Urinary Tract Infection Molecular Mechanisms and Clinical Translation
Abstract
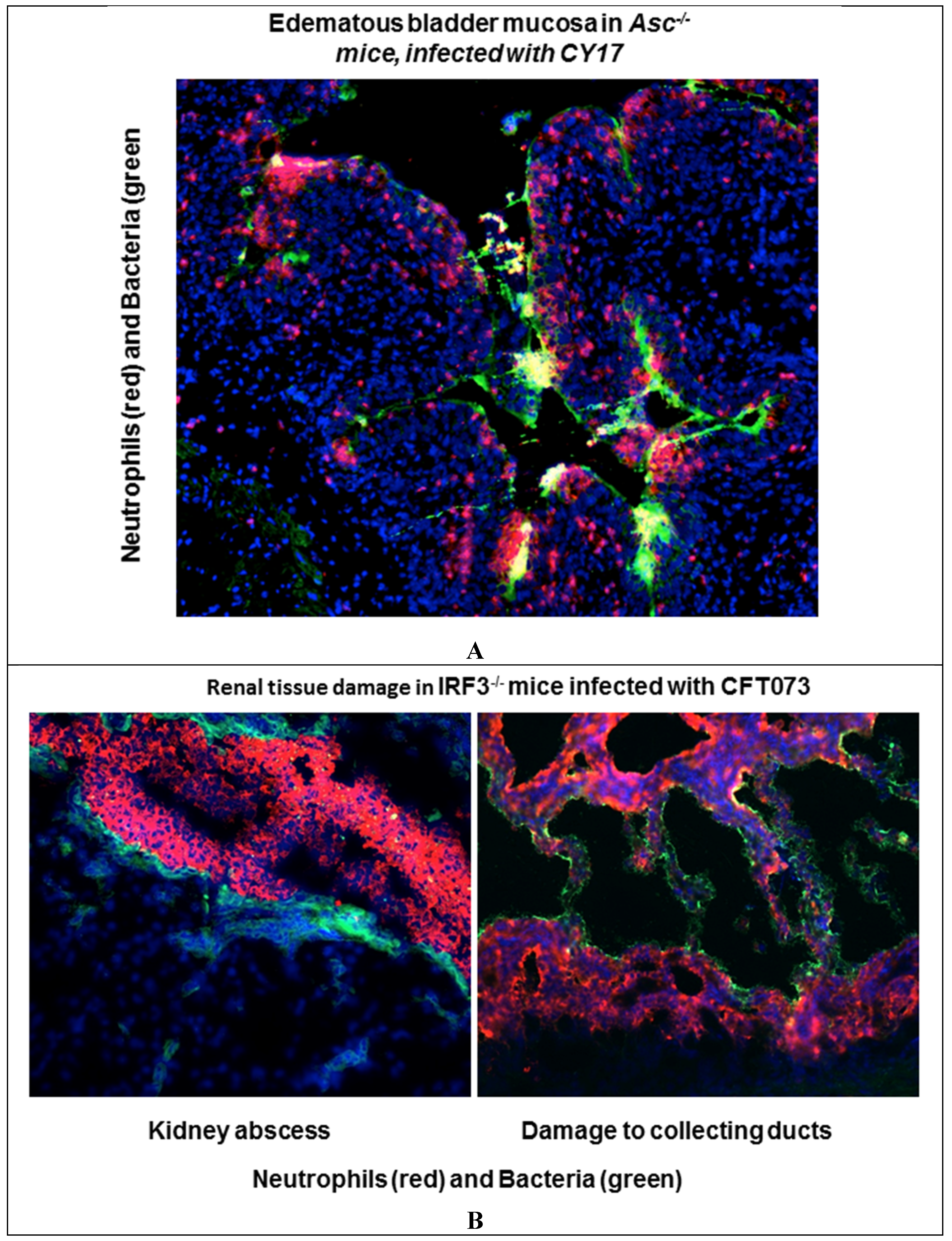
:1. Introduction
- Identify key, virulence factors that distinguish pathogens from commensals.
- Characterize critical host signaling pathways that define symptoms and pathology.
- Inactivate specific genes that control these pathways and characterize the consequences for protection and pathology.
- Confirm the relevance of these genetic variants for disease in susceptible or resistant patient groups.
- Address how ABU strains achieve the state of “commensalism”.
- Develop immune-modulatory therapies, as a complement to antibiotics.
