Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors
Abstract
1. Introduction
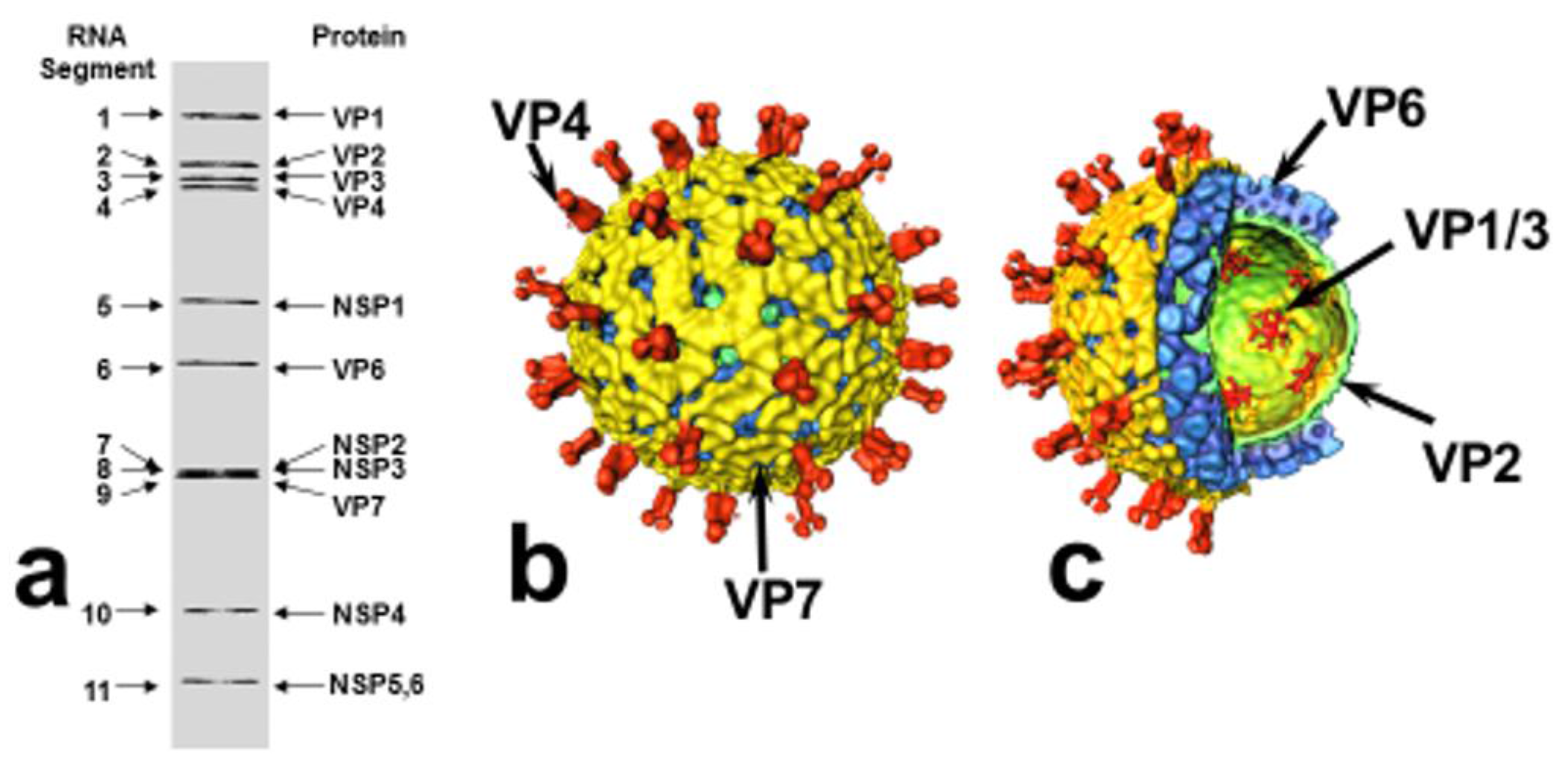
2. Rotavirus Structure and Classification
3. Rotavirus Replication Cycle
4. Rotavirus Pathogenesis
5. Rotavirus Molecular Epidemiology
6. Immune Responses to Rotavirus Infection/Vaccination
7. Prevention of Rotavirus Disease by Vaccination
8. Differences in Rotavirus Vaccine Effectiveness: Causes and Contributing Factors
8.1. Malnutrition
8.1.1. Zinc Deficiency
8.1.2. Avitaminoses
Vitamin A
Vitamin D
8.2. Gut Microbiota
8.3. Co-Infections
8.4. Immaturity/Functional Reduction of the Infant’s Immune System
8.5. Environmental Enteropathy
8.6. Passive Transfer of Maternal Antibodies
8.6.1. Rotavirus Antibody Transferred to Infants in Breast Milk
8.6.2. Transplacentally Acquired Maternal RV Specific Antibodies
8.7. Genetic Factors
9. Outlook and Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bishop, R.F.; Davidson, G.P.; Holmes, I.H.; Ruck, B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 1973, 2, 1281–1283. [Google Scholar] [CrossRef]
- Flewett, T.H.; Bryden, A.S.; Davies, H. Virus particles in gastroenteritis. Lancet 1973, 2, 1497. [Google Scholar] [CrossRef]
- Estes, M.K.; Greenberg, H.B. Rotaviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Armah, G.E.; Sow, S.O.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cunliffe, N.A.; Steele, D.; Witte, D.; Kirsten, M.; Louw, C.; Ngwira, B.; Victor, J.C.; Gillard, P.H.; Cheuvart, B.B.; et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N. Engl. J. Med. 2010, 362, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Dang, D.A.; Victor, J.C.; Shin, S.; Yunus, M.; Dallas, M.J.; Podder, G.; Vu, D.T.; Le, T.P.; Luby, S.P.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 615–623. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. World Health Organization-Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S96–S105. [Google Scholar] [PubMed]
- Pesavento, J.B.; Estes, M.K.; Prasad, B.V.V. Structural organization of the genome in rotavirus. In Viral Gastroenteritis; Desselberger, U., Gray, J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 115–127. [Google Scholar]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Mihalov-Kovács, E.; Gellért, Á.; Marton, S.; Farkas, S.L.; Fehér, E.; Oldal, M.; Jakab, F.; Martella, V.; Bányai, K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015, 21, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef] [PubMed]
- Rega Institute, KU Leuven, Belgium. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/7th-RCWG-meeting (accessed on 25 September 2017).
- Lundgren, O.; Peregrin, A.T.; Persson, K.; Kordasti, S.; Uhnoo, I.; Svensson, L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 2000, 287, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Hagbom, M.; Istrate, C.; Engblom, D.; Karlsson, T.; Rodriguez-Diaz, J.; Buesa, J.; Taylor, J.A.; Loitto, V.M.; Magnusson, K.E.; Ahlman, H.; et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011, 7, e1002115. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005, 15, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Iturriza-Gómara, M.; Dallman, T.; Bányai, K.; Böttiger, B.; Buesa, J.; Diedrich, S.; Fiore, L.; Johansen, K.; Koopmans, M.; Korsun, N.; et al. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol. Infect. 2011, 139, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Todd, S.; Page, N.A.; Duncan Steele, A.; Peenze, I.; Cunliffe, N.A. Rotavirus strain types circulating in Africa: Review of studies published during 1997–2006. J. Infect. Dis. 2010, 202, S34–S42. