Long-Term Breastfeeding: Protective Effects Against Triple-Negative Breast Cancer and the Role of the Breast Microbiota
Abstract
1. Introduction
2. Epidemiological Evidence Linking Breastfeeding and TNBC Risk
3. Breast Microbiota: Composition and Function in Health
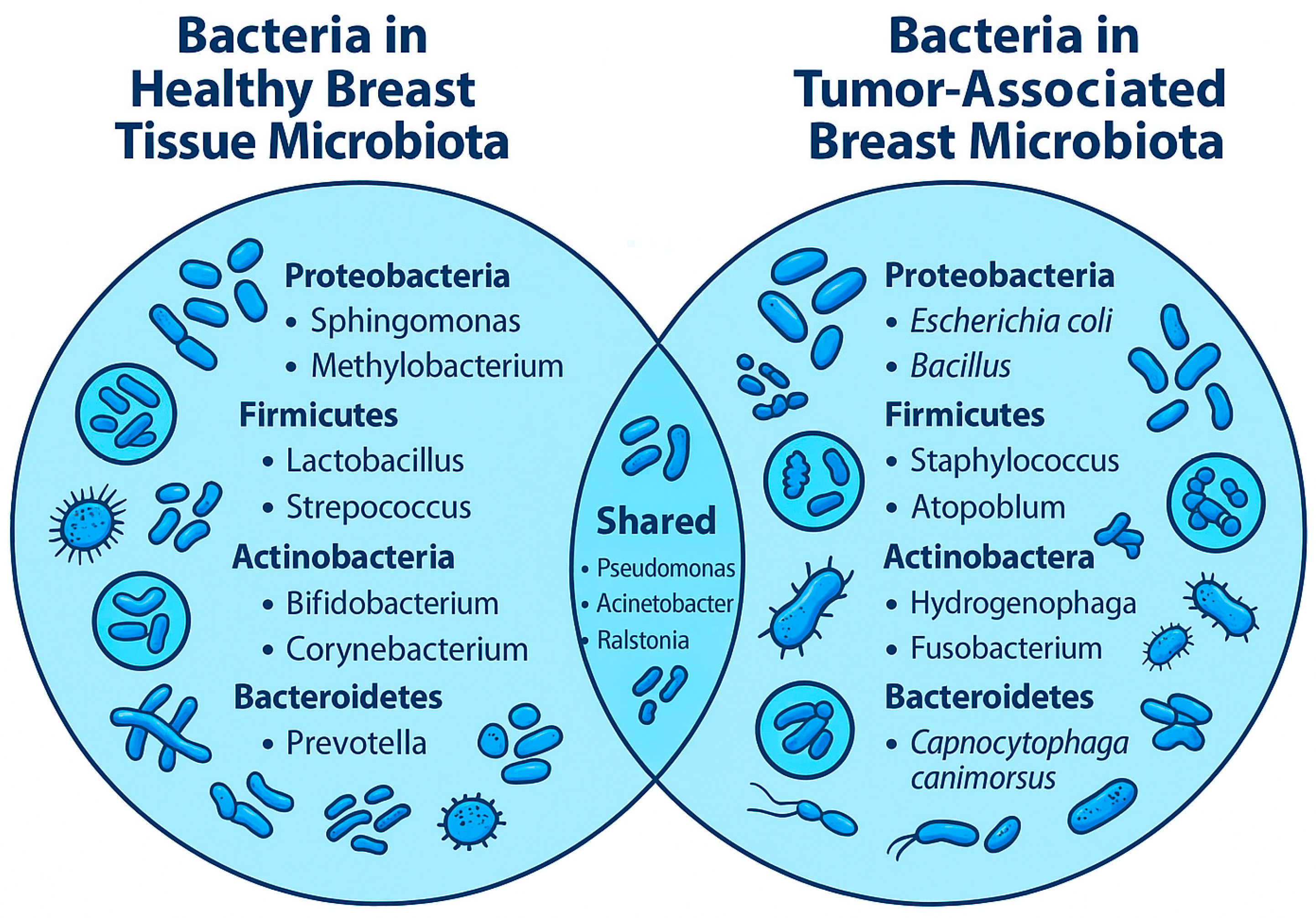
3.1. Composition of Breast Microbiota
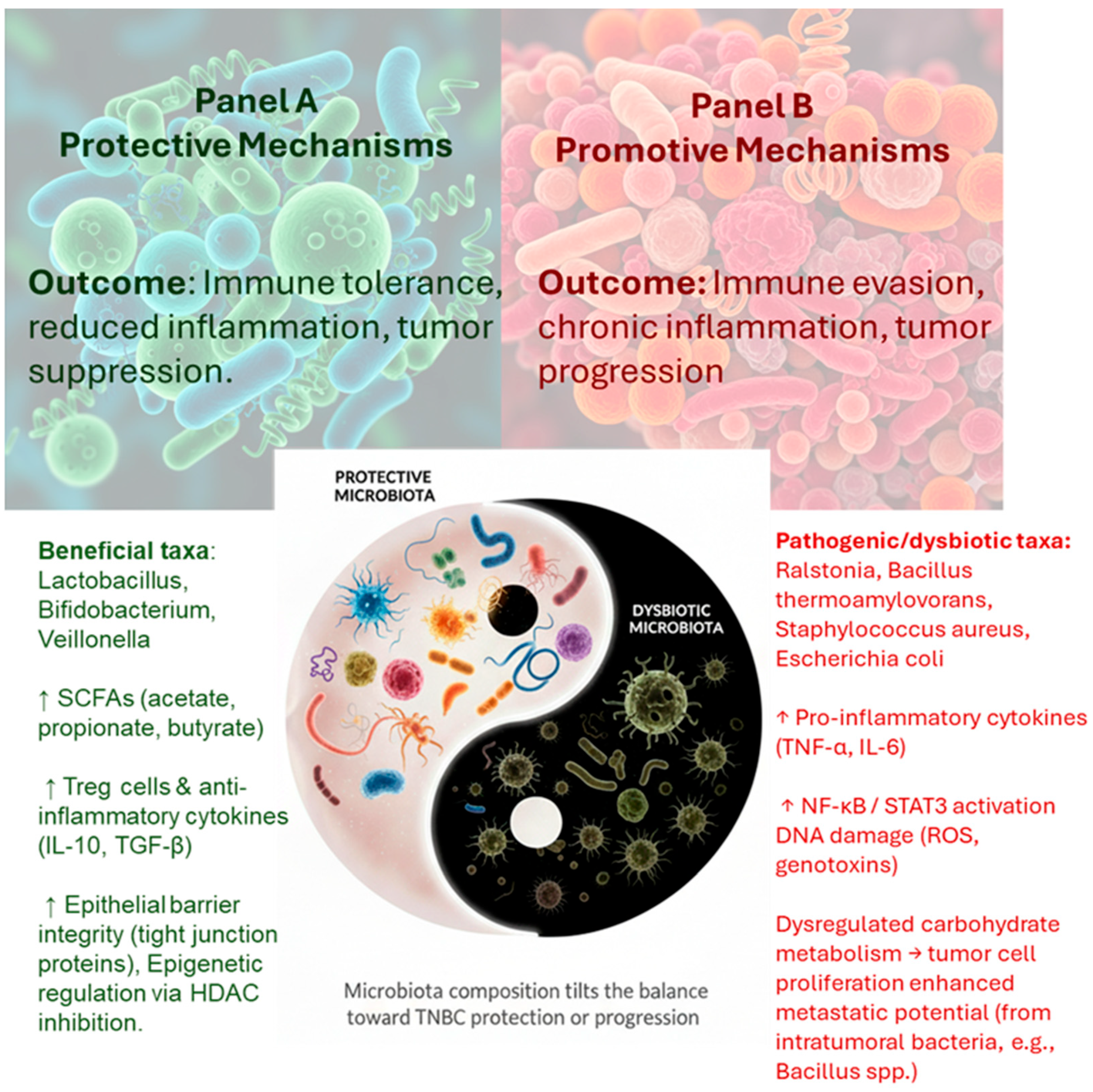
3.2. Functions of the Breast Microbiota
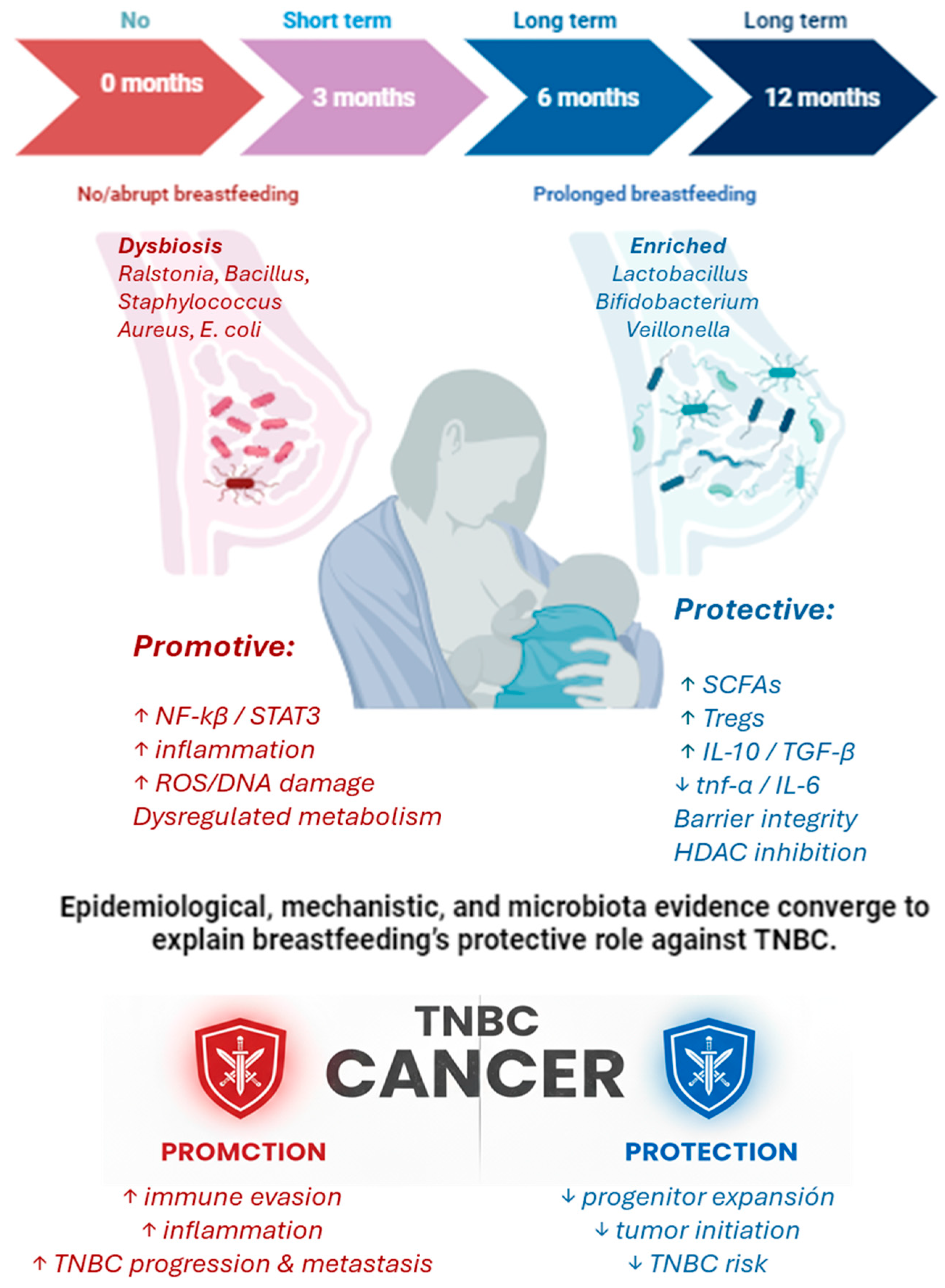
4. Long-Term Breastfeeding and Breast Cancer Risk Reduction
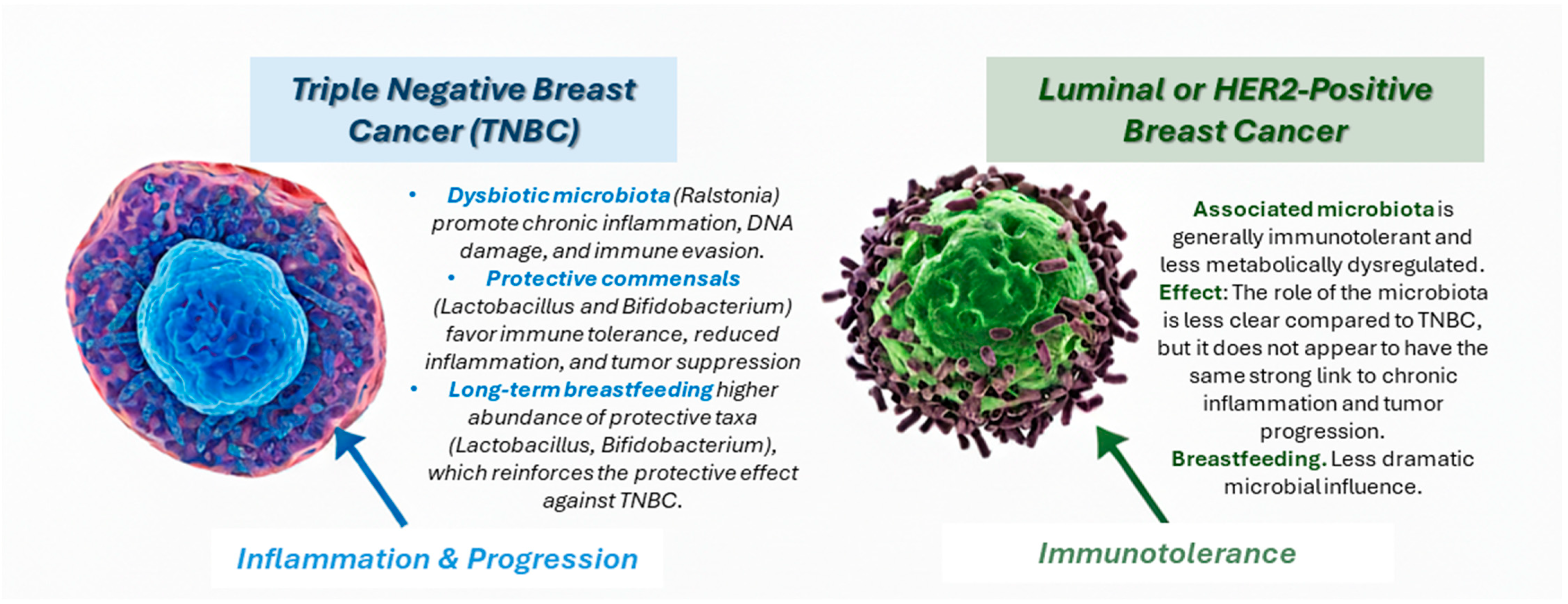
5. Integrative Mechanisms Linking Breastfeeding, Microbiota, and TNBC Protection
6. Therapeutic and Preventive Implications
7. Future Perspectives and Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, T.C.; Keene, S.D.; Patel, R.M. Early feeding factors associated with exclusive versus partial human milk feeding in neonates receiving intensive care. J. Perinatol. 2014, 34, 606–610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Modak, A.; Ronghe, V.; Gomase, K.P. The Psychological Benefits of Breastfeeding: Fostering Maternal Well-Being and Child Development. Cureus 2023, 15, e46730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, D.P.; Du, C.; Zhang, Z.M.; Li, G.X.; Yu, Z.F.; Wang, X.; Li, P.F.; Cheng, C.; Liu, Y.P.; Zhao, Y.S. Breastfeeding and ovarian cancer risk: A systematic review and meta-analysis of 40 epidemiological studies. Asian Pac. J. Cancer Prev. 2014, 15, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Stewart, C.J. Role of breastfeeding in disease prevention. Microb. Biotechnol. 2024, 17, e14520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tschiderer, L.; Seekircher, L.; Kunutsor, S.K.; Peters, S.A.E.; O’Keeffe, L.M.; Willeit, P. Breastfeeding Is Associated with a Reduced Maternal Cardiovascular Risk: Systematic Review and Meta-Analysis Involving Data from 8 Studies and 1,192,700 Parous Women. J. Am. Heart Assoc. 2022, 11, e022746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chehayeb, R.J.; Odzer, N.; Albany, R.A.; Ferrucci, L.; Sarpong, D.; Perez-Escamilla, R.; Lewis, J.B.; Phipps, A.I.; Meisner, A.; Pusztai, L. Breastfeeding attributable fraction of triple negative breast cancer in the US. npj Breast Cancer 2025, 11, 40. [Google Scholar] [CrossRef]
