Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland
Abstract
1. Introduction
2. Results
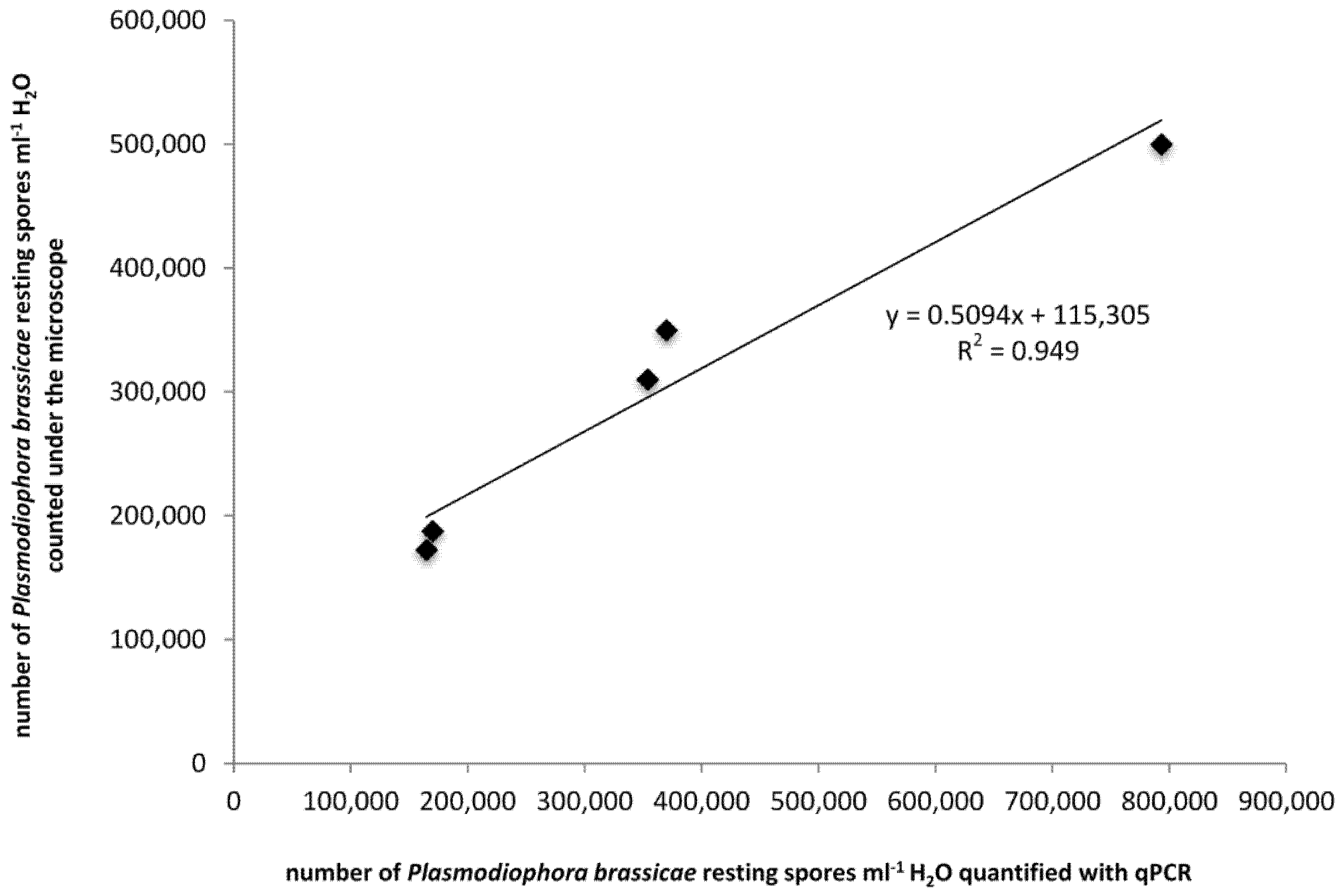
2.1. Establishment of the Linear Regression between Microscope Observations and qPCR Results
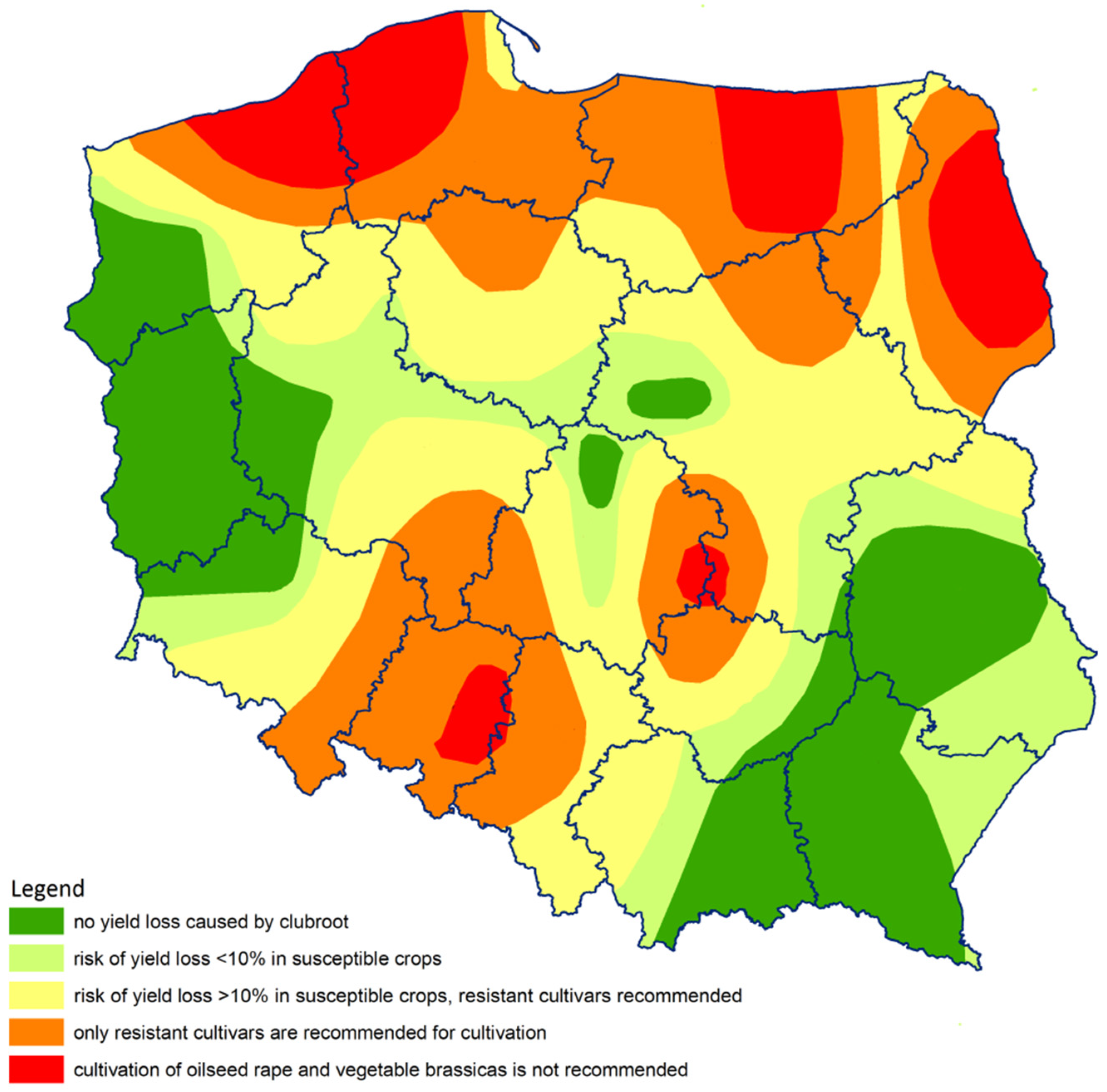
2.2. Quantification of P. brassicae in Soil Samples Using Real-Time qPCR
3. Discussion
4. Material and Methods
4.1. Soil Sampling
4.2. DNA Extraction from Soil
4.3. Real-Time qPCR
4.4. Quantification of Plasmodiophora Brassicae Spores
4.5. Cultivation Guidelines Based on qPCR Assay
4.6. Preparation of Maps
4.7. Statistical Analysis and Data Integration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woronin, M. Plasmodiophora brassicae—Organism, Pricziniajuszczij Kapustnym Rastienjam Boliezn Izwiestnuju Pod Nazvaniem Kily; Typographia, W.F., Ed.; Demakov: St. Petersburg, Russia, 1877; p. 24, (In Old Russian). [Google Scholar]
- Dixon, G.R. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant. Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Manolii, V.; Turnbull, G.D.; Fredua-Agyeman, R.; Hollman, K.; Kaus, S. Characterization of clubroot (Plasmodiophora brassicae) from canola (Brassica napus) in the Peace Country of Alberta, Canada. Can. J. Plant. Pathol. 2020. [Google Scholar] [CrossRef]
- Pageau, D.; Lajeunesse, J.; Lafond, J. Impact de l’hernie des crucifères [Plasmodiophora brassicae] sur la productivité et la qualité du canola. Can. J. Plant. Pathol. 2006, 28, 137–143. (In French) [Google Scholar] [CrossRef]
- Strelkov, S.E.; Manolii, V.P.; Cao, T.; Xue, S.; Hwang, S.F. Pathotype classification of Plasmodiophora brassicae and its occurrence in Brassica napus in Alberta, Canada. J. Phytopathol. 2007, 155, 706–712. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Gossen, B.D.; Turnbull, G.D.; Peng, G.; Howard, R.J. Seedling age and inoculum density affect clubroot severity and seed yield in canola. Can. J. Plant. Sci. 2010, 91, 183–190. [Google Scholar] [CrossRef]
- Faggian, R.; Strelkov, S.E. Detection and measurement of Plasmodiophora brassicae. J. Plant. Growth Regul. 2009, 28, 282–288. [Google Scholar] [CrossRef]
- Chai, A.L.; Xie, X.W.; Shi, Y.W.; Li, B.J. Research status of clubroot (Plasmodiophora brassicae) on cruciferous crops in China. Can. J. Plant. Pathol. 2014, 36, 142–153. [Google Scholar]
