Ciprofloxacin-Induced Antibacterial Activity Is Atteneuated by Pretreatment with Antioxidant Agents
Abstract
:1. Introduction
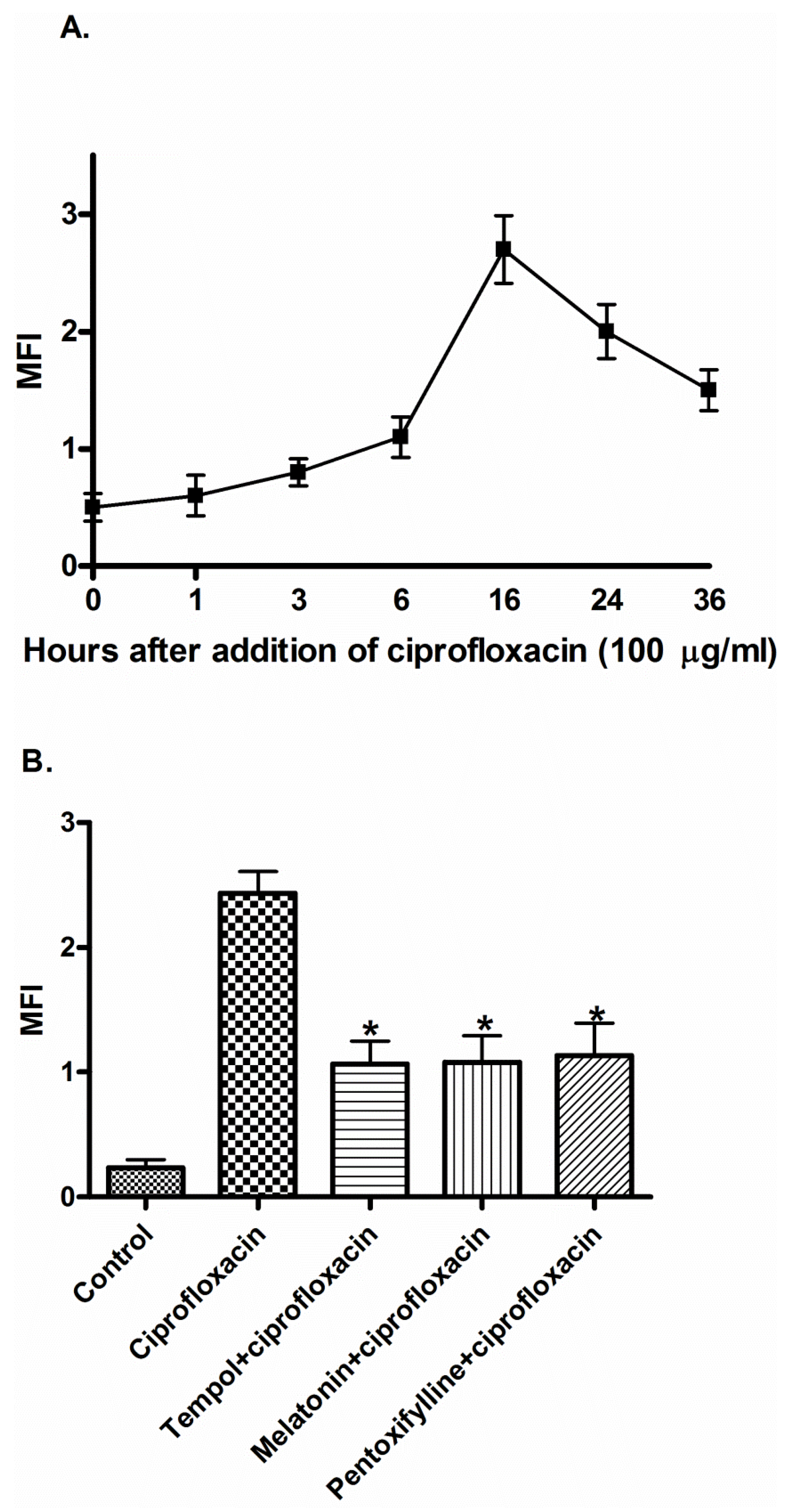
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Microbial Culture and Growth Conditions
4.3. Antimicrobial Susceptibility Test
4.4. Determination of Minimum Inhibitory Concentration (MIC)
4.5. Measurement of ROS Generation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Al-Soud, Y.A.; Al-Masoudi, N.A. A new class of dihaloquinolones bearing N′-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and á-amino ester precursors: Synthesis and antimicrobial activity. J. Braz. Chem. Soc. 2003, 14, 790–796. [Google Scholar] [CrossRef]
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar] [PubMed]
- Umezawa, N.; Arakane, K.; Ryu, A.; Mashiko, S.; Hirobe, M.; Nagano, T. Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch. Biochem. Biophys. 1997, 342, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Malik, M.; Snyder, M.; Drlica, K. DNA gyrase and topoisomerase IV on the bacterial chromosome: Quinolone-induced DNA cleavage. J. Mol. Biol. 1996, 258, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar] [PubMed]
- Pouzaud, F.; Bernard-Beaubois, K.; Thevenin, M.; Warnet, J.M.; Hayem, G.; Rat, P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: Involvement of oxidative stress. J. Pharmacol. Exp. Ther. 2004, 308, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Mallet, L.; Huang, A. Coadministration of gatifloxacin and multivitamin preparation containing minerals: Potential treatment failure in an elderly patient. Ann. Pharmacother. 2005, 39, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.J.; Lopez-Pingarron, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; Garcia-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramirez, J.M.; Bernal-Perez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal. Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Winkelsberg, D.; Pinson, A.; Hahn, S.M.; Mitchell, J.B.; Russo, A. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J. Clin. Invest. 1991, 87, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Chateauneuf, J.; Lusztyk, J.; Ingold, K.U. Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethylpiperidine-n-oxyl. J. Org. Chem. 1988, 53, 1629–1632. [Google Scholar] [CrossRef]