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Desai, R.; Arora, R.; Chitamabar, S.; Naik, T.N.; Krishnan, T.; Deshpande, J.; Gupte, M.D.; Venkatasubramaniam, S.; Gentsch, J.R.; et al. Diversity of circulating rotavirus strains in children hospitalized with diarrhea in India, 2005–2009. Vaccine 2013, 31, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.W.; Ettayebi, K.; Hardy, M.E. Rotavirus NSP1 inhibits NFkappaB activation by inducing proteasome-dependent degradation of beta-TrCP: A novel mechanism of IFN antagonism. PLoS Pathog. 2009, 5, e1000280. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.; Truong, T.T.; Coulson, B.S. Rotavirus antagonizes cellular antiviral responses by inhibiting the nuclear accumulation of STAT1, STAT2, and NF-kappaB. J. Virol. 2009, 83, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Angel, J.; Franco, M.A.; Greenberg, H.B. Rotavirus immune responses and correlates of protection. Curr. Opin. Virol. 2012, 2, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Offit, P.A. Rotaviruses: Immunological determinants of protection against infection and disease. Adv. Virus Res. 1994, 44, 161–202. [Google Scholar] [PubMed]
- Franco, M.A.; Angel, J.; Greenberg, H.B. Immunity and correlates of protection for rotavirus vaccines. Vaccine 2006, 24, 2718–2731. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Siadat-Pajouh, M.; Krishnaney, A.A.; Greenberg, H.B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 1996, 272, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Sapparapu, G.; Sims, A.L.; Aiyegbo, M.S.; Shaikh, F.Y.; Harth, E.M.; Crowe, J.E., Jr. Intracellular neutralization of a virus using a cell-penetrating molecular transporter. Nanomedicine 2014, 9, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U.; Huppertz, H.I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J. Infect. Dis. 2011, 203, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M.; Pérez-Schael, I.; Velázquez, F.R.; Abate, H.; Breuer, T.; Clemens, S.C.; Cheuvart, B.; Espinoza, F.; Gillard, P.; Innis, B.L.; et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 2006, 354, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Leshem, E.; Moritz, R.E.; Curns, A.T.; Zhou, F.; Tate, J.E.; Lopman, B.A.; Parashar, U.D. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics 2014, 134, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rha, B.; Tate, J.E.; Payne, D.C.; Cortese, M.M.; Lopman, B.A.; Curns, A.T.; Parashar, U.D. Effectiveness and impact of rotavirus vaccines in the United States—2006–2012. Expert Rev. Vaccines 2014, 13, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Jonesteller, C.L.; Burnett, E.; Yen, C.; Tate, J.E.; Parashar, U.D. Effectiveness of Rotavirus Vaccination: A systematic review of the first decade of global post-licensure data, 2006–2016. Clin. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pollard, S.L.; Malpica-Llanos, T.; Friberg, I.K.; Fischer-Walker, C.; Ashraf, S.; Walker, N. Estimating the herd immunity effect of rotavirus vaccine. Vaccine 2015, 33, 3795–3800. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Rongsen-Chandola, T.; Bavdekar, A.; John, J.; Antony, K.; Taneja, S.; Goyal, N.; Kawade, A.; Kang, G.; Rathore, S.S.; et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: A randomised, double-blind, placebo-controlled trial. Lancet 2014, 383, 2136–2143. [Google Scholar] [CrossRef]
- Tate, J.E.; Arora, R.; Kang, G.; Parashar, U.D. Rotavirus vaccines at the threshold of implementation in India. Natl. Med. J. India 2014, 27, 245–248. [Google Scholar] [PubMed]
