Tissue Proteases and Immune Responses: Influencing Factors of COVID-19 Severity and Mortality
Abstract
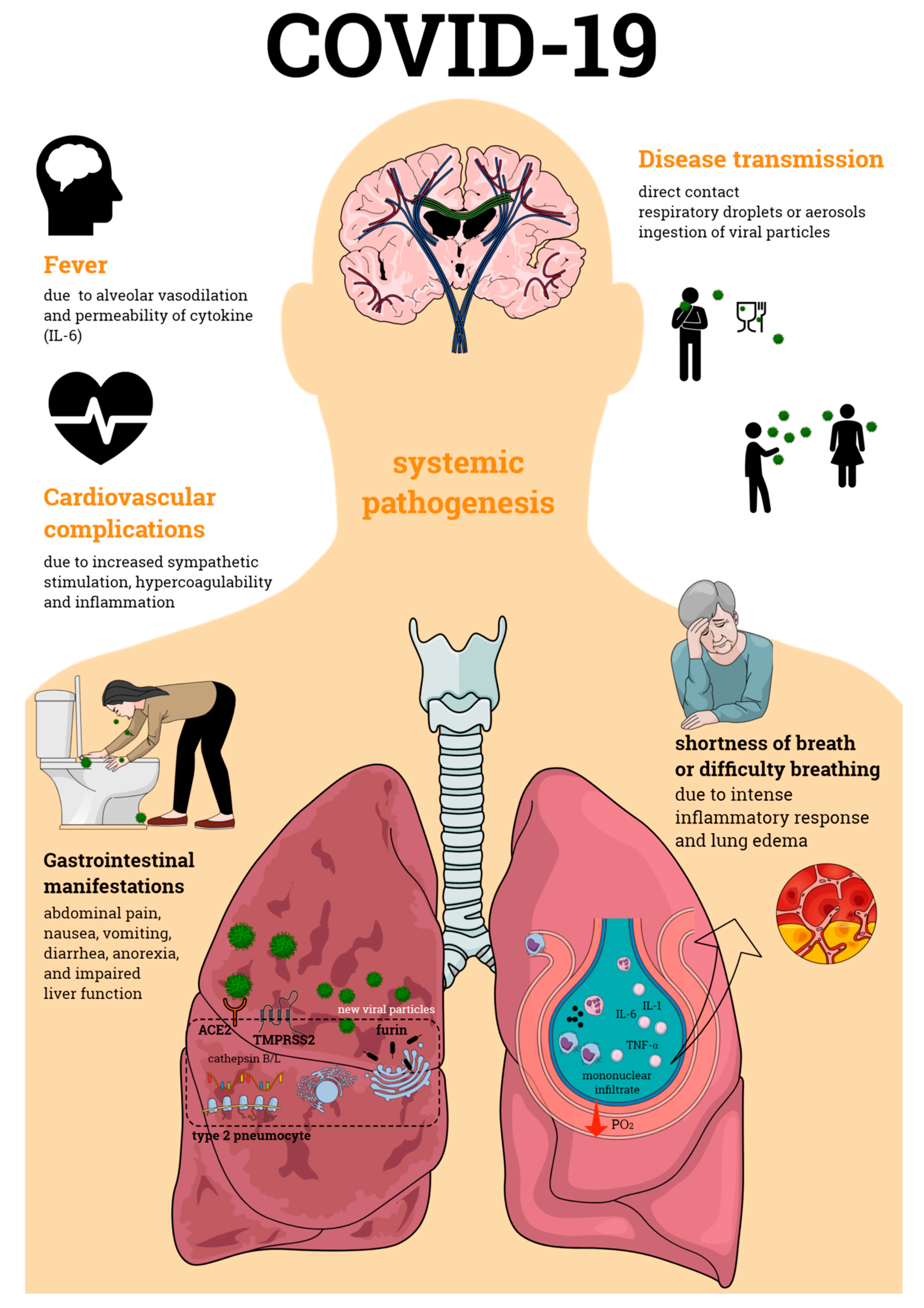
:1. Introduction
2. Pathogenesis of COVID-19
3. Illness Degree and Association with Host Tissue Proteins and Immune Responses
3.1. Tissue Protein Expression
3.2. Immune Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, H.; Schipper, D.; et al. Comparative Pathogenesis Of COVID-19, MERS And SARS In A Non-Human Primate Model. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.03.17.995639v1 (accessed on 20 March 2020). [CrossRef] [Green Version]
- Peck, K.M.; Burch, C.L.; Heise, M.T.; Baric, R.S. Coronavirus Host Range Expansion and Middle East Respiratory Syndrome Coronavirus Emergence: Biochemical Mechanisms and Evolutionary Perspectives. Annu. Rev. Virol. 2015, 2, 95–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wevers, B.A.; van der Hoek, L. Recently Discovered Human Coronaviruses. Clin. Lab. Med. 2009, 29, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. COVID-19 Rapid Response Team Guidance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/rtt-management-introduction.html (accessed on 5 October 2020).