- Mao, X.; Omeogu, C.; Karanth, S.; Joshi, A.; Meernik, C.; Wilson, L.; Clark, A.; Deveaux, A.; He, C.; Johnson, T.; et al. Association of reproductive risk factors and breast cancer molecular subtypes: A systematic review and meta-analysis. BMC Cancer 2023, 23, 644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dietze, E.C.; Sistrunk, C.; Miranda-Carboni, G.; O’Regan, R.; Seewaldt, V.L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 2015, 15, 248–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Xue, Y.; Lu, H.; Bai, J.; Cui, L.; Ning, Y.; Yuan, Q.; Jia, X.; Wang, S. Relationship between infant gastrointestinal microorganisms and maternal microbiome within 6 months of delivery. Microbiol. Spectr. 2024, 12, e0360823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Ning, H.; Li, Y.; Li, Y.; Ma, J. The microbiota in breast cancer: Dysbiosis, microbial metabolites, and therapeutic implications. Am. J. Cancer Res. 2025, 15, 1384–1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devoy, C.; Flores Bueso, Y.; Tangney, M. Understanding and harnessing triple-negative breast cancer-related microbiota in oncology. Front. Oncol. 2022, 12, 1020121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Ren, L.; Wang, Y.; Li, J.; Zhou, Q.; Peng, C.; Li, Y.; Cheng, R.; He, F.; Shen, X. The Effect of Breast Milk Microbiota on the Composition of Infant Gut Microbiota: A Cohort Study. Nutrients 2022, 14, 5397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alemu, B.K.; Azeze, G.G.; Wu, L.; Lau, S.L.; Wang, C.C.; Wang, Y. Effects of maternal probiotic supplementation on breast milk microbiome and infant gut microbiome and health: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. MFM 2023, 5, 101148. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef] [PubMed]
- Dombrowska-Pali, A.; Wiktorczyk-Kapischke, N.; Chrustek, A.; Olszewska-Słonina, D.; Gospodarek-Komkowska, E.; Socha, M.W. Human Milk Microbiome—A Review of Scientific Reports. Nutrients 2024, 16, 1420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basree, M.M.; Shinde, N.; Koivisto, C.; Cuitino, M.; Kladney, R.; Zhang, J.; Stephens, J.; Palettas, M.; Zhang, A.; Kim, H.K.; et al. Abrupt involution induces inflammation, estrogenic signaling, and hyperplasia linking lack of breastfeeding with increased risk of breast cancer. Breast Cancer Res. 2019, 21, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, H.; Ursin, G.; Xu, X.; Lee, E.; Togawa, K.; Duan, L.; Lu, Y.; Malone, K.E.; Marchbanks, P.A.; McDonald, J.A.; et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: A pooled analysis. Breast Cancer Res. 