11.6 | 13.3 | 15.2 | 15.0 | 17.0 | 16.2 | 14.8 | 13.5 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A16 | 18.8 | 15.5 | 17.4 | 16.6 | 17.9 | 19.2 | 19.3 | 18.1 | 16.7 | 16.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| A17 | 15.6 | 12.6 | 11.0 | 14.7 | 11.9 | 16.4 | 12.9 | 16.2 | 12.0 | 14.2 | 15.6 | - | - | - | - | - | - | - | - | - | - | - |
| A18 | 19.0 | 13.8 | 15.3 | 17.4 | 16.0 | 18.8 | 17.2 | 17.8 | 15.3 | 13.0 | 16.5 | 14.4 | - | - | - | - | - | - | - | - | - | - |
| A19 | 16.9 | 13.9 | 12.1 | 14.4 | 14.2 | 14.8 | 5.1 | 14.1 | 15.4 | 14.5 | 17.4 | 11.6 | 15.6 | - | - | - | - | - | - | - | - | - |
| A20 | 18.9 | 14.9 | 15.4 | 17.6 | 14.4 | 18.6 | 17.3 | 16.1 | 15.1 | 15.9 | 16.2 | 16.1 | 16.7 | 15.4 | - | - | - | - | - | - | - | - |
| A21 | 16.3 | 15.1 | 14.3 | 18.2 | 14.9 | 18.6 | 16.5 | 15.0 | 14.5 | 14.0 | 16.8 | 15.4 | 15.4 | 16.1 | 16.7 | - | - | - | - | - | - | - |
| A22 | 22.4 | 19.7 | 18.9 | 19.9 | 20.9 | 21.5 | 20.1 | 21.2 | 20.1 | 20.4 | 21.4 | 19.0 | 18.8 | 19.8 | 20.5 | 20.2 | - | - | - | - | - | - |
| 5560 | 16.0 | 15.1 | 12.5 | 13.9 | 14.2 | 18.1 | 14.3 | 16.2 | 15.4 | 15.1 | 16.1 | 12.8 | 17.2 | 12.0 | 13.9 | 15.1 | 16.6 | - | - | - | - | - |
| HE2 | 16,7 | 15.6 | 13.3 | 15.9 | 13.7 | 18.5 | 14.3 | 17.4 | 15.1 | 13.2 | 17.1 | 11.9 | 13.5 | 13.5 | 15.9 | 13.1 | 19.2 | 10.3 | - | - | - | - |
| BY1 | 16.2 | 14.9 | 14.1 | 15.5 | 14.4 | 17.8 | 14.0 | 17.4 | 15.0 | 13.6 | 16.6 | 12.2 | 14.5 | 13.2 | 14.9 | 14.3 | 18.4 | 9.9 | 2.2 | - | - | - |
| NW1 | 17.9 | 14.7 | 14.9 | 15.9 | 15.4 | 18.8 | 16.8 | 14.1 | 15.9 | 15.1 | 14.7 | 15.9 | 14.8 | 16.6 | 15.6 | 12.8 | 19.3 | 12.5 | 14.7 | 14.7 | - | - |
| B7 | 17.0 | 12.5 | 11.5 | 15.7 | 13.7 | 16.7 | 14.9 | 14.7 | 14.2 | 11.3 | 16.3 | 11.0 | 11.6 | 12.0 | 15.9 | 14.8 | 18.8 | 12.5 | 12.3 | 13.5 | 14.9 | - |
| A5 | 18.1 | 15.0 | 14.7 | 15.6 | 15.2 | 16.0 | 15.9 | 17.1 | 15.4 | 15.3 | 16.8 | 14.4 | 17.8 | 14.5 | 16.4 | 16.4 | 18.5 | 8.4 | 11.3 | 10.6 | 14.4 | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed. Pathogens 2020, 9, 992. https://doi.org/10.3390/pathogens9120992
Olech M, Kuźmak J. Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed. Pathogens. 2020; 9(12):992. https://doi.org/10.3390/pathogens9120992
Chicago/Turabian StyleOlech, Monika, and Jacek Kuźmak. 2020. "Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed" Pathogens 9, no. 12: 992. https://doi.org/10.3390/pathogens9120992
APA StyleOlech, M., & Kuźmak, J. (2020). Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed. Pathogens, 9(12), 992. https://doi.org/10.3390/pathogens9120992