- Matsuyama, R.; Nishiura, H.; Kutsuna, S.; Hayakawa, K.; Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): A systematic review and meta-analysis. BMC Public Health 2016, 16, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channappanavar, R.; Fett, C.; Mack, M.; Eyck., P.P.T.; Meyerholz, D.K.; Perlman, S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol 2017. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Zheng, W.; Wu, X.; Zhu, J.J.; Arnold, A.P.; Sandberg, K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol. Sex. Differ. 2010, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Department, S.R. “Number of Smokers by Age and Gender in Italy 2018.” Statista. Available online: www.statista.com/statistics/501615/italy-smokers-by-age-and-gender/ (accessed on 5 October 2020).
- Porcheddu, R.; Serra, C.; Kelvin, D.; Kelvin, N.; Rubino, S. Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J. Infect. Dev. Ctries. 2020, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA J. Am. Med. Assoc. 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Gabutti, G.; D’Anchera, E.; Sandri, F.; Savio, M.; Stefanati, A. Coronavirus: Update Related to the Current Outbreak of COVID-19. Infect. Dis. Ther. 2020, 9, 241–253. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, Q.; Casanova, J.L.; Su, H.C.; Abel, L.; Bastard, P.; Cobat, A.; Jouanguy, E.; Notarangelo, L. Severe COVID-19 in the young and healthy: Monogenic inborn errors of immunity? Nat. Rev. Immunol. 2020, 20, 455–456. [Google Scholar] [CrossRef]
- Fung, S.-Y.; Yuen, K.-S.; Ye, Z.-W.; Chan, C.-P.; Jin, D.-Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020, 9, 558–570. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020. [Google Scholar] [CrossRef] [Green Version]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, D.; Liu, G.; Gack, M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil. Med. Res. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xu, S.-B.; Lin, Y.-X.; Tian, D.; Zhu, Z.-Q.; Dai, F.-H.; Wu, F.; Song, Z.-G.; Huang, W.; Chen, J.; et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. (Engl). 2020, 133, 1039–1043. [Google Scholar] [CrossRef]
- Xing, Y.-H.; Ni, W.; Wu, Q.; Li, W.-J.; Li, G.-J.; Wang, W.-D.; Tong, J.-N.; Song, X.-F.; Wing-Kin Wong, G.; Xing, Q.-S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020, 53, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Xue, Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020, 92, 680–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, W.; Hu, G.; Xia, S.; Sun, Z.; Liu, Z.; Xie, Y.; Zhang, R.; Jiang, S.; Lu, L. RETRACTED ARTICLE: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Jabeen, N.; Raza, F.; Shabbir, S.; Baig, A.A.; Amanullah, A.; Aziz, B. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [Green Version]
- Asselta, R.; Paraboschi, E.M.; Mantovani, A.; Duga, S. ACE2 and TMPRSS2 Variants and Expression as Candidates to Sex and Country Differences in COVID-19 Severity in Italy. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3559608 (accessed on 5 October 2020). [CrossRef] [Green Version]