- Lopman, B.A.; Pitzer, V.E.; Sarkar, R.; Gladstone, B.; Patel, M.; Glasser, J.; Gambhir, M.; Athison, C.; Grenfell, B.T.; Edmunds, W.J.; et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS ONE 2012, 7, e41720. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.D.; Colgate, E.R.; Mychaleckyj, J.C.; Haque, R.; Dickson, D.M.; Carmolli, M.P.; Nayak, U.; Taniuchi, M.; Naylor, C.; Qadri, F.; et al. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: Description of methods of an interventional study designed to explore complex biologic problems. Am. J. Trop. Med. Hyg. 2015, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J. Malnutrition and vaccination in developing countries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Hoest, C.; Seidman, J.C.; Pan, W.; Ambikapathi, R.; Kang, G.; Kosek, M.; Knobler, S.; Mason, C.J.; Miller, M.; MAL-ED Network Investigators. Evaluating associations between vaccine response and malnutrition, gut function, and enteric infections in the MAL-ED cohort study: Methods and challenges. Clin. Infect. Dis. 2014, 59 (Suppl. 4), S273–S279. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Elia, M. Deprivation linked to malnutrition risk and mortality in hospital. Br. J. Nutr. 2006, 96, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Perez-Schael, I.; Salinas, B.; Tomat, M.; Linhares, A.C.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Bouckenooghe, A.; Yarzabal, J.P. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J. Infect. Dis. 2007, 196, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Savy, M.; Edmond, K.; Fine, P.E.; Hall, A.; Hennig, B.J.; Moore, S.E.; Mulholland, K.; Schaible, U.; Prentice, A.M. Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 2009, 139, 2154S–2218S. [Google Scholar] [CrossRef] [PubMed]
- Gastañaduy, P.A.; Contreras-Roldán, I.; Bernart, C.; López, B.; Benoit, S.R.; Xuya, M.; Muñoz, F.; Desai, R.; Quaye, O.; Tam, K.I.; et al. Effectiveness of Monovalent and Pentavalent Rotavirus Vaccines in Guatemala. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.F.; Hille, D.A.; Liu, G.F.; Kaplan, S.S.; Nelson, M.; Goveia, M.G.; Mast, T.C. Heterogeneity of Rotavirus Vaccine Efficacy Among Infants in Developing Countries. Pediatr. Infect. Dis. J. 2017, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [PubMed]
- Young, G.P.; Mortimer, E.K.; Gopalsamy, G.L.; Alpers, D.H.; Binder, H.J.; Manary, M.J.; Ramakrishna, B.S.; Brown, I.L.; Brewer, T.G. Zinc deficiency in children with environmental enteropathy-development of new strategies: Report from an expert workshop. Am. J. Clin. Nutr. 2014, 100, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Colgate, E.R.; Haque, R.; Dickson, D.M.; Carmolli, M.P.; Mychaleckyj, J.C.; Nayak, U.; Qadri, F.; Alam, M.; Walsh, M.C.; Diehl, S.A.; et al. Delayed Dosing of Oral Rotavirus Vaccine Demonstrates Decreased Risk of Rotavirus Gastroenteritis Associated With Serum Zinc: A Randomized Controlled Trial. Clin. Infect. Dis. 2016, 63, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Bosomprah, S.; Beach, L.B.; Beres, L.K.; Newman, J.; Kapasa, K.; Rudd, C.; Njobvu, L.; Guffey, B.; Hubbard, S.; Foo, K.; et al. Findings from a comprehensive diarrhoea prevention and treatment programme in Lusaka, Zambia. BMC Public Health 2016, 16, 475. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Siegismund, C.S.; Saif, L.J. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J. Immunol. 2013, 190, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Chattha, K.S.; Kandasamy, S.; Vlasova, A.N.; Saif, L.J. Vitamin A deficiency impairs adaptive B and T cell responses to a prototype monovalent attenuated human rotavirus vaccine and virulent human rotavirus challenge in a gnotobiotic piglet model. PLoS ONE 2013, 8, e82966. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Saif, L.J. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq®) in a neonatal gnotobiotic pig model. Vaccine 2014, 32, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Ndure, J.; Plebanski, M.; Flanagan, K.L. Heterologous and sex differential effects of administering vitamin A supplementation with vaccines. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Sprenger-Mähr, H.; Knoll, F.; Neyer, U.; Lhotta, K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 2012, 30, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Penkert, R.R.; Jones, B.G.; Sealy, R.E.; Hurwitz, J.L. Vitamin Supplementation at the Time of Immunization with a Cold-Adapted Influenza Virus Vaccine Corrects Poor Mucosal Antibody Responses in Mice Deficient for Vitamins A and D. Clin. Vaccine Immunol. 2016, 23, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bucak, I.H.; Ozturk, A.B.; Almis, H.; Cevik, M.Ö.; Tekin, M.; Konca, Ç.; Turgut, M.; Bulbul, M. Is there a relationship between low vitamin D and rotaviral diarrhea? Pediatr. Int. 2016, 58, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, B.; Mao, X.; He, J.; Huang, Z.; Zheng, P.; Yu, J.; Han, G.; Liang, X.; Chen, D. Dietary vitamin D supplementation attenuates immune responses of pigs challenged with rotavirus potentially through the retinoic acid-inducible gene I signalling pathway. Br. J. Nutr. 2014, 112, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lomberg, B. Making government smarter. How to set national priorities. For. Aff. 2017, 96, 90–98. [Google Scholar]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Bik, E.M.; Costello, E.K.; Dethlefsen, L.; Haque, R.; Relman, D.A.; Singh, U. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. Can. Med. Assoc. J. 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Rudensky, A.Y. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol. Rev. 2012, 245, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, I.; John, S.M.; Bandyopadhyay, R.; Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Azevedo, M.S.; Wen, K.; Gonzalez, A.; Saif, L.J.; Li, G.; Yousef, A.E.; Yuan, L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine 2008, 26, 3655–3661. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Liu, Z.; Esseili, M.; Shao, L.; Rajashekara, G.; Saif, L.J. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS ONE 2013, 8, e76962. [Google Scholar] [CrossRef] [PubMed]
- Chattha, K.S.; Vlasova, A.N.; Kandasamy, S.; Rajashekara, G.; Saif, L.J. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J. Immunol. 2013, 191, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, G.; Wen, K.; Wu, S.; Zhang, Y.; Bui, T.; Yang, X.; Kocher, J.; Sun, J.; Jortner, B.; et al. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Rajashekara, G.; Saif, L.J. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes 2014, 5, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J. Infect. Dis. 2017, 215, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W. The virome in mammalian physiology and disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.M.; Handley, S.A.; Virgin, H.W. Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities. Gastroenterology 2014, 146, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jovel, J.; Halloran, B.; Wine, E.; Patterson, J.; Ford, G.; O’Keefe, S.; Meng, B.; Song, D.; Zhang, Y.; et al. Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm. Bowel Dis. 2015, 21, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Haak, B.W.; Boele van Hensbroek, M.; Wiersinga, W.J. The Intestinal Microbiome in Infectious Diseases: The Clinical Relevance of a Rapidly Emerging Field. Open Forum Infect. Dis. 2017, 4, ofx144. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Heilig, H.G.; Molenaar, D.; Kajander, K.; Surakka, A.; Smidt, H.; de Vos, W.M. Development and application of the human intestinal tract chip, a phylogenetic microarray: Analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 2009, 11, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Shepherd, M.; Wen, K.; Li, G.; Yang, X.; Kocher, J.; Giri-Rachman, E.; Dickerman, A.; Settlage, R.; et al. Probiotics and virulent human rotavirus modulate the transplanted human gut microbiota in gnotobiotic pigs. Gut Pathog. 