Is Taenia crassiceps Cysticercosis a Threat to Dogs? Description of Macro- and Microscopic Lesions in a Dog Case Report and a Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Necropsy and Sampling
2.2. Cytology
2.3. Histopathology
2.4. Parasite Identification
3. Results
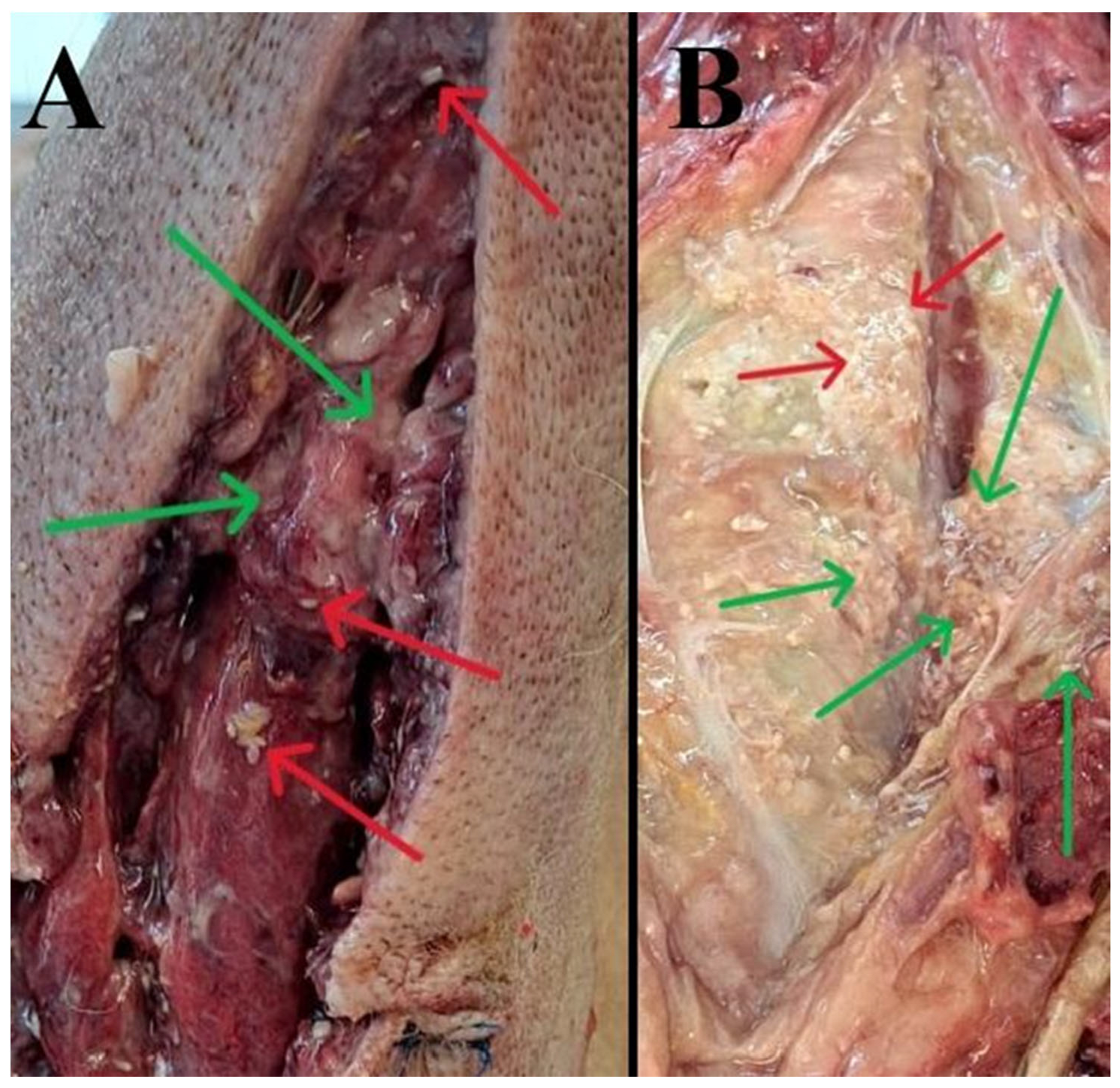
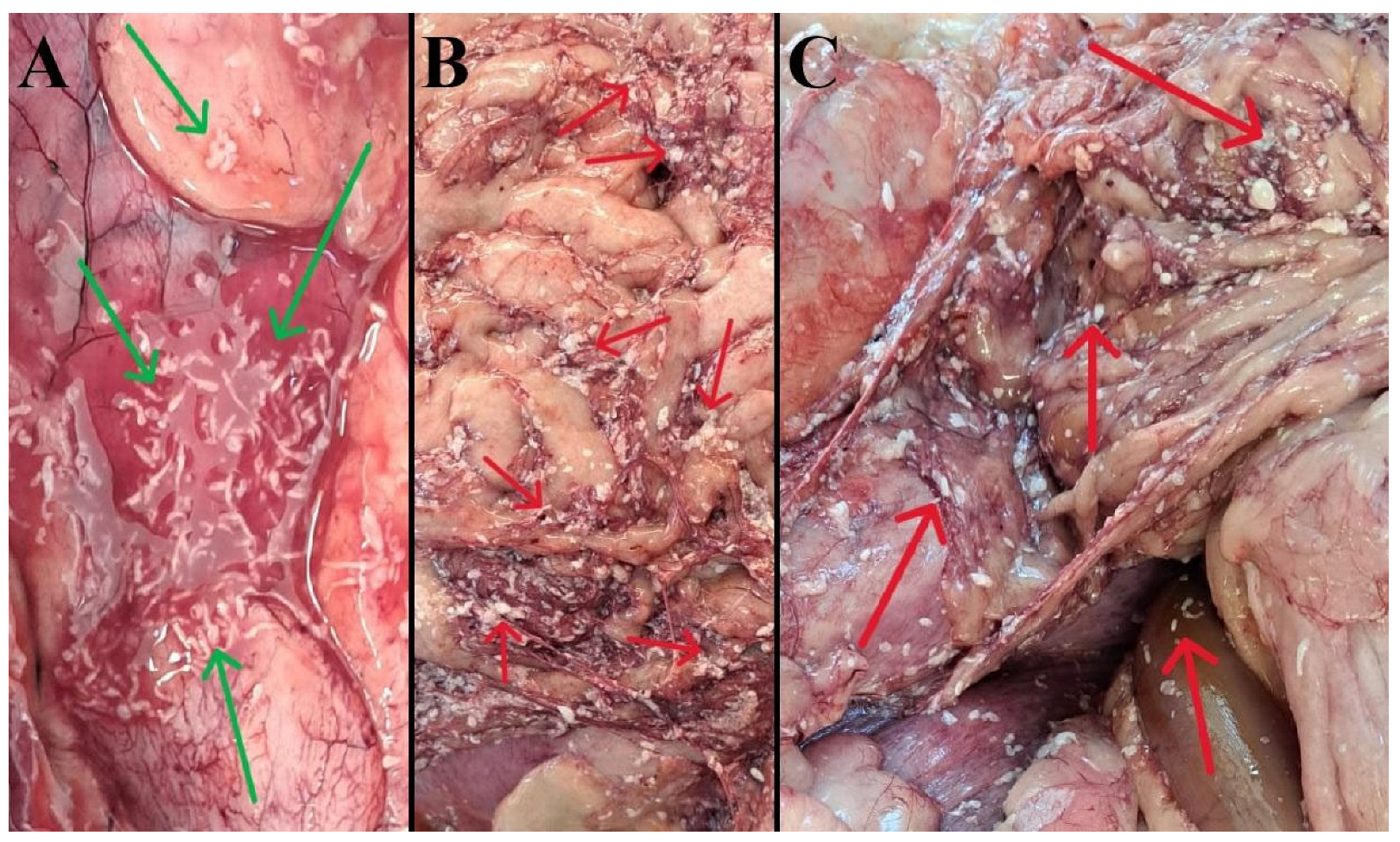
3.1. Gross (Necropsy) Findings
3.2. Cytological Findings
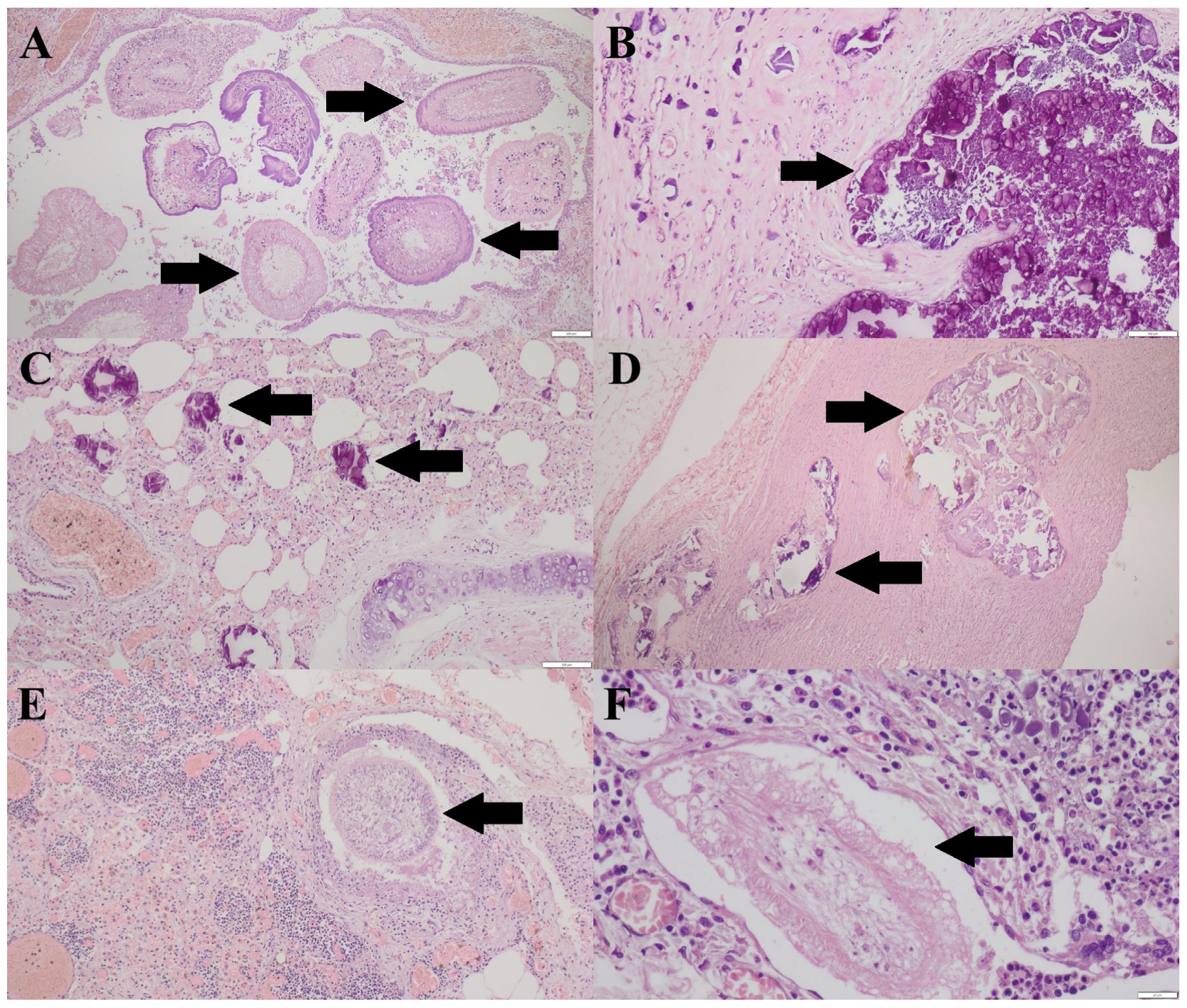
3.3. Histopathological Findings
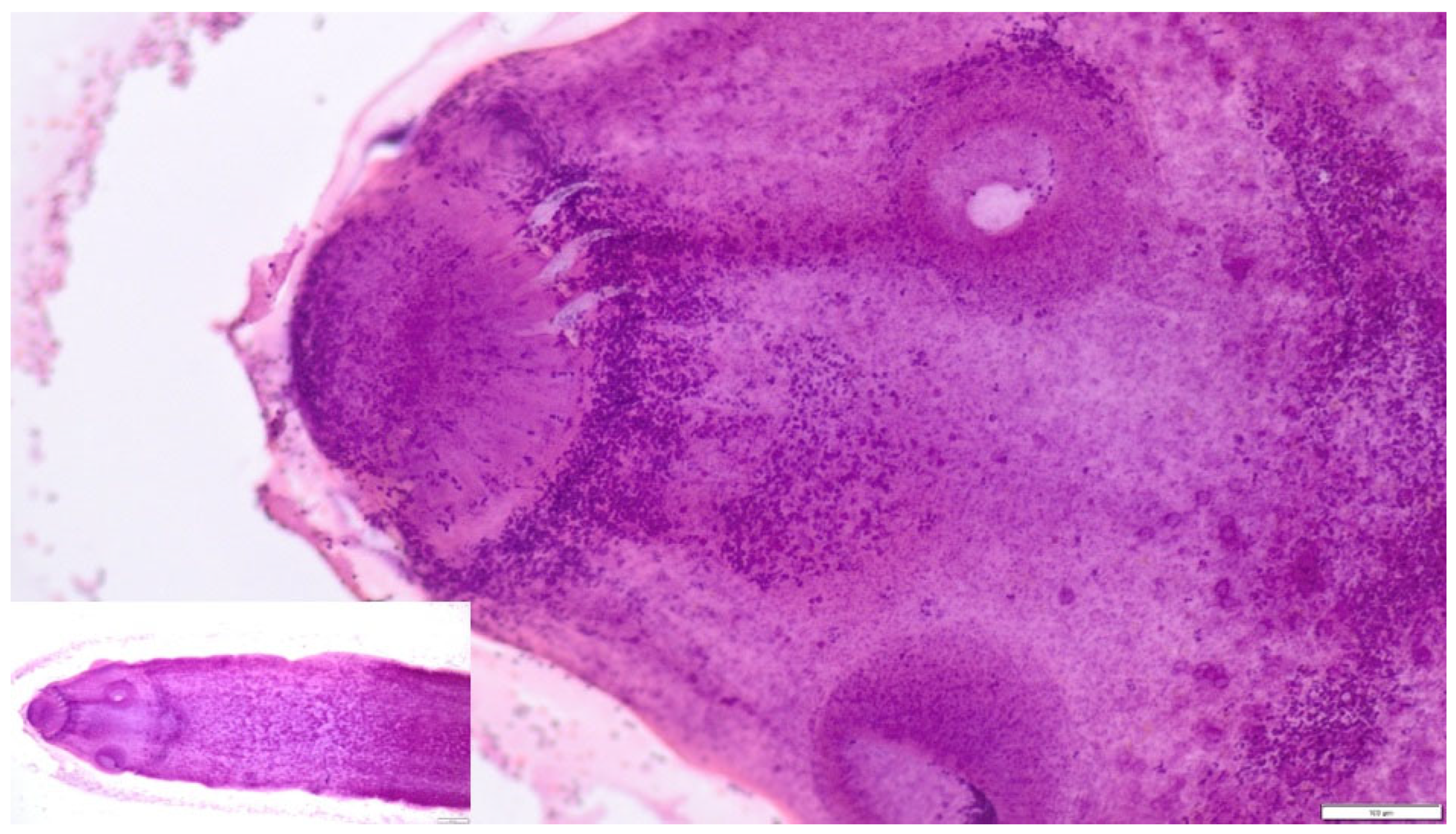
3.4. Parasitological Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCR | polymerase chain reaction |
| HE | hematoxylin and eosin |
| HIV | human immunodeficiency virus |
References
- Lescano, A.G.; Zunt, J. Other Cestodes. Sparganosis, Coenurosis and Taenia crassiceps Cysticercosis. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; pp. 335–345. [Google Scholar]
- Zhang, Y.; Abdu, A.; Wu, T.; Forzán, M.J.; Hammer, K.; Lejeune, M. Taenia crassiceps Cysticercosis in a Wild Muskrat and a Domestic Dog in the Northeastern United States. Pathogens 2023, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Rommel, M.; Eckert, J.; Kutzer, E.; Körting, W.; Schnieder, T. Veterinärmedizinische Parasitologie, 5th ed.; Parey Buchverlag: Berlin, Germany, 2000. [Google Scholar]
- Bowman, D.D. Georgis’ Parasitology for Veterinarians, 11th ed.; Philippa Saunders: Philadelphia, PA, USA, 2020; pp. 414–415. [Google Scholar]