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ehsan, S.; Banerjee, S.; Fernandez Perez, C.; Lhuilier, I.; Neuner, J.; Friebel-Klingner, T.; Fayanju, O.M.; Nair, B.; Niinuma, S.A.; et al. The unique risk factor profile of triple-negative breast cancer: A comprehensive meta-analysis. J. Natl. Cancer Inst. 2024, 116, 1210–1219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmer, J.R.; Boggs, D.A.; Wise, L.A.; Ambrosone, C.B.; Adams-Campbell, L.L.; Rosenberg, L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1883–1891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogony, J.W.; Radisky, D.C.; Ruddy, K.J.; Goodison, S.; Wickland, D.P.; Egan, K.M.; Knutson, K.L.; Asmann, Y.W.; Sherman, M.E. Immune Responses and Risk of Triple-negative Breast Cancer: Implications for Higher Rates among African American Women. Cancer Prev. Res. 2020, 13, 901–910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmer, J.R.; Viscidi, E.; Troester, M.A.; Hong, C.C.; Schedin, P.; Bethea, T.N.; Bandera, E.V.; Borges, V.; McKinnon, C.; Haiman, C.A.; et al. Parity, lactation, and breast cancer subtypes in African American women: Results from the AMBER Consortium. J. Natl. Cancer Inst. 2014, 106, dju237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- John, E.M.; Hines, L.M.; Phipps, A.I.; Koo, J.; Longacre, T.A.; Ingles, S.A.; Baumgartner, K.B.; Slattery, M.L.; Wu, A.H. Reproductive history, breast-feeding and risk of triple negative breast cancer: The Breast Cancer Etiology in Minorities (BEM) study. Int. J. Cancer 2018, 142, 2273–2285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Millikan, R.C.; Newman, B.; Tse, C.K.; Moorman, P.G.; Conway, K.; Dressler, L.G.; Smith, L.V.; Labbok, M.H.; Geradts, J.; Bensen, J.T.; et al. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 2008, 109, 123–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islami, F.; Liu, Y.; Jemal, A.; Zhou, J.; Weiderpass, E.; Colditz, G.; Boffetta, P.; Weiss, M. Breastfeeding and breast cancer risk by receptor status—A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2398–2407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotsopoulos, J.; Lubinski, J.; Salmena, L.; Lynch, H.T.; Kim-Sing, C.; Foulkes, W.D.; Ghadirian, P.; Neuhausen, S.L.; Demsky, R.; Tung, N.; et al. Hereditary Breast Cancer Clinical Study Group. Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2012, 14, R42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.R.; O’Brien, J.; Borges, V.F.; Conklin, M.W.; Keely, P.J.; Eliceiri, K.W.; Marusyk, A.; Tan, A.C.; Schedin, P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat. Med. 2011, 17, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jordan, I.; Hebestreit, A.; Swai, B.; Krawinkel, M.B. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: A case-control study in Northern Tanzania. Breast Cancer Res. Treat. 2013, 142, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Stordal, B. Breastfeeding reduces the risk of breast cancer: A call for action in high-income countries with low rates of breastfeeding. Cancer Med. 2023, 12, 4616–4625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ElShamy, W.M. The protective effect of longer duration of breastfeeding against pregnancy-associated triple negative breast cancer. Oncotarget 2016, 7, 53941–53950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002, 360, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Reid, G. The potential influence of the microbiota and probiotics on women during long spaceflights. Womens Health 2016, 12, 193–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Togo, A.; Dufour, J.C.; Lagier, J.C.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of human breast and milk microbiota: A systematic review. Future Microbiol. 2019, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Ixta, J.M.; Juárez-Castelán, C.J.; Ramírez-Sánchez, D.; Lázaro-Pérez, N.D.S.; Castro-Arellano, J.J.; Romero-Maldonado, S.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Salgado-Mancilla, M.; Gómez-Cruz, C.Y.; et al. Post Natal Microbial and Metabolite Transmission: The Path from Mother to Infant. Nutrients 2024, 16, 1990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moossavi, S.; Azad, M.B. Origins of human milk microbiota: New evidence and arising questions. Gut Microbes 2020, 12, 1667722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGuire, M.K.; McGuire, M.A. Human milk: Mother nature’s prototypical probiotic food? Adv. Nutr. 2015, 6, 112–123. [Google Scholar] [CrossRef]
- Rad, S.K.; Yeo, K.K.L.; Wu, F.; Li, R.; Nourmohammadi, S.; Tomita, Y.; Price, T.J.; Ingman, W.V.; Townsend, A.R.; Smith, E. A Systematic Review and Meta-Analysis of 16S rRNA and Cancer Microbiome Atlas Datasets to Characterize Microbiota Signatures in Normal Breast, Mastitis, and Breast Cancer. Microorganisms 2025, 13, 467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, J.E.; Price, W.J.; Shafii, B.; Yahvah, K.M.; Bode, L.; McGuire, M.A.; McGuire, M.K. Relationships Among Microbial Communities, Maternal Cells, Oligosaccharides, and Macronutrients in Human Milk. J. Hum. Lact. 2017, 33, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, S.; Fu, S.; Zhou, X.; Min, Y.; Lang, J.; Chen, M. Intratumoral microbiota composition in women’s cancers: A systematic review and meta-analysis. Front. Oncol. 