- Paradowski, P.T.; Zeman, K. Pentoxifylline. Postepy Hig. Med. Dosw. 1994, 49, 201–220. [Google Scholar]
- Davila-Esqueda, M.E.; Martinez-Morales, F. Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp. Diabetes. Res. 2004, 5, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sharma, R.K.; Agarwal, A.; Falcone, T. Antioxidant effect of pentoxifylline in reducing oxidative stress induced embryotoxicity. Fertil. Steril. 2004, 82, S324–S325. [Google Scholar] [CrossRef]
- Dinckan, A.; Sahin, E.; Ogus, M.; Emek, K.; Gumuslu, S. The effect of pentoxifylline on oxidative stress in CO2 pneumoperitoneum. Surg. Endosc. 2009, 23, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Albesa, I.; Becerra, M.C.; Battan, P.C.; Paez, P.L. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys Res. Commun. 2004, 317, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.C.; Albesa, I. Oxidative stress induced by ciprofloxacin in staphylococcus aureus. Biochem. Biophys Res. Commun. 2002, 297, 1003–1007. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Mhaidat, N.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Shaweesh, A.I. Ciprofloxacin-induced antibacterial activity is reversed by vitamin e and vitamin c. Curr. Microbiol. 2012, 64, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.; Alzoubi, K.; Al-Azzam, S. Flouroquinolones-induced antibacterial activity atteneuation by pretreatment with vitamin b12. Int. J. Pharmacol. 2015, 11, 67–71. [Google Scholar] [CrossRef]
- Gurbay, A.; Hincal, F. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug. Chem. Toxicol. 2004, 27, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Mangoli, S.H.; Jawali, N. Involvement of reactive oxygen species in the action of ciprofloxacin against escherichia coli. Antimicrob. Agents Chemother. 2006, 50, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Gootz, T.D.; Barrett, J.F.; Sutcliffe, J.A. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob. Agents Chemother. 1990, 34, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gellert, M. DNA topoisomerases. Annu. Rev. Biochem. 1981, 50, 879–910. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically; Approved Standard—Eighth Edition. Available online: http://simpleshowoflove.weebly.com/uploads/1/4/0/7/14073276/agar_dilution_assay.pdf (accessed on 9 March 2016).