- Bružinskaitė-Schmidhalter, R.; Šarkūnas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.V.; Obber, F.; Trevisiol, K.; Dellamaria, D.; Celva, R.; Bregoli, M.; Ormelli, S.; Sgubin, S.; Bonato, P.; Da Rold, G.; et al. Echinococcus multilocularis and other cestodes in red foxes (Vulpes vulpes) of northeast Italy, 2012–2018. Parasites Vectors 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Okulewicz, A.; Hildebrand, J.; Okulewicz, J.; Perec, A. Red fox (Vulpes vulpes) as a reservoir of parasites and source of zoonosis. Wiad. Parazytol. 2005, 51, 125–132. [Google Scholar]
- Sanchis-Monsonís, G.; Fanelli, A.; Martínez-Carrasco, C.; Tizzani, P. The typical cestodes of the red fox in eastern areas of the Iberian Peninsula have a grouped distribution. Vet. Parasitol. 2020, 283, 109168. [Google Scholar] [CrossRef]
- Schneider, A.; Moré, G.; Pewsner, M.; Frey, C.F.; Basso, W. Cestodes in Eurasian wolves (Canis lupus lupus) and domestic dogs (Canis lupus familiaris) in Switzerland. Int. J. Parasitol.-Parasit. Wildl. 2025, 26, 101027. [Google Scholar] [CrossRef]
- Delling, C.; Böttcher, D.; Schiffbauer, V.; Bernhard, A.; Schmäschke, R. First report of pulmonary cysticercosis caused by Taenia crassiceps in a Cape fur seal (Arctocephalus pusillus). Int. J. Parasitol.-Parasit. Wildl. 2019, 15, 83–86. [Google Scholar] [CrossRef]
- Ballweber, L.R. Taenia crassiceps subcutaneous cysticercosis in an adult dog. Vet. Rec. 2009, 165, 693–694. [Google Scholar] [CrossRef]
- Murphy, C.; Kursh, L.; Nolan, T.; Perry, J. Subcutaneous Taenia crassiceps Cysticercosis Mass Excision from an 11-Year-Old Mixed-Breed Dog. J. Am. Anim. Hosp. Assoc. 2021, 9, 57. [Google Scholar] [CrossRef]