- Robak, J. Variability of Plasmodiophora brassicae Wor Pathotypes. Occurring in Poland and Their Pathogenicity to Cultivars and Breeding Lines of Brassica oleracea; Habilitation monograph, No 6; Research Institute of Horticulture: Skierniewice, Poland, 1991. [Google Scholar]
- Korbas, M.; Jajor, E.; Budka, A. Clubroot (Plasmodiophora brassicae)—A threat for oilseed rape. J. Plant. Prot. Res. 2009, 49, 463–468. [Google Scholar] [CrossRef]
- Robak, J.; Gidelska, A. Epidemiology and new possibility of control of Plasmodiophora brassicae causal agent of clubroot of cruciferous crop in Poland. Prog. Plant. Prot. 2009, 49, 268–274. [Google Scholar]
- Jedryczka, M.; Korbas, M.; Jajor, E.; Danielewicz, J.; Kaczmarek, J. Occurrence of Plasmodiophora brassicae in Wielkopolska province. Prog. Plant. Prot. 2013, 53, 774–778. (In Polish) [Google Scholar]
- Jedryczka, M.; Kasprzyk, I.; Korbas, M.; Jajor, E.; Kaczmarek, J. Infestation of Polish agricultural soils by Plasmodiophora brassicae along the Polish-Ukrainian border. J. Plant. Prot. Res. 2014, 54, 238–241. [Google Scholar] [CrossRef]
- Eckert, M.; Gout, L.; Rouxel, T.; Blaise, F.; Jedryczka, M.; Fitt, B.D.L.; Balesdent, M.H. Identification and characterization of polymorphic minisatellites in the phytopathogenic ascomycete Leptosphaeria maculans. Curr. Genet. 2005, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ricarova, V.; Kaczmarek, J.; Strelkov, S.E.; Kazda, J.; Lueders, W.; Rysanek, P.; Manolii, V.; Jedryczka, M. Pathotypes of Plasmodiophora brassicae causing damage to oilseed rape in the Czech Republic and Poland. Eur. J. Plant. Pathol. 2016, 145, 559–572. [Google Scholar] [CrossRef]
- Zamani-Noor, N. Variation in pathotypes and virulence of Plasmodiophora brassicae populations in Germany. Plant. Pathol. 2017, 66, 316–324. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Oeng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and pathotype classification of Plasmodiophora brassicae populations collected from clubroot resistant canola (Brassica napus) in Canada. Can. J. Plant. Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Konieczny, W. Clubroot is present on 250 thousand hectares. Farmer 2012, 5, 38–42. (In Polish) [Google Scholar]
- Robak, J.; Czubatka, A.; Czajka, A. Integrated pest management of crucifer crops against clubroot. Prog. Plant. Prot. 2014, 54, 19–24. [Google Scholar]
- Dixon, G.R. Plasmodiophora brassicae in its environment. J. Plant. Growth Regul. 2009, 28, 212–228. [Google Scholar] [CrossRef]
