Intra Vitam Diagnosis of Neglected Gurltia paralysans Infections in Domestic Cats (Felis catus) by a Commercial Serology Test for Canine Angiostrongylosis and Insights into Clinical and Histopathological Findings—Four-Case Report
Abstract
1. Introduction
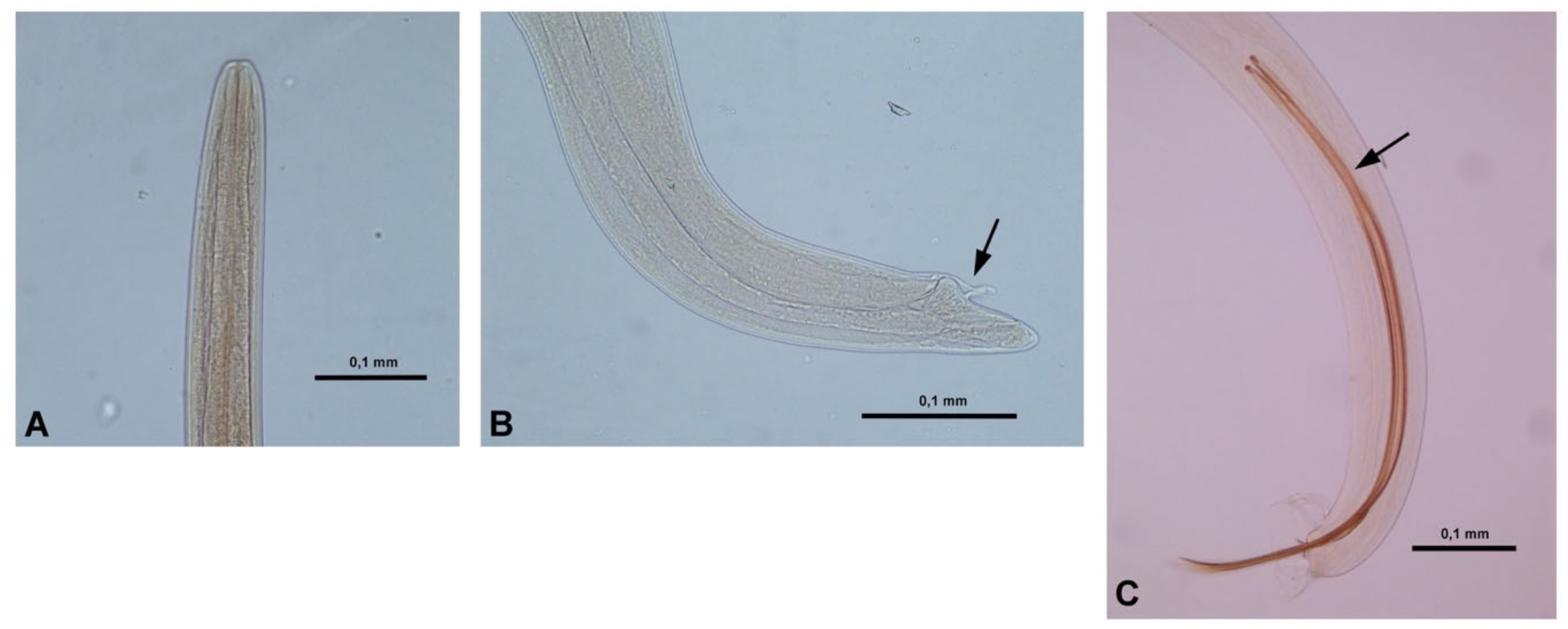
2. Results
3. Discussion
4. Materials and Methods
4.1. Case Definition and Selection
4.2. Cat Sera and Serologic Essay
4.3. Necropsies and Histological Tissue Sample Collection
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez, M.; Mieres, M.; Moroni, M.; Mora, A.; Barrios, N.; Simeone, C.; Lindsay, D. Meningomyelitis due to nematode infection in four cats. Vet. Parasitol. 2010, 170, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Moroni, M.; Muñoz, P.; Gómez, M.; Mieres, M.; Rojas, M.; Lillo, C.; Aguirre, F.; Acosta-Jamett, G.; Kaiser, M.; Lindsay, D. Gurltia paralysans (Wolffhügel, 1933): Description of adults and additional case reports of neurological diseases in three domestic cats from southern Chile. Vet. Parasitol. 2012, 184, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, C.C.; Santos, A.D.; Machado, T.P.; Ataíde, M.W.D.; Rodriguez, R.; Pereira, A.M.; Motta, A.C.D. First case report of nematode parasitic myelopathy in a wild feline in Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e014619. [Google Scholar] [CrossRef] [PubMed]
- Alzate, G.; Aranzazu, D.; Alzate, A.; Chaparro, J. Domestic cat paraplegia compatible with Gurltia paralysans nematode. First cases reported in Colombia. Rev. Colomb. Cienc. Pecua 2011, 24, 663–669. [Google Scholar]
- Rivero, R.; Matto, C.; Adrien, M.; Nan, F.; Bell, T.; Gardiner, C. Parasite meningomyelitis in cats in Uruguay. Rev. Bras. Parasitol. Vet. 2011, 20, 259–261. [Google Scholar] [CrossRef][Green Version]
- Bono, M.F.; Orcellet, V.; Marengo, R.; Bosio, A.; Junkers, E.; Plaza, D.; Marini, M.R.; Sanchez, A.; Rubio, M.G.; Candiotti, V. A description of three cases of parasitic meningomyelitis in felines from the province of Santa Fé, Argentina. Parasitaria 2016, 74, 1–4. [Google Scholar]
- Togni, M.; Panziera, W.; Souza, T.; Oliveira, J.; Mazzanti, A.; Barros, C.; Fighera, R. Epidemiological, clinical and pathological aspects of Gurltia paralysans infection in cats. Pesqui. Vet. Bras. 2013, 33, 363–371. [Google Scholar] [CrossRef]
- Udiz-Rodríguez, R.; García-Livia, K.; Valladares-Salmerón, M.; Dorta-Almenar, M.; Martín-Carrillo, N.; Martin-Alonso, A.; Isquierdo-Rodriguez, E.; Feliu, C.; Valladares, B.; Foronda, P. First ocular report of Gurltia paralysans (Wolffhügel, 1993) in cat. Vet. Parasitol. 2018, 255, 74–77. [Google Scholar] [CrossRef]