- Cheng, H.; Wang, Y.; Wang, G. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, W.; Juhong, Z.; Yang, J.; Wang, H.; Zhou, L.; Chang, J. Associations between genetic variations in the FURIN gene and hypertension. BMC Med. Genet. 2010, 11, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Yang, W.; Wu, J.; Chen, B.; Yang, X.; Chen, J.; McVey, D.G.; Andreadi, C.; Gong, P.; Webb, T.R.; et al. Influence of a Coronary Artery Disease–Associated Genetic Variant on FURIN Expression and Effect of Furin on Macrophage Behavior. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1837–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, W.; McVey, D.G.; Zhao, G.; Hu, J.; Poston, R.N.; Ren, M.; Willeit, K.; Coassin, S.; Willeit, J.; et al. FURIN Expression in Vascular Endothelial Cells Is Modulated by a Coronary Artery Disease–Associated Genetic Variant and Influences Monocyte Transendothelial Migration. J. Am. Heart Assoc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Mbewe-Campbell, N.; Wei, Z.; Zhang, K.; Friese, R.S.; Mahata, M.; Schork, A.J.; Rao, F.; Chiron, S.; Biswas, N.; Kim, H.-S.; et al. Genes and environment. J. Hypertens. 2012, 30, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.S.; Chakraborty, T.T.; Kumar, N.; Banerjee, I. Association between IFITM3 rs12252 polymorphism and influenza susceptibility and severity: A meta-analysis. Gene 2018, 674, 70–79. [Google Scholar] [CrossRef]
- Cai, G.; Bossé, Y.; Xiao, F.; Kheradmand, F.; Amos, C.I. Tobacco smoking increases the lung gene expression of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 201, 1557–1559. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.B.; Zhong, J.-C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, Z.; Moradi, M.; Nasri, H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J. Res. Med. Sci. 2014, 19. [Google Scholar]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K.; Karmouty-Quintana, H.; Davies, J.; Akkanti, B.; Harting, M.T. Understanding the age divide in COVID-19: Why are children overwhelmingly spared? Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L39–L44. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakala, G.K.; Cabrera-Fuentes, H.A.; Crespo-Avilan, G.E.; Rattanasopa, C.; Burlacu, A.; George, B.L.; Anand, K.; Mayan, D.C.; Corlianò, M.; Hernández-Reséndiz, S.; et al. FURIN Inhibition Reduces Vascular Remodeling and Atherosclerotic Lesion Progression in Mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Yin, J.; Wang, W.; Shi, H.; Shi, Y.; Xu, B.; Qiao, L.; Feng, Y.; Pang, L.; Wei, F.; et al. Downregulated Gene Expression Spectrum and Immune Responses Changed During the Disease Progression in Patients With COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e18. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science (80-. ) 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Song, X.; Delaney, M.; Bell, M.; Smith, K.; Pershad, J.; Ansusinha, E.; Hahn, A.; Hamdy, R.; Harik, N.; et al. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. J. Pediatr. 2020, 223, 199–203.e1. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; David, J.K.; Maden, S.K.; Wood, M.A.; Weeder, B.R.; Nellore, A.; Thompson, R.F. Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Zhang, Y.; Dorgham, K.; Béziat, V.; Puel, A.; Lorenzo, L.; Bizien, L.; Assant, S.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 4585, 1–19. [Google Scholar] [CrossRef]
| Protein | Species | Protein Expression/Activity | Variant/Polymorphism | Possible Effect | References |