- Solovieva, M.E.; Soloviev, V.V.; Akatov, V.S. Vitamin b12b increases the cytotoxicity of short-time exposure to ascorbic acid, inducing oxidative burst and iron-dependent DNA damage. Eur. J. Pharmacol. 2007, 566, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, M.E.; Solovyev, V.V.; Kudryavtsev, A.A.; Trizna, Y.A.; Akatov, V.S. Vitamin b12b enhances the cytotoxicity of dithiothreitol. Free Radic. Biol. Med. 2008, 44, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sasaki, T.; Matsuoka, H. Vitamin b(12) promotes cx40 and hcn4 gene expression at an early stage of cardiomyocyte differentiation. Exp. Anim. 2009, 58, 57–60. [Google Scholar] [CrossRef] [PubMed]
| Standard Bacterial Strains | Zone of Inhibition (mm)* | ||||||
|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Tempol | Ciprofloxacin + Tempol | Melatonin | Ciprofloxacin + Melatonin | Pentoxifylline | Ciprofloxacin + Pentoxifylline | |
| Gram +ve: | |||||||
| S. aureus | 22.7 ± 1.5 | 2.7 ± 0.6 | 8.7 ± 0.6 | 5.0 ± 1.2 | 12.0 ± 1.0 | 5. 3 ± 0.6 | 13. 3 ± 0.6 |
| S. epidermidis | 22.0 ± 1.0 | 3.3 ± 0.6 | 8.3 ± 0.6 | 2.7 ± 0.6 | 9.7 ± 0.6 | 2. 4 ± 0.6 | 12. 3 ± 0.6 |
| MRSA | 10.7 ± 1.5 | 2.7 ± 0.6 | 2.7 ± 0.6 | 3.7 ± 1.2 | 5.0 ± 1.0 | 1.2 ± 0.6 | 4.0 ± 1.0 |
| S. pneumoniae | 14.7 ± 0.6 | 4.3 ± 1.2 | 6.7 ± 0.6 | 4.7 ± 1.6 | 7.3 ± 0.6 | 4.7 ± 0.6 | 7.7 ± 0.6 |
| VRE | 16.7 ± 1.5 | 1.2 ± 0.6 | 1.7 ± 0.6 | 2.3 ± 1.2 | 2.3 ± 1.2 | 2.1 ± 1.6 | 3.0 ± 1.0 |
| S. pyogenes | 21.7 ± 1.5 | 4.3 ± 1.2 | 7.3 ± 1.2 | 4.7 ± 1.2 | 9.7 ± 0.6 | 4.7 ± 1.2 | 12.0 ± 1.0 |
| Gram –ve: | |||||||
| E. coli | 26.7 ± 2.0 | 5 .0 ± 2.0 | 8 .0 ± 1.0 | 5.0 ± 2.0 | 13.0 ± 1.0 | 2.7 ± 0.6 | 15.7 ± 0.6 |
| P. aeruginosa | 23.3 ± 1.2 | 2.7 ± 1.6 | 9.7 ± 0.6 | 2.0 ± 1.0 | 13.0 ± 1.0 | 4. 3 ± 2.1 | 13.3 ± 1.2 |
| P. mirabilis | 19.7 ± 2.1 | 1.7 ± 1.2 | 7.7 ± 1.2 | 2.7 ± 0.0 | 8.0 ± 0.0 | 1.7 ± 0.6 | 10.7 ± 0.6 |
| K. pneumoniae | 22.0 ± 2.0 | 4.7 ± 0.6 | 7.7 ± 0.6 | 2.0 ± 1.2 | 12.0 ± 1.0 | 4.3 ± 0.6 | 13.0 ± 1.0 |
| A. baumannii | 11.3 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 | 4.7 ± 1.6 | 3.7 ± 0.6 | 1.2 ± 0.6 | 5.3 ± 0.6 |
| Standard Bacterial Strains | MIC (µg/mL)* | |||