- Nolte, A.; Strube, C.; Raue, K.; Brämer, C.; Baumgärtner, W.; Wohlsein, P. Subkutane Taenia-crassiceps-Zystizerkose Bei Einem Hund Mit Cushing-Syndrom. [Subcutaneous Taenia crassiceps cysticercosis in a dog with Cushing’s syndrome]. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2016, 44, 53–58. [Google Scholar] [CrossRef]
- Umhang, G.; Comte, S.; Raton, V.; Hormaz, V.; Boucher, J.M.; Favier, S.; Combes, B.; Boue, F. Echinococcus multilocularis infections in dogs from urban and peri-urban areas in France. Parasitol. Res. 2014, 113, 2219–2222. [Google Scholar] [CrossRef]
- Samorek-Pieróg, M.; Karamon, J.; Brzana, A.; Sobieraj, L.; Włodarczyk, M.; Sroka, J.; Bełcik, A.; Korpysa-Dzirba, W.; Cencek, T. Molecular Confirmation of Taenia crassiceps Cysticercosis in a Captive Ring-Tailed Lemur (Lemur catta) in Poland. Pathogens 2022, 11, 835. [Google Scholar] [CrossRef]
- Karamon, J.; Sroka, J.; Dąbrowska, J.; Bilska-Zając, E.; Zdybel, J.; Kochanowski, M.; Różycki, M.; Cencek, T. First report of Echinococcus multilocularis in cats in Poland: A monitoring study in cats and dogs from a rural area and animal shelter in a highly endemic region. Parasites Vectors 2019, 12, 313. [Google Scholar] [CrossRef]
- Jańczak, D.; Kędziorek, J.; Ściubisz, K.; Tomasz, K.; Taborska, J.; Kowalczyk, K.; Lewicki, M.; Szaluś-Jordanow, O. First report of subcutaneous Taenia crassiceps cysticercosis in a dog in Poland. Ann. Agric. Environ. Med. 2025. [Google Scholar] [CrossRef]