|---|---|---|---|---|---|
| ACE2 | Human | - | rs143936283 (E329G) and rs73635825 (S19P) allele variant | mild to moderate COVID-19 | [50] |
| Human | - | high allele frequency in the QTL expression quantitative trait loci variants – associated with higher ACE2 expression | mild to moderate COVID-19 | [51] | |
| Human | increased lung expression | - | severe COVID-19 | [23,52] | |
| Human | no expression alteration | - | mild to moderate COVID-19 | [13,53] | |
| Human | decreased activity | - | severe COVID-19 | [54] | |
| TMPRSS2 | Human | increased activity | - | severe COVID-19 | [53] |
| Furin | Human | - | G allele of 1970C > G | severe COVID-19 | [55] |
| Human | increased expression | rs17514846 variant | severe COVID-19 | [56,57] | |
| CatL | Human | - | proximal CTSL1 promoter at position C-171A | severe COVID-19 | [58] |
| HLA-DR | Human | low expression | - | severe COVID-19 | [59] |
| IFN-γ | Human | - | rs12252-C/C in the gene IFITM3 | mild to severe COVID-19 | [30,60] |
| Disease Severity | Complications | Immune Response Intensity | Cellular Immune Response | Cytokine/Chemokine Responses | Disease Outcome | References |
|---|---|---|---|---|---|---|
| Mild | - | - | peripheral blood: normal levels of CD4+, CD8+, CD19+, and NK cells | normal plasma cytokine levels | - | [71] |
| - | - | peripheral blood: low neutrophil counts (2.34 (1.2–2.81) × 109/L) and normal lymphocyte counts | normal plasma cytokine levels | - | [70] | |
| - | - | peripheral blood: low neutrophil and normal lymphocyte counts | - | - | [12] | |
| fever, cough, diarrhea, myalgia, anosmia/ageusia | - | peripheral blood: increase of CD10LowCD101+ neutrophils, CD14HighCD16High monocytes, IFN-producing monocytes BALF: increase of monocyte/macrophage counts | low CXCL8 and increased IFN-α plasma levels | - | [73] | |
| Moderate | pneumonia, hepatic failure | hyperinflammation | peripheral blood: normal lymphocyte counts, normal CD4+ and CD8+ cell counts | increase of plasma IL-1ra, IL-6, IL-18, CTACK, MIG, M-CSF, IL-10, IP-10, IFN-γ | recovery | [41] |
| - | hyperinflammation | BALF: CD14+ cells, high % of plasmacytoid dendritic cells, T and B lymphocytes and NK cells | increase of BALF CXCL9, CXCL10, CXCL11, and CXCL16 | recovery | [72] | |
| dyspnea and pneumonia | - | peripheral blood: low CD14LowCD16High monocyte counts | increase of plasma calprotectin | - | [73] | |
| Severe | pneumonia, acute respiratory distress syndrome, RNAemia, acute cardiac and kidney injury, secondary infection, shock, median systolic pressure of 145, respiratory rate > 24 breaths/min, increased pro-thrombin time and D-dimer level | hyperinflammation | peripheral blood: high numbers of neutrophils: 10.6 (5.0–11.8) × 109/L, low lymphocyte counts: 0.4 (0.2–0.8) × 109/L | increase of plasma levels of IL-2, IL-7, IL-10, GSCF, IP-10, MCP-1, MIP-1α, and TNFα | increased mortality | [69] |
| pneumonia, acute respiratory distress syndrome, hepatic and kidney failure, cardiac failure, shock | hyperinflammation | peripheral blood: low % of neutrophils and lymphocytes, low counts of CD4+ (0.3 (0.2–0.4) × 109/L) and CD8+ cells (0.1 (0.1–0.2) × 109/L) | increase of plasma IL-1ra, IL-6, IL-18, CTACK, MIG, MCP-3, M-CSF, MIP-1α, HGF, IL-10, IP-10, IFN-γ | increased mortality | [41] | |
