H5N1 Clade 2.3.4.4b: Evolution, Global Spread, and Host Range Expansion
Abstract
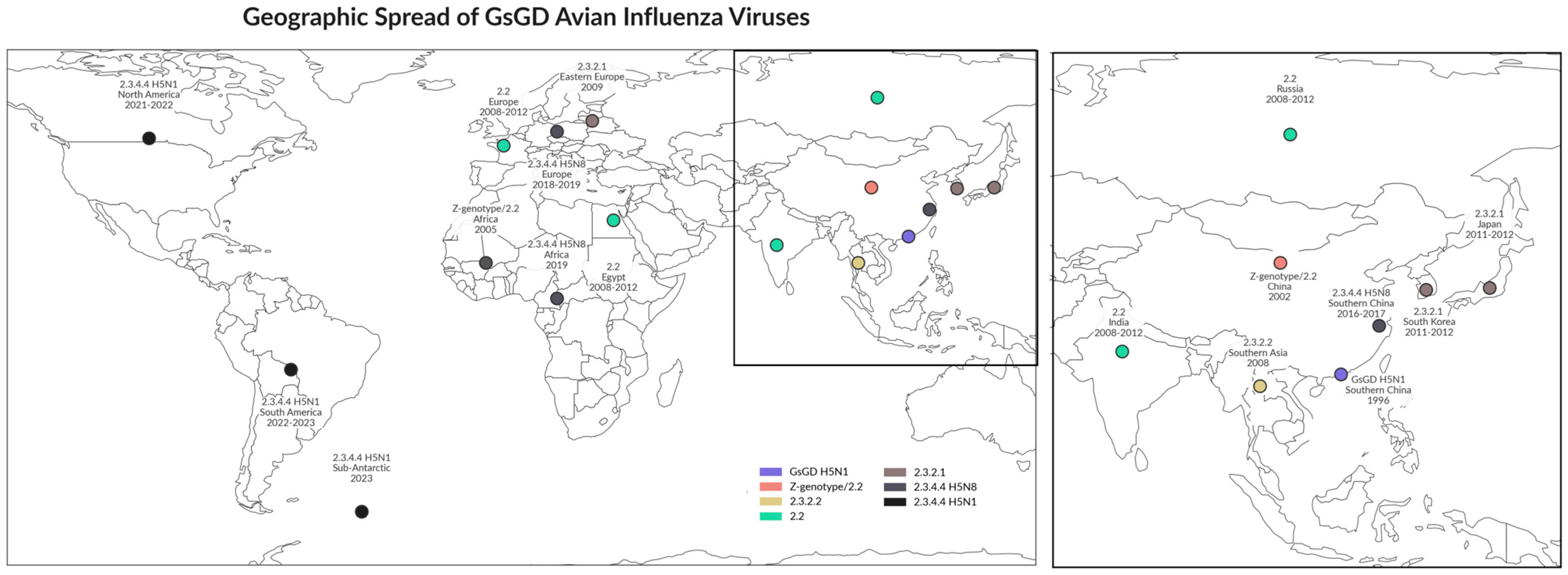
1. Emergence of 2.3.4.4b Clade Highly Pathogenic Avian Influenza Viruses (HPAIVs)
2. Global Spread of H5N1 HPAIV Clade 2.3.4.4b Since 2020
2.1. Europe
2.2. North and South America
2.3. Sub-Antarctic and Antarctic Region
2.4. Australia
3. H5N1 HPAIV Spillover to Mammals
3.1. Avian Influenza in Cattle and Small Ruminants
3.2. Avian Influenza in Seals and Fur Animals
3.3. Avian Influenza in Companion Animals
4. Human Cases of H5N1 Infection: Overview and Recent Developments
4.1. Cumulative Global Cases and Fatality
4.2. Recent Human Cases (2022–2025)
4.3. Paradigm Shift: Mammal-to-Human Transmission
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014, 22, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Subbarao, C.N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Chin, P.S.; Hoffmann, E.; Webby, R.; Webster, R.G.; Guan, Y.; Peiris, M.; Shortridge, K.F. Molecular evolution of H6 influenza viruses from poultry in Southeastern China: Prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)-like genes in poultry. J. Virol. 2002, 76, 507–516. [Google Scholar] [CrossRef]
- Cauthen, A.N.; Swayne, D.E.; Schultz-Cherry, S.; Perdue, M.L.; Suarez, D.L. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 2000, 74, 6592–6599. [Google Scholar] [CrossRef]
- Guan, Y.; Peiris, J.S.; Lipatov, A.S.; Ellis, T.M.; Dyrting, K.C.; Krauss, S.; Zhang, L.J.; Webster, R.G.; Shortridge, K.F. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 2002, 99, 8950–8955. [Google Scholar] [CrossRef]
- Guan, Y.; Peiris, M.; Kong, K.F.; Dyrting, K.C.; Ellis, T.M.; Sit, T.; Zhang, L.J.; Shortridge, K.F. H5N1 influenza viruses isolated from geese in Southeastern China: Evidence for genetic reassortment and interspecies transmission to ducks. Virology 2002, 292, 16–23. [Google Scholar] [CrossRef]
- Webster, R.G.; Guan, Y.; Peiris, M.; Walker, D.; Krauss, S.; Zhou, N.N.; Govorkova, E.A.; Ellis, T.M.; Dyrting, K.C.; Sit, T.; et al. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 2002, 76, 118–126. [Google Scholar] [CrossRef]
- Li, K.S.; Guan, Y.; Wang, J.; Smith, G.J.; Xu, K.M.; Duan, L.; Rahardjo, A.P.; Puthavathana, P.; Buranathai, C.; Nguyen, T.D.; et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 2004, 430, 209–213. [Google Scholar] [CrossRef]