- McDonald, M.R.; Sharma, K.; Gossen, B.D.; Deora, A.; Feng, J.; Hwang, S.-F. The role of primary and secondary infection in host response to Plasmodiophora brassicae. Phytopathology 2014, 104, 1078–1087. [Google Scholar] [CrossRef]
- Rashid, A.; Ahmed, H.U.; Xiao, Q.; Hwang, S.F.; Strelkov, S.E. Effects of root exudates and pH on Plasmodiophora brassicae resting spore germination and infection of canola (Brassica napus L.) root hairs. Crop. Protect. 2013, 48, 16–23. [Google Scholar] [CrossRef]
- Walerowski, P.; Gündel, A.; Yahaya, N.; Truman, W.; Sobczak, M.; Olszak, M.; Rolfe, S.; Borisjuk, L.; Malinowski, R. Clubroot disease stimulates early steps of phloem differentiation and recruits SWEET sucrose transporters within developing galls. Plant. Cell 2018, 30, 3058–3073. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, R.; Truman, W.; Blicharz, S. Genius architect or clever thief—How Plasmodiophora brassicae reprograms host development to establish a pathogen oriented physiological sink. MPMI 2019, 32, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, B. Zróżnicowanie Biologiczne Plasmodiophora brassicae Wor. w Polsce oraz Podatność Uprawianych Roślin Krzyżowych na Wykryte Patotypy Grzyba; SGGW-AR: Warsaw, Poland, 1984; Volume 34, pp. 5–17. (In Polish) [Google Scholar]
- Dixon, G.R. Interactions of soil nutrient environment, pathogenesis and host resistance. Plant. Prot. Sci. 2002, 38, 87–94. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant. Pathol. 2011, 60, 830–838. [Google Scholar] [CrossRef]
- Rimmer, S.R.; Shattuck, V.I.; Buchwaldt, L. Compendium of Brassica Diseases; The APS: St. Paul, MN, USA, 2008; pp. 1–117. [Google Scholar]
- Gossen, B.D.; Deora, A.; Peng, G.; Hwang, S.; McDonald, M.R. Effect of environmental parameters on clubroot development and the risk of pathogen spread. Can. J. Plant. Pathol. 2014, 36, 37–48. [Google Scholar] [CrossRef]
- Rennie, D.C.; Manoli, V.P.; Cao, T.; Hwang, S.F.; Howard, R.J.; Strelkov, S.E. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant. Pathol. 2011, 60, 811–819. [Google Scholar] [CrossRef]
- Chai, A.L.; Li, J.P.; Xie, X.W.; Shi, Y.X.; Li, B.J. Dissemination of Plasmodiophora brassicae in livestock manure detected by qPCR. Plant. Pathol. 2016, 65, 137–144. [Google Scholar] [CrossRef]
- Niemann, J.; Kaczmarek, J.; Książczyk, T.; Wojciechowski, A.; Jedryczka, M. Chinese cabbage (Brassica rapa ssp. pekinensis)—A valuable source of resistance to clubroot (Plasmodiophora brassicae). Eur. J. Plant. Pathol. 2017, 147, 181–198. [Google Scholar] [CrossRef]