- Flammer-Anikpeh, Y.; Grimm, F.; Lindenblatt, N.; Zinkernagel, A. It isn’t always caviar. BMJ Case. Rep. 2014. [Google Scholar] [CrossRef]
- Floß, N.; Dolff, S.; Junker, A.; Blau, T.; Rauchenbach, L.; Sure, U.; Witzke, O.; Tappe, D.; Schönfeld, A. Cerebral Taenia crassiceps larvae infection in a 71-year-old immunocompetent male. Infection 2023, 51, 277–281. [Google Scholar] [CrossRef]
- Wiesner, M.; Glawischnig, W.; Lutzmann, I.; Grimm, F.; Blutke, A. Autochthonous Taenia crassiceps infection in a ring-tailed lemur (Lemur catta) in the Salzburg Zoo. Wien. Tierarztl. Monatsschr. 2019, 5–6, 109–115. [Google Scholar]
- Grbavac, L.; Šiki’c, A.; Kosteši’c, P.; Šoštari’c-Zuckermann, I.-C.; Moicec Perko, V.; Boras, J.; Bata, I.; Musulin, A.; Kostanjšak, T.; Živicnjak, T. Comprehensive Diagnosis, Treatment, and Outcome of Taenia crassiceps Cysticercosis in a Ring-Tailed Lemur (Lemur catta) from a Croatian Zoo: No Longer Unusual? Pathogens 2024, 13, 283. [Google Scholar] [CrossRef]
- Luzón, M.; de la Fuente-López, C.; Martínez-Nevado, E.; Fernández-Morán, J.; Ponce-Gordo, F. Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta). J. Zoo. Wildl. Med. 2010, 41, 327–330. [Google Scholar] [CrossRef]
- Cuccato, M.; Rubiola, S.; Rossi, L.; Piga, S.; Scaglione, F.E. Case-report: Massive infection by Cysticercus longicollis in a captive Lemur catta from Italy. Front. Vet. Sci. 2023, 10, 1288451. [Google Scholar] [CrossRef] [PubMed]