- Chotpitayasunondh, T.; Lochindarat, S.; Srisan, P.; Chokepaibulkit, K.; Weerakul, J.; Maneerattanaporn, M.; Sawanpanyalert, P. Cases of influenza A (H5N1)—Thailand, 2004. Morb. Mortal. Wkly. Rep. 2004, 53, 100–102. [Google Scholar]
- Tran, T.H.; Nguyen, T.L.; Nguyen, T.D.; Luong, T.S.; Pham, P.M.; Nguyen, V.V.; Pham, T.S.; Vo, C.D.; Le, T.Q.M.; Hgo, T.T.; et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 2004, 350, 1179–1188. [Google Scholar] [CrossRef]
- WHO/OIE/FAO. H5N1 Evolution Working Group. Continued evolution of highly pathogenic avian influenza A (H5N1): Updated nomenclature. Influenza Other Respir. Viruses 2012, 6, 1–5. [Google Scholar] [CrossRef]
- WHO/OIE/FAO. H5N1 Evolution Working Group. Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, e1. [Google Scholar] [CrossRef]
- Saad, M.D.; Ahmed, L.S.; Gamal-Eldein, M.A.; Fouda, M.K.; Khalil, F.; Yingst, S.L.; Parker, M.A.; Montevillel, M.R. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg. Infect. Dis. 2007, 13, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, M.F.; Olinger, C.M.; Owoade, A.A.; de Landtsheer, S.; Ammerlaan, W.; Niesters, H.G.M.; Osterhaus, A.D.; Fouchier, R.A.M.; Muller, C.P. Multiple introductions of H5N1 in Nigeria—Phylogenetic analysis reveals that this deadly virus first arrived in Africa from different sources. Nature 2006, 442, 37. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Olinger, C.M.; Owoade, A.A.; Tarnagda, Z.; Tahita, M.C.; Sow, A.; De Landtsheer, S.; Ammerlaan, W.; Ouedraogo, J.B.; Osterhaus, A.D.; et al. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J. Gen. Virol. 2007, 88, 2297–2306. [Google Scholar] [CrossRef]
- Daniels, P.; Wiyono, A.; Sawitri, E.; Poermadjaja, B.; Sims, L.D. H5N1 highly pathogenic avian influenza in Indonesia: Retrospective considerations. Curr. Top Microbiol. Immunol. 2013, 365, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Feeroz, M.M.; Hasan, M.K.; Akhtar, S.; Walker, D.; Seiler, P.; Barman, S.; Franks, J.; Jones-Engel, L.; McKenzie, P.; et al. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg. Microbes Infect. 2017, 6, e12. [Google Scholar] [CrossRef]
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Review analysis and impact of co-circulating H5N1 and H9N2 avian influenza viruses in Bangladesh. Epidemiol. Infect. 2018, 146, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. H5N1 Avian Influenza: Timeline of Major Event. 2011. Available online: https://cdn.who.int/media/docs/default-source/influenza/avian-and-other-zoonotic-influenza/h5n1_avian_influenza_update20141204.pdf (accessed on 10 July 2025).