2014, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Vlasova, A.N.; Fischer, D.D.; Chattha, K.S.; Shao, L.; Kumar, A.; Langel, S.N.; Rauf, A.; Huang, H.C.; Rajashekara, G.; et al. Unraveling the Differences between Gram-Positive and Gram-Negative Probiotics in Modulating Protective Immunity to Enteric Infections. Front. Immunol. 2017, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Twitchell, E.L.; Tin, C.; Wen, K.; Zhang, H.; Becker-Dreps, S.; Azcarate-Peril, M.A.; Vilchez, S.; Li, G.; Ramesh, A.; Weiss, M.; et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Platts-Mills, J.A.; Begum, S.; Uddin, M.J.; Sobuz, S.U.; Liu, J.; Kirkpatrick, B.D.; Colgate, E.R.; Carmolli, M.P.; Dickson, D.M.; et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine 2016, 34, 3068–3075. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, D.; Goldstick, J.E.; Cevallos, W.; Trueba, G.; Eisenberg, J.N.S. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: Evidence from a community-based study in Northwestern Ecuador. Am. J. Epidemiol. 2012, 176, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Vasco, G.; Trueba, G.; Atherton, R.; Calvopiña, M.; Cevallos, W.; Andrade, T.; Eguiguren, M.; Eisenberg, J.N. Identifying etiological agents causing diarrhea in low income Ecuadorian communities. Am. J. Trop. Med. Hyg. 2014, 91, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Y.; Liu, M.C.; Hsu, C.F.; Lin, Y.C. Rotavirus infection increases the risk of bacteremia in children with nontyphoid Salmonella gastroenteritis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.; Lin, P.C.; Lin, L.C.; Chen, H.L.; Yang, R.C. Salmonella/rotavirus coinfection in hospitalized children. Kaohsiung J. Med. Sci. 2012, 28, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Valentini, D.; Vittucci, A.C.; Grandin, A.; Tozzi, A.E.; Russo, C.; Onori, M.; Menichella, D.; Bartuli, A.; Villani, A. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.I.; Murch, S.H.; Elia, M.; Sullivan, P.B.; Sanyang, M.S.; Jobarteh, B.; Lunn, P.G. Chronic T cell-mediated enteropathy in rural west African children: Relationship with nutritional status and small bowel function. Pediatr. Res. 2003, 54, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.I.; Elia, M.; Lunn, P.G. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J. Nutr. 2003, 133, 1332–1338. [Google Scholar] [PubMed]
- Naylor, C.; Lu, M.; Haque, R.; Mondal, D.; Buonomo, E.; Nayak, U.; Mychaleckyj, J.C.; Kirkpatrick, B.; Colgate, R.; Carmolli, M.; et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015, 2, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Becker-Dreps, S.; Vilchez, S.; Bucardo, F.; Twitchell, E.; Choi, W.S.; Hudgens, M.G.; Perez, J.; Yuan, L. The association between fecal biomarkers of environmental enteropathy and rotavirus vaccine response in Nicaraguan infants. Pediatr. Infect. Dis. J. 2017, 36, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Goveia, M.G.; DiNubile, M.J.; Dallas, M.J.; Heaton, P.; Kuter, B. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr. Infect. Dis. J. 2008, 27, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Bouckenooghe, A.; Damaso, S.; Han, H.H. Efficacy and immunogenicity of live-attenuated human rotavirus vaccine in breast-fed and formula-fed European infants. Pediatr. Infect. Dis. J. 2012, 31, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Rennels, M.B.; Wasserman, S.S.; Glass, R.I.; Keane, V.A. Comparison of immunogenicity and efficacy of rhesus rotavirus reassortant vaccines in breastfed and nonbreastfed children. US Rotavirus Vaccine Efficacy Group. Pediatrics 1995, 96, 1132–1136. [Google Scholar] [PubMed]