|---|---|---|---|---|
| Ciprofloxacin | Ciprofloxacin + Tempol | Ciprofloxacin + Melatonin | Ciprofloxacin + Pentoxifylline | |
| Gram +ve: | ||||
| E. coli | 0.07 ± 0.04 | 80.00 ± 0.00 | 133.33 ± 57.73 | 133.33 ± 57.73 |
| S. aureus | 0.14 ± 0.09 | 106.60 ± 46.18 | 166.67 ± 57.73 | 166.67 ± 57.73 |
| S. epidermidis | 0.10 ± 0.04 | 106.60 ± 46.18 | 133.33 ± 57.73 | 133.33 ± 57.73 |
| MRSA | 0.49 ± 0.00 | 320.00 ± 0.00 | 666.67 ± 230.9 | 533.33 ± 230.90 |
| S. pneumonia | 0.37 ± 0.17 | 266.60 ± 92.370 | 800.00 ± 0.00 | 666.67 ± 230.90 |
| VRE | 0.99 ± 0.00 | 640.00 ± 0.00 | 133.33 ± 461.80 | 133.33 ± 461.80 |
| S. pyogenes | 0.16 ± 0.07 | 133.30 ± 46.18 | 133.33 ± 57.73 | 133.33 ± 57.73 |
| Gram –ve: | ||||
| P. aeruginosa | 0.14 ± 0.09 | 133.30 ± 46.18 | 333.33 ± 115.4 | 266.67 ± 115.4 |
| P. mirabilis | 0.21 ± 0.07 | 160.00 ± 0.00 | 266.67 ± 115.4 | 200.00 ± 0.00 |
| K. pneumonia | 0.19 ± 0.08 | 133.30 ± 46.18 | 133.33 ± 57.73 | 266.67 ± 115.40 |
| A. baumannii | 0.49 ± 0.00 | 426.60 ± 184.70 | 800.00 ± 0.00 | 166.67 ± 461.80 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masadeh, M.M.; Alzoubi, K.H.; Al-azzam, S.I.; Khabour, O.F.; Al-buhairan, A.M. Ciprofloxacin-Induced Antibacterial Activity Is Atteneuated by Pretreatment with Antioxidant Agents. Pathogens 2016, 5, 28. https://doi.org/10.3390/pathogens5010028
Masadeh MM, Alzoubi KH, Al-azzam SI, Khabour OF, Al-buhairan AM. Ciprofloxacin-Induced Antibacterial Activity Is Atteneuated by Pretreatment with Antioxidant Agents. Pathogens. 2016; 5(1):28. https://doi.org/10.3390/pathogens5010028
Chicago/Turabian StyleMasadeh, Majed M., Karem H. Alzoubi, Sayer I. Al-azzam, Omar F. Khabour, and Ahlam M. Al-buhairan. 2016. "Ciprofloxacin-Induced Antibacterial Activity Is Atteneuated by Pretreatment with Antioxidant Agents" Pathogens 5, no. 1: 28. https://doi.org/10.3390/pathogens5010028
APA StyleMasadeh, M. M., Alzoubi, K. H., Al-azzam, S. I., Khabour, O. F., & Al-buhairan, A. M. (2016). Ciprofloxacin-Induced Antibacterial Activity Is Atteneuated by Pretreatment with Antioxidant Agents. Pathogens, 5(1), 28. https://doi.org/10.3390/pathogens5010028