- Reid, S.M.; Shell, W.M.; Barboi, G.; Onita, I.; Turcitu, M.; Cioranu, R.; Marinova-Petkova, A.; Goujgoulova, G.; Webby, R.J.; Webster, R.G.; et al. First reported incursion of highly pathogenic notifiable avian influenza A H5N1 viruses from clade 2.3.2 into European poultry. Transbound. Emerg. Dis. 2011, 58, 76–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Squires, R.B.; Noronha, J.; Hunt, V.; García-Sastre, A.; Macken, C.; Baumgarth, N.; Suarez, D.; Pickett, B.E.; Zhang, Y.; Larsen, C.N.; et al. Influenza research database: An integrated bioinformatics resource for influenza research and surveillance. Influenza Other Respir. Viruses 2012, 6, 404–416. [Google Scholar] [CrossRef]
- Brown, I.H. Summary of avian influenza activity in Europe, Asia, and Africa, 2006–2009. Avian Dis. 2010, 54, 187–193. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.M.; Lee, E.K.; Song, B.M.; Jeong, J.; Kwon, Y.K.; Kim, H.R.; Lee, K.J.; Hong, M.S.; Jang, I.; et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg. Infect. Dis. 2014, 20, 1087–1089. [Google Scholar] [CrossRef]
- Kanehira, K.; Uchida, Y.; Takemae, N.; Hikono, H.; Tsunekuni, R.; Saito, T. Characterization of an H5N8 influenza A virus isolated from chickens during an outbreak of severe avian influenza in Japan in April 2014. Arch. Virol. 2015, 160, 1629–1643. [Google Scholar] [CrossRef]
- Marchenko, V.; Goncharova, N.; Susloparov, I.; Kolosova, N.; Gudymo, A.; Svyatchenko, S.; Danilenko, A.; Durymanov, A.; Gavrilova, E.; Maksyutov, R.; et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology 2018, 525, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Pasick, J.; Berhane, Y.; Joseph, T.; Bowes, V.; Hisanaga, T.; Handel, K.; Alexandersen, S. Reassortant Highly Pathogenic Influenza A H5N2 Virus Containing Gene Segments Related to Eurasian H5N8 in British Columbia, Canada 2014. Sci. Rep. 2015, 5, 9484. [Google Scholar] [CrossRef]
- Torchetti, M.K.; Killian, M.L.; Dusek, R.J.; Pedersen, J.C.; Hines, N.; Bodenstein, B.; White, C.L.; Ip, H.S. Novel H5 clade 2.3.4.4 reassortant (H5N1) virus from a green-winged teal in Washington, USA. Genome Announc. 2015, 3, e00195-15. [Google Scholar] [CrossRef] [PubMed]
- Bevins, S.N.; Dusek, R.J.; White, C.L.; Gidlewski, T.; Bodenstein, B.; Mansfield, K.G.; DeBruyn, P.; Kraege, D.; Rowan, E.; Gillin, C.; et al. Widespread detection of highly pathogenic H5 influenza viruses in wild birds from the Pacific Flyway of the United States. Sci. Rep. 2016, 6, 28980. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Ke, C.W.; Li, Q.; Yuan, R.Y.; Xiang, D.; Jia, W.X.; Yu, Y.D.; Liu, L.; Huang, C.; Qi, W.B. Novel Reassortant Avian Influenza A(H5N6) Viruses in Humans, Guangdong, China, 2015. Emerg. Infect. Dis. 2016, 22, 1507–1509. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Huang, Y.; Zhu, W.; Yang, L.; Gao, L.; Li, X.; Bi, F.; Huang, C.; Kang, N.; et al. Human infections with novel reassortant H5N6 avian influenza viruses in China. Emerg. Microbes Infect. 2017, 6, e50. [Google Scholar] [CrossRef]
- Claes, F.; Morzaria, S.P.; Donis, R.O. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the Asian HPAI H5 lineage maintained. Curr. Opin. Virol. 2016, 16, 158–163. [Google Scholar] [CrossRef]