- Simin, S.; Vračar, V.; Kozoderović, G.; Stevanov, S.; Alić, A.; Lalošević, D.; Lalošević, V. Subcutaneous Taenia crassiceps Cysticercosis in a Ring-Tailed Lemur (Lemur catta) in a Serbian Zoo. Acta Parasit. 2023, 68, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Alić, A.; Hodžić, A.; Škapur, V.; Alić, A.Š.; Prašović, S.; Duscher, G.G. Fatal pulmonary cysticercosis caused by Cysticercus longicollis in a captive ring-tailed lemur (Lemur catta). Vet. Parasitol. 2017, 241, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ježková, J.; Zikmundová, V.; Příhodová, P.; Kostka, F.; Sak, B.; Kváč, M. Massive, disseminated and lethal cysticercosis caused by Taenia crassiceps (Cestoda) in captive western grey bamboo lemur (Hapalemur occidentalis Rumpler) in Zoo in the Czech Republic. Folia Parasitol. 2025, 72, 026. [Google Scholar] [CrossRef]
- Hiepe, T.; Lucius, R.; Gottstein, B. Allgemeine Parasitologie mit den Grundzügen der Immunologie, Diagnostik und Bekämpfung; Parey: Stuttgart, Germany, 2006. [Google Scholar]
- Ntoukas, V.; Tappe, D.; Pfütze, D.; Simon, M.; Holzmann, T. Cerebellar Cysticercosis Caused by Larval Taenia crassiceps Tapeworm in Immunocompetent Woman, Germany. Emerg. Infect. Dis. 2013, 19, 2008–2011. [Google Scholar] [CrossRef]
- Chermette, R.; Bussieras, J.; Mialot, M.; Raynal, P.C. Subcutaneous Taenia crassiceps cysticercosis in a dog. J. Am. Vet. Med. Assoc. 1993, 203, 263–265. [Google Scholar] [CrossRef]
- Hofmannova, L.; Mikes, L.; Jedlickova, L.; Pokorny, J.; Svobodova, V. Unusual cases of Taenia crassipceps in naturally infected animals in the Czech Republic. Vet. Med.-Czech. 2018, 63, 73–80. [Google Scholar] [CrossRef]