2025, 15, 1544786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Yang, J. Investigating the Anna Karenina principle of the breast microbiome. BMC Microbiol. 2025, 25, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerbec, Z.J.; Serapio-Palacios, A.; Metcalfe-Roach, A.; Krekhno, Z.; Bar-Yoseph, H.; Woodward, S.E.; Pena-Díaz, J.; Nemirovsky, O.; Awrey, S.; Moreno, S.H.; et al. Identification of intratumoral bacteria that enhance breast tumor metastasis. mBio 2025, 16, e0359524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M.; et al. Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019, 9, 11940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Actis, S.; Cazzaniga, M.; Bounous, V.E.; D’Alonzo, M.; Rosso, R.; Accomasso, F.; Minella, C.; Biglia, N. Emerging evidence on the role of breast microbiota on the development of breast cancer in high-risk patients. Carcinogenesis 2023, 44, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Hoskinson, C.; Zheng, K.; Gabel, J.; Kump, A.; German, R.; Podicheti, R.; Marino, N.; Stiemsma, L.T. Composition and Functional Potential of the Human Mammary Microbiota Prior to and Following Breast Tumor Diagnosis. mSystems 2022, 7, e0148921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated with Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Luo, G.; Jiang, F.; Jia, W.; Li, J.; Huang, T.; Zhang, X.; Mao, Y.; Su, S.; Han, W.; et al. Early life bifidobacterial mother-infant transmission: Greater contribution from the infant gut to human milk revealed by microbiomic and culture-based methods. mSystems 2025, 10, e0048025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ait Zenati, F.; Baldi, S.; Di Gloria, L.; Djoudi, F.; Bertorello, S.; Ramazzotti, M.; Niccolai, E.; Amedei, A. Compositional and Functional Disparities in the Breast Oncobiome Between Patients Living in Urban or Rural Areas. Genes 2025, 16, 806. [Google Scholar] [CrossRef] [PubMed]
- Samarra, A.; Alcañiz, A.J.; Martínez-Costa, C.; Marina, A.; Comas, I.; Segata, N.; Quijada, N.M.; Collado, M.C. Breastfeeding and early Bifidobacterium-driven microbial colonization shape the infant gut resistome. Nat. Commun. 2025, 16, 6099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues, M.F.; Carvalho, É.; Pezzuto, P.; Rumjanek, F.D.; Amoêdo, N.D. Reciprocal modulation of histone deacetylase inhibitors sodium butyrate and trichostatin A on the energy metabolism of breast cancer cells. J. Cell. Biochem. 2015, 116, 797–808. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C.; Zunszain, P.A.; Mann, G.E. Control of Redox Homeostasis by Short-Chain Fatty Acids: Implications for the Prevention and Treatment of Breast Cancer. Pathogens 2023, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Cao, X.; Gu, Q.; Kubi Amos-Abanyie, E.; Tolley, E.A.; Vidal, G.; Lyn-Cook, B.; Starlard-Davenport, A. Characterization of the Metabolome of Breast Tissues from Non-Hispanic Black and Non-Hispanic White Women Reveals Correlations between Microbial Dysbiosis and Enhanced Lipid Metabolism Pathways in Triple-Negative Breast Tumors. Cancers 2022, 14, 4075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Huang, S.; Yuan, Y. Butyrate inhibits the malignant biological behaviors of breast cancer cells by facilitating cuproptosis-associated gene expression. J. Cancer Res. Clin. Oncol. 2024, 150, 287. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, W.; Sun, D.; Zhao, X.; Huang, Z.; Liu, C.; Sheng, Y. Bacterial metabolites: Effects on the development of breast cancer and therapeutic efficacy (Review). Oncol. Lett. 2025, 29, 210. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Ujlaki, G.; Sári, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, S.; Chen, B.; Yang, J.; Wang, J.; Zhu, D.; Meng, Q.; Zhang, L. Study of Microbiomes in Aseptically Collected Samples of Human Breast Tissue Using Needle Biopsy and the Potential Role of in situ Tissue Microbiomes for Promoting Malignancy. Front. Oncol. 2018, 8, 318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Moreno de LeBlanc, A.; Matar, C.; Thériault, C.; Perdigón, G. Effects of milk fermented by Lactobacillus helveticus R389 on immune cells associated to mammary glands in normal and a breast cancer model. Immunobiology 2005, 210, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Soltan Dallal, M.M.; Hassan, Z.M.; Holakuyee, M.; Agha Amiri, S.; Abolhassani, M.; Mahdavi, M. Oral administration of Lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br. J. Nutr. 2010, 104, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Sribenja, S.; Maguire, O.; Attwood, K.; Buas, M.F.; Palmer, J.R.; Ambrosone, C.B.; Higgins, M.J. Deletion of Foxa1 in the mouse mammary gland results in abnormal accumulation of luminal progenitor cells: A link between reproductive factors and ER-/TNBC breast cancer? Am. J. Cancer Res. 2021, 11, 3263–3270. [Google Scholar] [PubMed] [PubMed Central]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, Y.; Lei, Y.; Wang, C.; Wang, J.; Wang, L.; Liu, L.; Liu, L.; Gao, X.; Li, Q. Epigenetic Regulation of miR-29s Affects the Lactation Activity of Dairy Cow Mammary Epithelial Cells. J. Cell Physiol. 