- Butler, J.; Stewart, C.R.; Layton, D.S.; Phommachanh, P.; Harper, J.; Payne, J. Novel reassortant H5N6 influenza A virus from the Lao People’s Democratic Republic is highly pathogenic in chickens. PLoS ONE. 2016, 11, e0162375. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, W.; Li, X.; Bo, H.; Zhang, Y.; Zou, S.; Gao, R.; Dong, J.; Zhao, X.; Chen, W. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. J. Virol. 2017, 91, e02199-16. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.H.; Okamatsu, M.; Matsuno, K.; Hiono, T.; Ogasawara, K.; Nguyen, L.T.; Van Nguyen, L.; Nguyen, T.N.; Nguyen, T.T.; Van Pham, D. Genetic and antigenic characterization of H5, H6 and H9 avian influenza viruses circulating in live bird markets with intervention in the center part of Vietnam. Vet. Microbiol. 2016, 192, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Chen, Q.; Wang, Q.; Chen, J.; Jin, T.; Wong, G.; Quan, C.; Liu, J.; Wu, J.; Yin, R.; et al. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China. Cell Host Microbe 2016, 20, 810–821. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Bai, T.; Zhao, X.; Zhao, X.; Zhang, Y.; Guo, J.; Li, Z.; Yang, L.; Wang, D.; et al. A fatal case of infection with a further reassortant, highly pathogenic avian influenza (HPAI) H5N6 virus in Yunnan, China. Infect. Genet. Evol. 2016, 40, 63–66. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Li, M.; Zhao, L.; Wang, D.; Tian, J.; Bai, X.; Ci, Y.; Wu, S.; Wang, F. Evolution and extensive reassortment of H5 influenza viruses isolated from wild birds in China over the past decade. Emerg. Microbes Infect. 2020, 9, 1793–1803. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Dong, J.; Bo, H.; Liu, J.; Yang, J.; Zhang, Y.; Wei, H.; Huang, W.; Zhao, X.; et al. Epidemiologic, Clinical, and Genetic Characteristics of Human Infections with Influenza A(H5N6) Viruses, China. Emerg. Infect. Dis. 2022, 28, 1332–1344. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antigenic and Genetic Characteristics of Zoonotic Influenza A Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness. 2022. Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2022-2023/202203_zoonotic_vaccinevirusupdate.pdf (accessed on 10 July 2025).
- World Organization for Animal Health (WOAH). Highly Pathogenic Avian Influenza (HPAI). HPAI SITUATION—Update. Available online: https://www.woah.org/app/uploads/2021/03/hpai-asof07052020.pdf (accessed on 8 September 2025).
- Swieton, E.; Fusaro, A.; Shittu, I.; Niemczuk, K.; Zecchin, B.; Joannis, T.; Bonfante, F.; Smietanka, K.; Terregino, C. Sub-Saharan Africa and Eurasia Ancestry of Reassortant Highly Pathogenic Avian Influenza A(H5N8) Virus, Europe, December 2019. Emerg. Infect. Dis. 2020, 26, 1557–1561. [Google Scholar] [CrossRef]
- Zeng, J.; Du, F.; Xiao, L.; Sun, H.; Lu, L.; Lei, W.; Zheng, J.; Wang, L.; Shu, S.; Li, Y.; et al. Spatiotemporal genotype replacement of H5N8 avian influenza viruses contributed to H5N1 emergence in 2021/2022 panzootic. J. Virol. 2024, 98, e0140123. [Google Scholar] [CrossRef] [PubMed]
- The Centers for Disease Control and Prevention (CDC). Technical Report: December 2023 Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-122923.html (accessed on 1 June 2025).