- Donald, E.C.; Porter, I.J. Integrated control of clubroot. J. Plant. Growth Regul. 2009, 28, 289–303. [Google Scholar] [CrossRef]
- Hwang, S.F.; Howard, R.J.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Can. J. Plant. Pathol. 2014, 36, 49–65. [Google Scholar] [CrossRef]
- Dixon, G.R. Clubroot (Plasmodiophora brassicae Woronin)—An agricultural and biological challenge worldwide. Can. J. Plant. Pathol. 2014, 23, 5–18. [Google Scholar] [CrossRef]
- Fedotova, T. Contribution to the evolution of a method for the evaluation of soil infection with clubroot (Plasmodiophora brassicae Wor.). Trudjj Zashchite Rastenii 1933, 2, 51–83. (In Russian) [Google Scholar]
- Naiki, T.; Takahashi, K.; Kageyama, K. The relationship between toot hair infection with Plasmodiophora brassicae Wor. and subsequent club formation among cruciferous species. Ann. Phytopathol. Soc. Jpn. 1984, 50, 211–215. [Google Scholar] [CrossRef][Green Version]
- Takahashi, K.; Yamaguchi, T. Assessment of pathogenicity of resting spores of Plasmodiophora brassicae in soil by fluorescence microscopy. Ann. Phytopathol. Soc. Jpn. 1987, 55, 621–628. [Google Scholar] [CrossRef][Green Version]
- Colhoun, J. A technique for examining soil for the presence of Plasmodiophora brassicae Woron. Ann. Appl. Biol. 1957, 45, 559–565. [Google Scholar] [CrossRef]
- Melville, I.E.; Hawken, R.W. Soil testing for club root in Devon and Cornwall. Plant. Pathol. 1967, 16, 145–147. [Google Scholar] [CrossRef]
- Friberg, H. Persistence of Plasmodiophora brassicae. Influence of Non-Host Plants, Soil Fauna and Organic Material. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015. [Google Scholar]
- Lange, L.; Heide, M.; Hobolth, L.; Olson, L.W. Serological detection of Plasmodiophora brassicae by Dot immunobinding and visualization of the serological reaction by scanning electron microscopy. Phytopathology 1989, 79, 1066–1071. [Google Scholar] [CrossRef]
- White, J.G.; Wakeham, A.J. Serological detection of resting spores of Plasmodiophora brassicae in soil. EPPO Bulletin. 1996, 25, 75–80. [Google Scholar] [CrossRef]
- Wakeham, A.; Faggian, R.; Kennedy, R. Development and validation of ‘‘in field’’ detection kits for the clubroot pathogen Plasmodiophora brassicae. Plant Pathol. J. 2008, 90, 425. [Google Scholar]