2015, 230, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.M.; Al-Sharif, S.; Ala’ Bereshy, R.; Al-Eitan, S.F.; Banikhaled, S.H.; Al-Qudimat, A.R.; Al-Zoubi, R.M.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubinstein, M.M.; Brown, K.A.; Iyengar, N.M. Targeting obesity-related dysfunction in hormonally driven cancers. Br. J. Cancer 2021, 125, 495–509. [Google Scholar] [CrossRef]
- Mir, R.; Albarqi, S.A.; Albalawi, W.; Alatwi, H.E.; Alatawy, M.; Bedaiwi, R.I.; Almotairi, R.; Husain, E.; Zubair, M.; Alanazi, G.; et al. Emerging Role of Gut Microbiota in Breast Cancer Development and Its Implications in Treatment. Metabolites 2024, 14, 683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filippou, C.; Themistocleous, S.C.; Marangos, G.; Panayiotou, Y.; Fyrilla, M.; Kousparou, C.A.; Pana, Z.D.; Tsioutis, C.; Johnson, E.O.; Yiallouris, A. Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach. Int. J. Mol. Sci. 2024, 25, 1110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Han, W.; Feng, Y.; Wang, Y.; Sun, T.; Xu, J. Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review). Int. J. Oncol. 2024, 65, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohseni, A.H.; Taghinezhad-S, S.; Casolaro, V.; Lv, Z.; Li, D. Potential links between the microbiota and T cell immunity determine the tumor cell fate. Cell Death Dis. 2023, 14, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Sig. Transduct. Target Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Nutrition Intervention and Microbiome Modulation in the Management of Breast Cancer. Nutrients 2024, 16, 2644. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int. J. Cancer 2014, 135, 529–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Kisková, T.; Mikeš, J.; Demečková, V.; Orendáš, P.; Bojková, B.; Péč, M.; Kubatka, P.; et al. Preventive effects of probiotic bacteria Lactobacillus plantarum and dietary fiber in chemically-induced mammary carcinogenesis. Anticancer Res. 2014, 34, 4969–4975. [Google Scholar] [PubMed]
- Imani Fooladi, A.A.; Yazdi, M.H.; Pourmand, M.R.; Mirshafiey, A.; Hassan, Z.M.; Azizi, T.; Mahdavi, M.; Soltan Dallal, M.M. Th1 Cytokine Production Induced by Lactobacillus acidophilus in BALB/c Mice Bearing Transplanted Breast Tumor. Jundishapur. J. Microbiol. 2015, 8, e17354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Study to Investigate Efficacy of a Novel Probiotic on the Bacteriome and Mycobiome of Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT04362826 (accessed on 5 July 2025).