- Dzimira, S.; Prządka, P. Cytological diagnostics of cases of subcutaneous dirofilariosis imitating proliferative lesions in dogs. Vet. Med.-Czech. 2020, 65, 537–542. [Google Scholar] [CrossRef]
- Kamal, M.M.; Grover, S.V. Cytomorphology of subcutaneous cysticercosis. A report of 10 cases. Acta Cytol. 1995, 39, 190. [Google Scholar]
- Rao, R.N.; Krishnani, N.; Malhotra, K.; Suresh, B.; Meghrotra, R. Dilemmas in cytodiagnosis of subcutaneous wellings: Mimics and look-alikes of cysticercosis. J. Clin. Pathol. 2010, 63, 926–929. [Google Scholar] [CrossRef]
- Ratnika; Adil, M.; Amin, S.S.; Kulhari, M. Disseminated Cysticercosis Masquerading as Mucocutaneous Nodules. Indian Dermatol. Online J. 2022, 5, 629–632. [Google Scholar] [CrossRef] [PubMed]
- da Silva Santana, R.C.; Prudente, T.P.; de Sousa Guerra, C.H.; de Lima, N.F.; de Souza Lino Junior, R.; Vinaud, M.C. Albendazole–ivermectin combination decreases inflammation in experimental neurocysticercosis. Exp. Parasitol. 2023, 251, 108568. [Google Scholar] [CrossRef]
- Jansen, F.; Dorny, P.; Gabriël, S.; Dermauw, V.; Vang Johansen, M.; Trevisan, C. The survival and dispersal of Taenia eggs in the environment: What are the implications for transmission? A systematic review. Parasites Vectors 2021, 14, 88. [Google Scholar] [CrossRef]
- Sitali, M.C.; Schmidt, V.; Mwenda, R.; Sikasunge, C.S.; Mwape, K.E.; Simuunza, M.C.; da Costa, C.P.; Winkler, A.S.; Phir, I.K. Experimental animal models and their use in understanding cysticercosis: A systematic review. PLoS ONE 2022, 17, e0271232. [Google Scholar] [CrossRef]