- Ito, S.; Maehara, T.; Maruno, E.; Tanaka, S.; Kameya-Iwaki, M.; Kishi, F. Development of a PCR-based assay for the detection of Plasmodiophora brassicae in soil. J. Phytopathol. 2008, 147, 83–88. [Google Scholar] [CrossRef]
- Faggian, R.; Bulman, S.R.; Lawrie, A.C.; Porter, I.J. Specific polymerase chain reaction primers for the detection of Plasmodiophora brassicae in soil and water. Phytopathology 1999, 89, 392–439. [Google Scholar] [CrossRef] [PubMed]
- Staniaszek, M.; Robak, J.; Marczewski, W. Detection of Plasmodiophora brassicae Wor. by bioassay and nested PCR methods. Veg. Crop. Res. Bull. 2001, 54, 131–136. [Google Scholar]
- Wallenhammar, A.C.; Arwidsson, O. Detection of Plasmodiophora brassicae by PCR in naturally infested soils. Eur. J. Plant Pathol. 2001, 107, 313–321. [Google Scholar] [CrossRef]
- Cao, T.; Tewari, J.; Strelkov, S.E. Molecular detection of Plasmodiophora brassicae, causal agent of clubroot of crucifers, in plant and soil. Plant. Dis. 2007, 91, 80–87. [Google Scholar] [CrossRef]
- Sundelin, T.; Christensen, C.B.; Larsen, J.; Moller, K.; Bodker, L.; Jensen, B. In-planta quantification of Plasmodiophora brassicae using signature fatty acids and real time PCR. Plant Dis. 2010, 94, 432–438. [Google Scholar] [CrossRef]
- Wallenhammar, A.C.; Almquist, C.; Jonsson, A. In-field distribution of Plasmodiophora brassicae measured using quantitative real-time PCR. Plant Pathol. 2012, 61, 16–28. [Google Scholar] [CrossRef]
- Li, J.P.; Li, Y.; Shi, Y.X.; Xie, X.W.; A-li, C.; Li, B.J. Development of a Real-Time PCR assay for Plasmodiophora brassicae and its detection in soil samples. J. Integr. Agric. 2013, 12, 1799–1806. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Irzykowski, W.; Burzyński, A.; Jędryczka, M. The detection of Plasmidiophora brassicae using Loop-mediated isotermal DNA amplification. Acta Agtobot. 2014, 67, 59–66. [Google Scholar] [CrossRef][Green Version]
- Almquist, C. Monitoring Important Soil-Borne Plant Pathogens in Swedish Crop Production Using Real-Time PCR. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Eurofins Agro Testing Sweden AB. Klumprotsjuka. Analys av Plasmodiphora brassicae i Jord med Snabb och Specific Kvantifiering med DNA-Baserad Teknik. 2016. Available online: https://cdnmedia.eurofins.com/european-east/media/681411/folder-rapssjukdomar20150707.pdf (accessed on 6 December 2020). (In Swedish).
- National Agricultural Census 2019—Horticultural Crops; The Central Statistical Office (GUS) Warsaw: Warsaw, Poland, 2019.