2. Key, Virulence Factors that Distinguish Pathogens from Commensals
3. Critical Signaling Pathways that Define Symptoms and Pathology
4. Inactivate Specific Genes that Control These Pathways and Characterize the Consequences for Protection and Pathology
5. Relevance of Genetic Variants for Disease in Susceptible or Resistant Patient Groups
6. Address how ABU Strains Achieve the State of “Commensalism”
7. Immunomodulatory Therapies as a Complement to Antibiotics
- siRNA interference to attenuate the exaggerated inflammatory response in Irf3−/− mice with severe acute pyelonephritis.
- Inhibitors of IL-1 and IL-1 processors, to attenuate the IL-1-dependent hyper-inflammatory state in acute cystitis.
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Special Issue “Molecular Aspects of Urinary Tract Infection”. Available online: https://www.mdpi.com/journal/pathogens/special_issues/urinary-tract-infection (accessed on 23 February 2016).
- Eden, C.S.; Hanson, L.A.; Jodal, U.; Lindberg, U.; Akerlund, A.S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet 1976, 1, 490–492. [Google Scholar] [PubMed]
- Lomberg, H.; Eden, C.S. Influence of p blood group phenotype on susceptibility to urinary tract infection. FEMS Microbiol. Immunol. 1989, 1, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991, 4, 80–128. [Google Scholar] [PubMed]
- Svensson, M.; Platt, F.M.; Svanborg, C. Glycolipid receptor depletion as an approach to specific antimicrobial therapy. FEMS Microbiol. Lett. 2006, 258, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, G.; Samuelsson, M.; Wullt, B.; Leijonhufvud, I.; Fischer, H.; Svanborg, C. Papg-dependent adherence breaks mucosal inertia and triggers the innate host response. J. Infect. Dis. 2004, 189, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Orskov, I.; Svanborg Eden, C.; Orskov, F. Aerobactin production of serotyped Escherichia coli from urinary tract infections. Med. Microbiol. Immunol. 1988, 177, 9–14. [Google Scholar] [PubMed]
- Caugant, D.A.; Levin, B.R.; Orskov, I.; Orskov, F.; Svanborg Eden, C.; Selander, R.K. Genetic diversity in relation to serotype in Escherichia coli. Infect. Immun. 1985, 49, 407–413. [Google Scholar] [PubMed]
- Plos, K.; Hull, S.I.; Hull, R.A.; Levin, B.R.; Orskov, I.; Orskov, F.; Svanborg-Eden, C. Distribution of the p-associated-pilus (pap) region among Escherichia coli from natural sources: Evidence for horizontal gene transfer. Infect. Immun. 1989, 57, 1604–1611. [Google Scholar] [PubMed]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Caugant, D.A.; Levin, B.R.; Selander, R.K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics 1981, 98, 467–490. [Google Scholar] [PubMed]
- Nielubowicz, G.R.; Mobley, H.L. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- De Man, P.; van Kooten, C.; Aarden, L.; Engberg, I.; Linder, H.; Svanborg Eden, C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect. Immun. 1989, 57, 3383–3388. [Google Scholar] [PubMed]
- Agace, W.; Hedges, S.; Andersson, U.; Andersson, J.; Ceska, M.; Svanborg, C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect. Immun. 1993, 61, 602–609. [Google Scholar] [PubMed]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ambite, I.; Lutay, N.; Godaly, G.; Svanborg, C. Urinary tract infections and the mucosal immune system. In Mucosal Immunology, 4th ed.; Jiri Mestecky, W.S., Russell, M.W., Cheroutre, H., Lambrecht, B.N., Kelsall, B.L., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 2039–2058. [Google Scholar]