| acute respiratory distress syndrome | hyperinflammation | peripheral blood: normal neutrophil counts, low % of lymphocytes and eosinophils, low counts of T cells (0.5 × 109/L), CD8+ cells (0.15 × 109/L) and regulatory T cells | increase of serum IL-6, decrease of IL-8 | - | [29] | |
| acute respiratory distress syndrome | hyperinflammation | BALF: increased % of neutrophils, reduced % of dendritic cells, presence of M1 and M2 macrophages | increase of BALF IL-8, IL-6, IL-1β, CXCL9, CXCL10, and CXCL11 | increased mortality | [72] | |
| - | - | peripheral blood: low numbers of CD4+, CD8+, CD19+, and NK cells | increase of plasma IL-6 | - | [71] | |
| acute respiratory distress syndrome | - | peripheral blood: normal neutrophils, low CD4+ and CD8+ cell counts | increase of plasma IL-6 | - | [70] | |
| acute respiratory distress syndrome | - | peripheral blood: low lymphocyte CD8+ cell counts | increase of IL-6, IL-10, IL-2, and IFN-γ | - | [12] | |
| - | hyperinflammation | - | neglected production of IFNs, increase of lung (CCL2, CCL8, and CCL11) and systemic (CCL2, CCL8 CXCL2, CXCL8, CXCL9, and CXCL16) chemokines, and systemic IL-6 and IL-1ra | - | [74] | |
| acute respiratory distress syndrome | - | peripheral blood: increase of total neutrophils, and CD10LowCD101− and CD10LowCD16Low neutrophil counts, low numbers of CD14LowCD16High monocytes, low frequencies of CD4+, CD8+, and CD19+ cells, increase of ROS- and NO-producing monocytes BALF: increase of ROS- and NO-producing monocytes/macrophages, accumulation of immature neutrophils | increase of plasma calprotectin, CXCL-8, CXCL-12, and IL-6 levels | - | [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulinari Turin de Oliveira, N.; Fernandes da Silva Figueiredo, I.; Cristine Malaquias da Silva, L.; Sauruk da Silva, K.; Regis Bueno, L.; Barbosa da Luz, B.; Rita Corso, C.; Paula Werner, M.F.; Soares Fernandes, E.; Maria-Ferreira, D. Tissue Proteases and Immune Responses: Influencing Factors of COVID-19 Severity and Mortality. Pathogens 2020, 9, 817. https://doi.org/10.3390/pathogens9100817
Mulinari Turin de Oliveira N, Fernandes da Silva Figueiredo I, Cristine Malaquias da Silva L, Sauruk da Silva K, Regis Bueno L, Barbosa da Luz B, Rita Corso C, Paula Werner MF, Soares Fernandes E, Maria-Ferreira D. Tissue Proteases and Immune Responses: Influencing Factors of COVID-19 Severity and Mortality. Pathogens. 2020; 9(10):817. https://doi.org/10.3390/pathogens9100817
Chicago/Turabian StyleMulinari Turin de Oliveira, Natália, Isabella Fernandes da Silva Figueiredo, Liziane Cristine Malaquias da Silva, Karien Sauruk da Silva, Laryssa Regis Bueno, Bruna Barbosa da Luz, Cláudia Rita Corso, Maria Fernanda Paula Werner, Elizabeth Soares Fernandes, and Daniele Maria-Ferreira. 2020. "Tissue Proteases and Immune Responses: Influencing Factors of COVID-19 Severity and Mortality" Pathogens 9, no. 10: 817. https://doi.org/10.3390/pathogens9100817
APA StyleMulinari Turin de Oliveira, N., Fernandes da Silva Figueiredo, I., Cristine Malaquias da Silva, L., Sauruk da Silva, K., Regis Bueno, L., Barbosa da Luz, B., Rita Corso, C., Paula Werner, M. F., Soares Fernandes, E., & Maria-Ferreira, D. (2020). Tissue Proteases and Immune Responses: Influencing Factors of COVID-19 Severity and Mortality. Pathogens, 9(10), 817. https://doi.org/10.3390/pathogens9100817