- Mieres, M.; Gómez, M.; Lillo, C.; Rojas, M.; Moroni, M.; Muñoz, P.; Acosta-Jamett, G.; Wiegand, R. Clinical, imaging and pathologic characteristics of Gurltia paralysans myelopathy in domestic cats from Chile. Vet. Radiol. Ultrasound 2013, 54, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Hirzmann, J.; Rodriguez, E.; Moroni, M.; Taubert, A.; Gibbons, L.; Hermosilla, C.; Gómez, M. Redescription and first molecular characterization of the Little known feline neurotropic nematode Gurltia paralysans (Nematoda: Metastrongyloidea). Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 119–125. [Google Scholar] [CrossRef]
- Moroni, M.; Muñoz, P.; Mieres, M.; Gómez, M.; Vera, F. Severe spinal cord thrombophlebitis and meningomyelitis by Gurltia paralysans in a cat. Vet. Rec. Case Rep. 2016, 4, e000327. [Google Scholar] [CrossRef]
- Hermosilla, C.; Kleinertz, S.; Silva, L.M.R.; Hirzmann, J.; Huber, D.; Kusak, J.; Taubert, A. Protozoan and helminth parasite fauna of free-ranging Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet. Parasitol. 2017, 233, 14–19. [Google Scholar] [CrossRef]
- Gavrilović, P.; Marinković, D.; Todorović, I.; Gravilović, A. First report of pneumonia caused by Angiostrongylus vasorum in a golden jackal. Acta Parasitol. 2017, 62, 880–884. [Google Scholar] [CrossRef]
- Schug, K.; Krämer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C.; Taubert, A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in Central Germany. Parasites Vectors 2018, 11, 85. [Google Scholar] [CrossRef]
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care 2010, 20, 98–109. [Google Scholar] [CrossRef]
- Gueldner, E.K.; Schuppisser, C.; Borel, N.; Hilbe, M.; Schnyder, M. First case of a natural infection in a domestic cat (Felis catus) with the canid heart worm Angiostrongylus vasorum. Vet. Parasitol. Reg. Stud. Reports 2019, 18, 100342. [Google Scholar] [CrossRef]
- Schnyder, M.; Stebler, K.; Naucke, T.; Lorentz, S.; Deplazes, P. Evaluation of a rapid device or serological in-clinic diagnosis of canine angiostrongylosis. Parasites Vectors 2014, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Schnyder, M.L.; Willesen, J.; Potter, A.; Chandrashekar, R. Performance of the Angio Detect TM in-clinic test kit for detection of Angiostrongylus vasorum infection in dog samples from Europe. Vet. Parasitol. 2017, 7, 45–47. [Google Scholar]
- Deak, G.; Gherman, C.; Ionica, A.; Daskalaki, A.; Matei, I.; D’Amico, G.; Domsa, C.; Pantchev, N.; Mihalca, A.; Cozma, V. Use of a commercial serologic test for Angiostrongylus vasorum for the detection of A. chabaudi in wildcats and A. daskalovi in badgers. Vet. Parasitol. 2017, 233, 107–110. [Google Scholar] [CrossRef]
- Diakou, A.; Psalla, D.; Migli, D.; Di Cesare, A.; Youlatos, D.; Marcer, F.; Traversa, D. First evidence of the European wildcat (Felis silvestris silvestris) as definitive host of Angiostrongylus chabaudi. Parasitol. Res. 2016, 115, 1235–1244. [Google Scholar] [CrossRef]