- Hedlund, M.; Wachtler, C.; Johansson, E.; Hang, L.; Somerville, J.E.; Darveau, R.P.; Svanborg, C. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol. Microbiol. 1999, 33, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Lutay, N.; Ragnarsdottir, B.; Yadav, M.; Jonsson, K.; Urbano, A.; Al Hadad, A.; Ramisch, S.; Storm, P.; Dobrindt, U.; et al. Pathogen specific, irf3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog. 2010, 6, e1001109. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Yamamoto, M.; Akira, S.; Beutler, B.; Svanborg, C. Mechanism of pathogen-specific tlr4 activation in the mucosa: Fimbriae, recognition receptors and adaptor protein selection. Eur. J. Immunol. 2006, 36, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Hull, R.; Hull, S.; McGhee, J.R.; Michalek, S.M.; Svanborg Eden, C. Difference in susceptibility to gram-negative urinary tract infection between c3h/hej and c3h/hen mice. Infect. Immun. 1984, 46, 839–844. [Google Scholar] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective lps signaling in c3h/hej and c57bl/10sccr mice: Mutations in tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Briles, D.E.; Svanborg-Eden, C. Evidence for separate genetic defects in c3h/hej and c3heb/fej mice, that affect susceptibility to gram-negative infections. J. Immunol. 1985, 134, 4118–4122. [Google Scholar] [PubMed]
- Shahin, R.D.; Engberg, I.; Hagberg, L.; Svanborg Eden, C. Neutrophil recruitment and bacterial clearance correlated with lps responsiveness in local gram-negative infection. J. Immunol. 1987, 138, 3475–3480. [Google Scholar] [PubMed]
- Hopkins, W.J.; Gendron-Fitzpatrick, A.; Balish, E.; Uehling, D.T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 1998, 66, 2798–2802. [Google Scholar] [PubMed]
- Frendeus, B.; Wachtler, C.; Hedlund, M.; Fischer, H.; Samuelsson, P.; Svensson, M.; Svanborg, C. Escherichia coli p fimbriae utilize the toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 2001, 40, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Frendeus, B.; Wachtler, C.; Hang, L.; Fischer, H.; Svanborg, C. Type 1 fimbriae deliver an lps- and tlr4-dependent activation signal to cd14-negative cells. Mol. Microbiol. 2001, 39, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.D.; Martin, S.M.; Hung, C.S.; Lorenz, R.G.; Hultgren, S.J. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2003, 100, 4203–4208. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdottir, B.; Lutay, N.; Gronberg-Hernandez, J.; Koves, B.; Svanborg, C. Genetics of innate immunity and uti susceptibility. Nat. Rev. Urol. 2011, 8, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Frendeus, B.; Godaly, G.; Hang, L.; Karpman, D.; Lundstedt, A.C.; Svanborg, C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J. Exp. Med. 2000, 192, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Frendeus, B.; Godaly, G.; Svanborg, C. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J. Infect. Dis. 2000, 182, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Irjala, H.; Alm, P.; Holmqvist, B.; Lundstedt, A.C.; Svanborg, C. Natural history of renal scarring in susceptible mil-8rh-/- mice. Kidney Int. 2005, 67, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.; Braconier, J.; Andreasson, A.; Svanborg, C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 1999, 179, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Godaly, G.; Otto, G.; Burdick, M.D.; Strieter, R.M.; Svanborg, C. Fimbrial lectins influence the chemokine repertoire in the urinary tract mucosa. Kidney Int. 2007, 71, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Agace, W.W.; Hedges, S.R.; Ceska, M.; Svanborg, C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Investig. 1993, 92, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Wullt, B.; Bergsten, G.; Fischer, H.; Godaly, G.; Karpman, D.; Leijonhufvud, I.; Lundstedt, A.C.; Samuelsson, P.; Samuelsson, M.; Svensson, M.L.; et al. The host response to urinary tract infection. Infect. Dis. Clin. N. Am. 2003, 17, 279–301. [Google Scholar] [CrossRef]
- Ragnarsdottir, B.; Jonsson, K.; Urbano, A.; Gronberg-Hernandez, J.; Lutay, N.; Tammi, M.; Gustafsson, M.; Lundstedt, A.C.; Leijonhufvud, I.; Karpman, D.; et al. Toll-like receptor 4 promoter polymorphisms: Common tlr4 variants may protect against severe urinary tract infection. PLoS ONE 2010, 5, e10734. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, A.C.; McCarthy, S.; Gustafsson, M.C.; Godaly, G.; Jodal, U.; Karpman, D.; Leijonhufvud, I.; Linden, C.; Martinell, J.; Ragnarsdottir, B.; et al. A genetic basis of susceptibility to acute pyelonephritis. PLoS ONE 2007, 2, e825. [Google Scholar] [CrossRef] [PubMed]