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ippolito, M.; Monge, T.; Violi, R.; Cappello, P.; Ferrocino, I.; Cocolin, L.S.; De Francesco, A.; Bo, S.; Finocchiaro, C. Gut microbiota composition after diet and probiotics in overweight breast cancer survivors: A randomized open-label pilot intervention trial. Nutrition 2020, 74, 110749. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B.; Uberseder, B.; Clear, K.Y.J.; Wilson, A.S.; Chiba, A.; Tooze, J.A.; Cook, K.L. Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep. 2018, 25, 47–56.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karin, M.; Jobin, C.; Balkwill, F. Chemotherapy, immunity and microbiota—A new triumvirate? Nat. Med. 2014, 20, 126–127. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Chen, Z.; Yu, X.; Luo, S.; Peng, X.; Li, X. Gut microbiota reshapes the TNBC immune microenvironment: Emerging immunotherapeutic strategies. Pharmacol. Res. 2025, 215, 107726. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) [Ref.] | Design/Population | Exposure Metric | Outcome/Subtype | Effect Size (95% CI) |
|---|---|---|---|---|
| Collaborative Group, Lancet (2002) [35] | Collaborative re-analysis, 47 studies (30 countries) | Per 12 months of breastfeeding (lifetime) | All breast cancer | RR↓ 4.3% (95% CI 2.9–5.8; p < 0.0001) per 12 months; ↓7% (5.0–9.0; p < 0.0001) per birth |
| Islami et al., Ann. Oncol. (2015) [28] | Systematic review & meta-analysis (27 studies; 36,881 cases) | Ever vs. never breastfeeding (parous women) | TNBC | OR 0.78 (0.66–0.91) |
| Ma, et al., Breast Cancer Res. (2017) [20] | Pooled analysis of 3 population-based case-control studies | ≥1 year vs. never (parous) | TNBC | OR 0.69 (0.50–0.96) |
| John, et al., BEM Study, Int. J. Cancer (2018) [26] | Multiethnic case-control (<50 y focus) | ≥24 mo vs. 0 mo (parous, <50 y) | TNBC | OR 0.52 (0.26–1.04) (borderline) |
| Kotsopoulos, et al., Cancer Res. (2012) [29] | Case-control, BRCA carriers | ≥1 y vs. <1 y; ≥2 y | BRCA1-associated BC | OR 0.68 (0.52–0.91); ≥2 y: OR 0.51 (0.35–0.74) |
| Chehayeb, et al., NPJ Breast Cancer (2025) [6] | Population-level modelling (US) | Attributable fraction estimate | TNBC | AF: ~15% (Black) & ~12% (White) potentially avoidable with BF support |
| AMBER Consortium (Palmer, et al., 2014, JNCI) [24] | Multi-study consortium of African-American women | Parity × breastfeeding (ever/never; by number of births) | ER- and TNBC | Parous vs. nulliparous: OR 1.33 (1.11–1.59) for ER-; among never-breastfed, ≥4 y vs. 1 birth OR 1.68 (1.15–2.44); breastfeeding attenuated the parity-associated ER- risk |
| Black Women’s Health Study (Palmer, et al., 2011, CEBP) [22] | Prospective cohort (AA women) | Parity; breastfeeding (ever/never) | ER-/PR- | 3+ vs. 0 births: HR 1.48 (0.98–1.84) for ER-/PR-; among women who had breastfed, high parity was no longer associated with increased ER-/PR- incidence |
| Carolina Breast Cancer Study (Millikan et al., 2008, BCRT) [27] | Population-based case-control | Never vs. ever breastfeeding; central adiposity (WHR) | Basal-like (≈TNBC) | Joint PAF ≈53% For Basal-Like from Never breastfeeding + elevated WHR; up to 68% preventable in younger AA women with breastfeeding promotion & adiposity reduction |
| Pregnancy-associated TNBC (ElShamy, 2016, Oncotarget) [34] | Narrative synthesis + experiment | Breastfeeding duration | Pregnancy-associated TNBC | Summarizes evidence that longer lactation lowers risk; cites does-response for overall BC (~4.3% lower RR per 12 months BF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Roca, J.; Climent, J. Long-Term Breastfeeding: Protective Effects Against Triple-Negative Breast Cancer and the Role of the Breast Microbiota. Pathogens 2025, 14, 946. https://doi.org/10.3390/pathogens14090946
Sierra-Roca J, Climent J. Long-Term Breastfeeding: Protective Effects Against Triple-Negative Breast Cancer and the Role of the Breast Microbiota. Pathogens. 2025; 14(9):946. https://doi.org/10.3390/pathogens14090946
Chicago/Turabian StyleSierra-Roca, Julia, and Joan Climent. 2025. "Long-Term Breastfeeding: Protective Effects Against Triple-Negative Breast Cancer and the Role of the Breast Microbiota" Pathogens 14, no. 9: 946. https://doi.org/10.3390/pathogens14090946
APA StyleSierra-Roca, J., & Climent, J. (2025). Long-Term Breastfeeding: Protective Effects Against Triple-Negative Breast Cancer and the Role of the Breast Microbiota. Pathogens, 14(9), 946. https://doi.org/10.3390/pathogens14090946