- Acuña-Olea, F.; Sacristán, I.; Aguilar, E.; García, S.; López, M.J.; Oyarzún-Ruiz, P.; Brito, J.L.; Fredes, F.; Napolitano, C. Gastrointestinal and cardiorespiratory endoparasites in the wild felid guigna (Leopardus guigna) in Chile: Richness increases with latitude and first records for the host species. Int. J. Parasitol. Parasites Wild. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.; Hendrix, C.; Lindsay, D.; Barr, S. Metastrongyloidea. In Feline Clinical Parasitology, 1st ed.; Wiley-Blackwell Publishing: Ames, IA, USA, 2002; pp. 272–273. [Google Scholar]
- Penagos-Tabares, F.; Lange, M.K.; Chaparro-Gutiérrez, J.J.; Taubert, A.; Hermosilla, C. Angiostrongylus vasorum and Aelurostrongylus abstrusus: Neglected and underestimated parasites in South America. Parasites Vectors 2018, 11, 208. [Google Scholar] [CrossRef]
- Elsheikha, H.; Schnyder, M.; Traversa, D.; Di Cesare, A.; Wright, I.; Lacher, D. Updates on feline aelurostrongylosis and research priorities for de next decade. Parasites Vectors 2016, 9, 389. [Google Scholar] [CrossRef]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is Angiostrongylosis a Realistic Threat for Domestic Cats? Front. Vet. Sci. 2020, 15, 195. [Google Scholar] [CrossRef]
- Zottler, E.M.; Strube, C.; Schnyder, M. Detection of specific antibodies in cats infected with the lung nematode Aelurostrongylus abstrusus. Vet. Parasitol. 2017, 75–82. [Google Scholar] [CrossRef]
- Al-Sabi, M.N.; Deplazes, P.; Webster, P.; Willesen, J.L.; Davidson, R.K.; Kapel, C.M. PCR detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol. Res. 2010, 107, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Verzberger-Epshtein, I.; Markham, R.J.; Sheppard, J.A.; Stryhn, H.; Whitney, H.; Conboy, G.A. Serologic detection of Angiostrongylus vasorum infection indogs. Vet. Parasitol. 2008, 151, 53–60. [Google Scholar] [CrossRef]
- Jefferies, R.; Morgan, E.; Helm, J.; Robinson, M.; Shaw, S. Improved Detection of Canine Angiostrongylus vasorum Infection Using Real-Time PCR and Indirect ELISA. Parasitol. Res. 2011, 109, 1577–1583. [Google Scholar] [CrossRef]
- Marioni-Henry, K.; Vite, C.H.; Newton, A.L.; Van Winkle, T.J. Prevalence of diseases of the spinal cord of cats. J. Vet. Intern. Med. 2004, 18, 851–858. [Google Scholar] [CrossRef]
- Schucan, A.; Schnyder, M.; Tanner, I.; Barutzki, D.; Traversa, D.; Deplazes, P. Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Vet. Parasitol. 2012, 185, 216–224. [Google Scholar] [CrossRef][Green Version]
- López-Contreras, F.; Rojas-Barón, L.; Gómez, M.; Morera, F.; Sepúlveda, P.; Moroni, M.; Muñoz, P.; Acosta-Jammet, G.; Mieres, M.; Hirzmann, J.; et al. Molecular Detection of Gurltia paralysans by Semi-Nested PCR in Cerebrospinal Fluid and Serum Samples from Domestic Cats (Felis catus). Animals 2020, 10, E1169. [Google Scholar] [CrossRef] [PubMed]


| ID | Gender | Neurologic Signs Duration | Baermann Test | Angio Detect TM® | Nº of Adult G. paralysans | Necropsy Findings |
|---|---|---|---|---|---|---|
| 1 | F | 5 m | n/d | +++ | 6 | Submeningeal congestion, tortuous veins |
| 2 | M | 12 m | + | - | n/d | |
| 3 | F | 2 m | - | + | 1 | Submeningeal congestion, tortuous veins |