- Crête, R. Worldwide importance of clubroot. Clubroot Newsl. 1981, 11, 6–7. [Google Scholar]
- Botero, A.; García, C.; Gossen, B.D.; Strelkov, S.E.; Todd, C.D.; Bonham-Smith, P.C.; Pérez-López, E. Clubroot disease in Latin America: Distribution and management strategies. Plant. Pathol. 2019, 68, 827–833. [Google Scholar] [CrossRef]
- Gossen, B.D.; Strelkov, S.E.; Manolii, V.P.; Rennie, D.C.; Cao, T.; Hwang, S.F.; Peng, G.; McDonald, M.R. Spread of Plasmodiophora brassicae on canola in Canada, 2003–2014: Old pathogen, new home. Can. J. Plant. Pathol. 2015, 37, 403–413. [Google Scholar] [CrossRef]
- Wallenhammar, A.C.; Gunnarson, A.; Hansson, F.; Jonsson, A. Quantification of Plasmodiophora brassicae using a DNA-based soil test facilitates sustainable oilseed rape production. Plants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- McGrann, G.R.D.; Gladders, P.; Smith, J.A.; Burnett, F. Control of clubroot (Plasmodiophora brassicae) in oilseed rape using varietal resistance and soil amendments. Field Crops Res. 2016, 186, 146–156. [Google Scholar] [CrossRef]
- Kazda, J.; Ricarova, V.; Prokinova, E.; Grimova, L.; Baranyk, P. Nádorovitost kořenů brukvovitých ohrožuje ozimou řepku. Uroda 2013, 2016, 28–32. [Google Scholar]
- Strelkov, S.E.; Hwang, S.F. Clubroot in the Canadian canola crop: 10 years into the outbreak. Can. J. Plant. Pathol. 2014, 36, 27–36. [Google Scholar] [CrossRef]
- Muirhead, K.; Todd, C.D.; Wei, Y.; Bonham-Smith, P.; Pérez-López, E. ClubrootTracker: A Resource to Plan a Clubroot-Free Farm. Plant. Health Prog. 2020, 21, 185–187. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Ludwig-Müller, J. Clubroot disease management challenges from a German perspective. Can. J. Plant. Pathol. 2014, 36, 85–98. [Google Scholar]
- Korbas, M.; Jajor, E.; Kaczmarek, J.; Perek, A.; Jedryczka, M. Infestation of Polish agricultural soils by Plasmodiophora brassicae on the Polish-Belarussian border in Podlasie province. IOBC/Wprs Bulletin. 2014, 104, 171–175. [Google Scholar]
- Jadczyszyn, J. Ocena użytkowania gruntów na obszarach specyficznych oraz charakterystyka czynników ograniczających produkcję rolniczą. In Rolnictwo na Obszarach Specyficznych; GUS: Warsaw, Poland, 2013; pp. 49–59. (In Polish) [Google Scholar]
- Wallenhammar, A.C. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant. Pathol. 1996, 45, 710–719. [Google Scholar] [CrossRef]