- European Food and Safety Authority (EFSA). Avian Influenza Overview March–June 2024. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8930 (accessed on 15 June 2025).
- European Food and Safety Authority (EFSA). Avian Influenza Overview December 2023–March 2024. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8754 (accessed on 15 June 2025).
- European Food and Safety Authority (EFSA). Avian Influenza Overview June–September 2023. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8328 (accessed on 5 September 2025).
- Lublin, A.; Shkoda, I.; Simanov, L.; Hadas, R.; Berkowitz, A.; Lapin, K.; Farnoushi, Y.; Katz, R.; Nagar, S.; Kharboush, C.; et al. The History of Highly-Pathogenic Avian Influenza in Israel (H5-subtypes): From 2006 to 2023. Israel, J. Vet. Med. 2023, 78, 2. [Google Scholar]
- Yang, Q.; Xue, X.; Zhang, Z.; Wu, M.J.; Ji, J.; Wang, W.; Yin, H.; Li, S.; Dai, H.; Duan, B.; et al. Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China. Viruses 2022, 15, 38. [Google Scholar] [CrossRef]
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764. [Google Scholar] [CrossRef]
- Carrique, L.; Fan, H.; Walker, A.P.; Keown, J.R.; Sharps, J.; Staller, E.; Barclay, W.S.; Fodor, E.; Grimes, J.M. Host ANP32A mediates the assembly of the influenza virus replicase. Nature 2020, 587, 638–643. [Google Scholar] [CrossRef]
- Waningen University and Research. WUR November 2023. Available online: www.wur.nl/en/ (accessed on 7 June 2025).
- Domanska-Blicharz, K.; Swieton, E.; Swiatalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Stys-Fijol, N.; Giza, A.; Pietruk, M.; et al. A(H5N1) Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Euro Surveill. 2023, 28, 31. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control); EURL (European Union Reference Laboratory for Avian Influenza); Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T. Scientific report: Avian influenza overview December 2024–March 2025. EFSA J. 2025, 23, 9352. [Google Scholar] [CrossRef]
- Byrne, A.M.P.; James, J.; Mollett, B.C.; Meyer, S.M.; Lewis, T.; Czepiel, M.; Seekings, A.H.; Mahmood, S.; Thomas, S.S.; Ross, C.S.; et al. Investigating the Genetic Diversity of H5 Avian Influenza Viruses in the United Kingdom from 2020–2022. Microbiol. Spectr. 2023, 11, e0477622. [Google Scholar] [CrossRef]
- Department for Environment Food and Rural Affairs (DEFRA). Available online: https://www.gov.uk/government/news/bird-flu-avian-influenza-latest-situation-in-england (accessed on 2 May 2024).
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef]
- Bevins, S.N.; Shriner, S.A.; Cumbee, J.C., Jr.; Dilione, K.E.; Douglass, K.E.; Ellis, J.W.; Killian, M.L.; Torchetti, M.K.; Lenoch, J.B. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg. Infect. Dis. 2022, 28, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Canada Government. Status of Ongoing Avian Influenza Response by Province. Available online: https://inspection.canada.ca/en/animal-health/terrestrial-animals/diseases/reportable/avian-influenza/latest-bird-flu-situation/status-ongoing-avian-influenza-response (accessed on 10 April 2025).
- The Centers for Disease Control and Prevention (CDC). Technical Report: April 2024 Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-04262024.html (accessed on 10 May 2025).
- The Centers for Disease Control and Prevention (CDC). Technical Report: March 2023 Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-031723.html (accessed on 10 May 2025).