- Willms, K.; Zurabian, R. Taenia crassiceps: In Vivo and in vitro models. Parasitology 2010, 137, 335–346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kandefer-Gola, M.; Żebrowski, K.; Ciaputa, R.; Demkowska-Kutrzepa, M.; Dzimira, S. Is Taenia crassiceps Cysticercosis a Threat to Dogs? Description of Macro- and Microscopic Lesions in a Dog Case Report and a Review of the Literature. Pathogens 2026, 15, 25. https://doi.org/10.3390/pathogens15010025
Kandefer-Gola M, Żebrowski K, Ciaputa R, Demkowska-Kutrzepa M, Dzimira S. Is Taenia crassiceps Cysticercosis a Threat to Dogs? Description of Macro- and Microscopic Lesions in a Dog Case Report and a Review of the Literature. Pathogens. 2026; 15(1):25. https://doi.org/10.3390/pathogens15010025
Chicago/Turabian StyleKandefer-Gola, Małgorzata, Kacper Żebrowski, Rafał Ciaputa, Marta Demkowska-Kutrzepa, and Stanisław Dzimira. 2026. "Is Taenia crassiceps Cysticercosis a Threat to Dogs? Description of Macro- and Microscopic Lesions in a Dog Case Report and a Review of the Literature" Pathogens 15, no. 1: 25. https://doi.org/10.3390/pathogens15010025
APA StyleKandefer-Gola, M., Żebrowski, K., Ciaputa, R., Demkowska-Kutrzepa, M., & Dzimira, S. (2026). Is Taenia crassiceps Cysticercosis a Threat to Dogs? Description of Macro- and Microscopic Lesions in a Dog Case Report and a Review of the Literature. Pathogens, 15(1), 25. https://doi.org/10.3390/pathogens15010025