| Name | Title |
|---|---|
| Bruce Beutler | KEY NOTE LECTURE Pathogens, Commensals, And Immunity: From the Perspective of The Urinary Bladder |
| HOST SUSCEPTIBILITY TO INFECTION | |
| Catharina Svanborg | Urinary Tract Infection Molecular Mechanisms and Clinical Translation |
| David Hains | Genetic Variation in Vesicoureteral Reflux and its Sequelae |
| Christian Kurts, Daniel Engel | Neutrophil-Migration into the Infected Uroepithelium is Regulated by the Crosstalk between Resident and Helper Macrophages |
| HOST RESPONSE MODULATION BY BACTERIA | |
| Soman Abraham | Why Serological Responses During Cystitis are Limited |
| Thomas Miethke | A comparative analysis of the mechanism of Toll-like receptor-disruption by TIR-containing protein C from uropathogenic Escherichia coli * |
| Ines Ambite | Bacterial control of host gene expression through RNA polymerase II * |
| David Hunstad | Subversion of host innate immunity by uropathogenic Escherichia coli binocular |
| ASYMPTOMATIC BACTERIAL CARRIAGE | |
| Lindsay Nicolle | The Paradigm Shift to Nontreatment of Asymptomatic Bacteriuria * |
| Björn Wullt | Asymtomatic Bacteriuria as a Model to Study the Coevolution of Hosts and Bacteria |
| Tommaso Cai | Asymptomatic bacteriuria in clinical urological practice: antibiotic preoperative prophylaxis and treatment of recurrent UTI |
| BACTERIAL VIRULENCE | |
| Harry Mobley | Measuring E. coli Gene Expression During Human Urinary Tract Infections |
| Matthew Mulvey | Histone Deacetylase 6 Regulates Bladder Architecture and Host Susceptibility to Uropathogenic Escherichia coli |
| Swaine Chen | Application and optimization of relE as a negative selection marker for making definitive genetic constructs in uropathogenic Escherichia coli strain UTI89 |
| Swaine Chen | Brighter fluorescent derivatives of UTI89 utilizing a monomeric vGFP |
| Eric Klein | Perspective: Adhesion mediated signal transduction in uropathogenic E. coli |
| NOVEL THERAPEUTIC APPROACHES | |
| Annelie Brauner | Novel Strategies in the Prevention and Treatment of Urinary Tract Infections |
| Ann Stapleton | Cytoprotective effect of Lactobacillus crispatus CTV-05 against uropathogenic Escherichia coli * |
| Scott Hultgren | Adhesive Pili in UTI Pathogenesis and Drug Development * |
| Susanne Geerlings | Non-antibiotic prophylaxis for urinary tract infections * |
| Harry Mobley | Development of a Vaccine against Escherichia coli Urinary Tract Infections |
| Clara Maria Pichl | Biomickry of UPEC cytoinvasion: a novel concept for improved drug delivery in UTI |
| ANTIBIOTIC RESISTANCE | |
| Mark Schembri | Molecular characterization of the multidrug resistant E. coli ST131 clone |
| Florian Wagenlehner | The Global Prevalence of Infections in Urology (GPIU) study: A long term, world wide surveillance study on urological infections |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godaly, G.; Ambite, I.; Puthia, M.; Nadeem, A.; Ho, J.; Nagy, K.; Huang, Y.; Rydström, G.; Svanborg, C. Urinary Tract Infection Molecular Mechanisms and Clinical Translation. Pathogens 2016, 5, 24. https://doi.org/10.3390/pathogens5010024
Godaly G, Ambite I, Puthia M, Nadeem A, Ho J, Nagy K, Huang Y, Rydström G, Svanborg C. Urinary Tract Infection Molecular Mechanisms and Clinical Translation. Pathogens. 2016; 5(1):24. https://doi.org/10.3390/pathogens5010024
Chicago/Turabian StyleGodaly, Gabriela, Ines Ambite, Manoj Puthia, Aftab Nadeem, James Ho, Karoly Nagy, Yujing Huang, Gustav Rydström, and Catharina Svanborg. 2016. "Urinary Tract Infection Molecular Mechanisms and Clinical Translation" Pathogens 5, no. 1: 24. https://doi.org/10.3390/pathogens5010024
APA StyleGodaly, G., Ambite, I., Puthia, M., Nadeem, A., Ho, J., Nagy, K., Huang, Y., Rydström, G., & Svanborg, C. (2016). Urinary Tract Infection Molecular Mechanisms and Clinical Translation. Pathogens, 5(1), 24. https://doi.org/10.3390/pathogens5010024