- Rastas, M.; Latvala, S.; Hannukkala, A. Occurrence of Plasmodiophora brassicae in Finnish turnip rape and oilseed rape fields. Agric. Food Sci. 2012, 21, 141–158. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.F.; McDonald, M.R. Interaction of pH and temperature affect infection and symptom development of Plasmodiophora brassicae in canola. Can. J. Plant. Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Kasinathan, H. Influence of pH, Temperature, and Biofungicides on Clubroot of Canola. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Strehlow, B.; de Mol, F.; Struck, C. Risk potential of clubroot disease on winter oilseed rape. Plant. Dis. 2015, 99, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Tsushima, S.; Shishido, Y. Soil suppressiveness to clubroot disease of Chinese cabbage caused by Plasmodiophora brassicae. Soil Biol. Biochem. 2000, 32, 1637–1642. [Google Scholar] [CrossRef]
- Tsushima, S.; Murakami, H.; Akimoto, T.; Katahira, M.; Kuroyanagi, Y.; Shishido, Y. A practical estimating method of the dose-response curve between inoculum density of Plasmodiophora brassicae and the disease severity for long-term IPM strategies. JARQ 2010, 44, 383–390. [Google Scholar] [CrossRef]
- Donald, E.; Cross, S.; Lawrence, J.; Porter, I. Pathotypes of Plasmodiophora brassicae, the cause of clubroot, in Australia. Ann. Appl. Biol. 2006, 148, 239–244. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubatka-Bieńkowska, A.; Kaczmarek, J.; Marzec-Schmidt, K.; Nieróbca, A.; Czajka, A.; Jędryczka, M. Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland. Pathogens 2020, 9, 1070. https://doi.org/10.3390/pathogens9121070
Czubatka-Bieńkowska A, Kaczmarek J, Marzec-Schmidt K, Nieróbca A, Czajka A, Jędryczka M. Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland. Pathogens. 2020; 9(12):1070. https://doi.org/10.3390/pathogens9121070
Chicago/Turabian StyleCzubatka-Bieńkowska, Anna, Joanna Kaczmarek, Katarzyna Marzec-Schmidt, Anna Nieróbca, Agnieszka Czajka, and Małgorzata Jędryczka. 2020. "Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland" Pathogens 9, no. 12: 1070. https://doi.org/10.3390/pathogens9121070
APA StyleCzubatka-Bieńkowska, A., Kaczmarek, J., Marzec-Schmidt, K., Nieróbca, A., Czajka, A., & Jędryczka, M. (2020). Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland. Pathogens, 9(12), 1070. https://doi.org/10.3390/pathogens9121070