| 4 | F | 6 m | - | + | 1 | Submeningeal congestion, tortuous veins |
| 5 | F | 36 m | - | + | n/d | |
| 6 | F | 12 m | + | ++ | n/d | |
| 7 | F | 36 m | - | - | n/d | |
| 8 | M | 10 m | n/d | - | n/d | |
| 9 | F | 12 m | + | +++ | 4 | Submeningeal congestion, tortuous veins |
| 10 | F | 4 m | - | + | n/d |
| Neurologic Signs | n = | ID (F) |
|---|---|---|
| Paraparesis | 10 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 |
| Ataxia (hindlimbs) | 10 | Symmetric ataxia: 1, 2, 3, 6, 8, 9, 10 asymmetric ataxia: 4, 5, 7 |
| Tail paralysis | 8 | mild: 2, 3, 5, 6, 8, 9complete: 4, 10 |
| Plantigrade stance | 2 | 2, 4 |
| Proprioception deficit (hindlimbs) | 10 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 |
| Muscular atrophy (hindlimbs) | 2 | 3, 9 |
| Urinary incontinence | 3 | 3, 4, 10 |
| Fecal incontinence | 1 | 4 |
| Lumbosacral hyperesthesia | 4 | 4, 5, 8, 9 |
| Perianal hyperesthesia | 2 | 9, 10 |
| Hindlimbs hyperesthesia | 1 | 1 |
| Patellar reflex | 5 | Increased: 6, 7, 10. Decreased: 8, 9 |
| Cross-extensor reflex | 3 | 7, 8, 10 |
| Positive Babinski reflex | 1 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, M.; García, C.; Maldonado, I.; Pantchev, N.; Taubert, A.; Hermosilla, C.; Moroni, M.; Muñoz, P.; Duran, A.; Mieres, M.; et al. Intra Vitam Diagnosis of Neglected Gurltia paralysans Infections in Domestic Cats (Felis catus) by a Commercial Serology Test for Canine Angiostrongylosis and Insights into Clinical and Histopathological Findings—Four-Case Report. Pathogens 2020, 9, 921. https://doi.org/10.3390/pathogens9110921
Gómez M, García C, Maldonado I, Pantchev N, Taubert A, Hermosilla C, Moroni M, Muñoz P, Duran A, Mieres M, et al. Intra Vitam Diagnosis of Neglected Gurltia paralysans Infections in Domestic Cats (Felis catus) by a Commercial Serology Test for Canine Angiostrongylosis and Insights into Clinical and Histopathological Findings—Four-Case Report. Pathogens. 2020; 9(11):921. https://doi.org/10.3390/pathogens9110921
Chicago/Turabian StyleGómez, Marcelo, Catalina García, Isabel Maldonado, Nikola Pantchev, Anja Taubert, Carlos Hermosilla, Manuel Moroni, Pamela Muñoz, Alejandra Duran, Marcelo Mieres, and et al. 2020. "Intra Vitam Diagnosis of Neglected Gurltia paralysans Infections in Domestic Cats (Felis catus) by a Commercial Serology Test for Canine Angiostrongylosis and Insights into Clinical and Histopathological Findings—Four-Case Report" Pathogens 9, no. 11: 921. https://doi.org/10.3390/pathogens9110921
APA StyleGómez, M., García, C., Maldonado, I., Pantchev, N., Taubert, A., Hermosilla, C., Moroni, M., Muñoz, P., Duran, A., Mieres, M., & Ojeda, J. (2020). Intra Vitam Diagnosis of Neglected Gurltia paralysans Infections in Domestic Cats (Felis catus) by a Commercial Serology Test for Canine Angiostrongylosis and Insights into Clinical and Histopathological Findings—Four-Case Report. Pathogens, 9(11), 921. https://doi.org/10.3390/pathogens9110921