- Rongsen-Chandola, T.; Strand, T.A.; Goyal, N.; Flem, E.; Rathore, S.S.; Arya, A.; Winje, B.A.; Lazarus, R.; Shanmugasundaram, E.; Babji, S.; et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014, 32 (Suppl. 1), A134–A139. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Moon, S.S.; Velasquez, D.; Jones, S.; Koen, A.; van Niekerk, N.; Jiang, B.; Parashar, U.D.; Madhi, S.A. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: A randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull. World Health Organ. 2014, 92, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Becker-Dreps, S.; Vilchez, S.; Velasquez, D.; Moon, S.S.; Hudgens, M.G.; Zambrana, L.E.; Jiang, B. Rotavirus-specific IgG antibodies from mothers’ serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr. Infect. Dis. J. 2015, 34, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Appaiahgari, M.B.; Glass, R.; Singh, S.; Taneja, S.; Rongsen-Chandola, T.; Bhandari, N.; Mishra, S.; Vrati, S. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine 2014, 32, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S.; Groome, M.J.; Velasquez, D.E.; Parashar, U.D.; Jones, S.; Koen, A.; van Niekerk, N.; Jiang, B.; Madhi, S.A. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin. Infect. Dis. 2016, 62, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Kirkwood, C.D.; Bines, J.; Cowley, D.; Pavlic, D.; Lee, K.J.; Orsini, F.; Watts, E.; Barnes, G.; Danchin, M. Rotavirus specific maternal antibodies and immune response to RV3-BB neonatal rotavirus vaccine in New Zealand. Hum. Vaccines Immunother. 2017, 13, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Crawford, S.E.; Czako, R.; Cortes-Penfield, N.W.; Smith, D.F.; Le Pendu, J.; Estes, M.K.; Prasad, B.V. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 2012, 485, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Marcille, B.M.; Barbé, L.; Dupé, M.; Le Moullac-Vaidye, B.; Besse, B.; Peltier, C.; Ruvoën-Clouet, N.; Le Pendu, J. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J. Infect. Dis. 2014, 209, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Hu, L.; Venkataram Prasad, B.V.; Estes, M.K. Diversity in Rotavirus-Host Glycan Interactions: A “Sweet” Spectrum. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Sharma, S.; Bucardo, F.; Nasir, W.; Günaydın, G.; Ouermi, D.; Nitiema, L.W.; Becker-Dreps, S.; Simpore, J.; Hammarström, L.; et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin. Infect. Dis. 2014, 59, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.M.; Cortese, M.M.; Yu, Y.; Lopman, B.; Morrow, A.L.; Fleming, J.A.; McNeal, M.M.; Steele, A.D.; Parashar, U.D.; Zaidi, A.K.M.; et al. Secretor and Salivary ABO Blood Group Antigen Status Predict Rotavirus Vaccine Take in Infants. J. Infect. Dis. 2017, 215, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Blutt, S.E.; Ettayebi, K.; Zeng, X.L.; Broughman, J.R.; Crawford, S.E.; Karandikar, U.C.; Sastri, N.P.; Conner, M.E.; Opekun, A.R.; et al. Human Intestinal Enteroids: A New Model to Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J. Virol. 2015, 90, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Komoto, S.; Kawagishi, T.; Nouda, R.; Nagasawa, N.; Onishi, M.; Matsuura, Y.; Taniguchi, K.; Kobayashi, T. Entirely plasmid-based reverse genetics system for rotaviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desselberger, U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens 2017, 6, 65. https://doi.org/10.3390/pathogens6040065
Desselberger U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens. 2017; 6(4):65. https://doi.org/10.3390/pathogens6040065
Chicago/Turabian StyleDesselberger, Ulrich. 2017. "Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors" Pathogens 6, no. 4: 65. https://doi.org/10.3390/pathogens6040065
APA StyleDesselberger, U. (2017). Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens, 6(4), 65. https://doi.org/10.3390/pathogens6040065