- Reischak, D.; Rivetti, A.V., Jr.; Otaka, J.N.P.; Domingues, C.S.; Freitas, T.L.; Cardoso, F.G.; Montesino, L.O.; da Silva, A.L.S.; Malta, F.; Amgarten, D.; et al. First report and genetic characterization of the highly pathogenic avian influenza A(H5N1) virus in Cabot’s tern (Thalasseus acuflavidus), Brazil. Vet Anim Sci. 2023, 22, 100319. [Google Scholar] [CrossRef]
- Araújo, A.C.; Silva, L.M.N.; Cho, A.Y.; Repenning, M.; Amgarten, D.; Moraes, A.P.; Malta, F.; Miller, M.; Dorlass, E.G.; Palameta, S.; et al. Incursion of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus, Brazil, 2023. Emerg. Infect. Dis. 2024, 30, 619–621. [Google Scholar] [CrossRef]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef] [PubMed]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef]
- Lisovski, S.; Günther, A.; Dewar, M.; Ainley, D.; Aldunate, F.; Arce, R.; Ballard, G.; Bauer, S.; Belliure, J.; Banyard, A.C.; et al. Unexpected Delayed Incursion of Highly Pathogenic Avian Influenza H5N1 (Clade 2.3.4.4b) Into the Antarctic Region. Influenza Other Respir. Viruses 2024, 18, e70010. [Google Scholar] [CrossRef]
- Ohlopkova, O.V.; Goncharov, A.E.; Aslanov, B.I.; Fadeev, A.V.; Davidyuk, Y.N.; Moshkin, A.D.; Stolbunova, K.A.; Stepanyuk, M.A.; Sobolev, I.A.; Tyumentseva, M.A.; et al. First detection of influenza A virus subtypes H1N1 and H3N8 in the Antarctic region: King George Island, 2023. Vopr. Virusol. 2024, 69, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Mautinho, S. Deadly Avian Flu Strain Is Spreading Rapidly in Antarctica. 2025. Available online: https://www.science.org/content/article/deadly-avian-flu-strain-spreading-rapidly-antarctica (accessed on 30 June 2025).
- Ogrzewalska, M.; Vanstreels, R.T.; Pereira, E.; Campinas, E.; Correa, L.; Melo, J.O.; Macedo, L.; Appolinario, L.; Arantes, I.; Brandao, M.L.; et al. Genomic Analysis of High Pathogenicity Avian Influenza Viruses from Antarctica Reveals Multiple Introductions from South America; Research Square: Durham, NC, USA, 2025. [Google Scholar] [CrossRef]
- Animal Health Australia. Available online: https://animalhealthaustralia.com.au/avian-influenza/ (accessed on 30 June 2025).
- Deng, Y.M.; Wille, M.; Dapat, C.; Xie, R.; Lay, O.; Peck, H.; Daley, A.J.; Dhanasakeran, V.; Barr, I.G. Influenza A(H5N1) Virus Clade 2.3.2.1a in Traveler Returning to Australia from India, 2024. Emerg. Infect. Dis. 2025, 31, 135–138. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Highly Pathogenicity Avian Influenza (HPAI) Situation Report 73. Available online: https://www.woah.org/app/uploads/2025/08/hpai-report-73-1.pdf (accessed on 10 June 2025).
- The Centers for Disease Control and Prevention (CDC). Technical Report June 2024: Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-06052024.html (accessed on 5 June 2024).
- The Centers for Disease Control and Prevention (CDC). Current H5N1 Bird Flu Situation in Dairy Cows. Available online: https://www.cdc.gov/bird-flu/situation-summary/mammals.html (accessed on 1 July 2024).
- The Centers for Disease Control and Prevention (CDC). Weekly US Influenza Surveillance Report: Key Updates for Week 32, Ending 9 August 2025. Available online: https://www.cdc.gov/fluview/surveillance/2025-week-32.html (accessed on 30 June 2025).
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Alkie, T.N.; Nasheri, N.; Romero-Barrios, P.; Catford, A.; Krishnan, J.; Pama, L.; Hooper-McGrevy, K.; Nfon, C.; Cutts, T.; Berhane, Y. Effectiveness of pasteurization for the inactivation of H5N1 influenza virus in raw whole milk. Food Microbiol. 2025, 125, 104653. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.C.; Webby, R.; Diefenbach, E.; Kanipe, C.; Anderson, T.K.; Baker, A.L. Timing and Molecular Characterisation of the Transmission to Cattle of H5N1 Influenza A Virus Genotype D1.1, Clade 2.3.4.4b. 2024. Available online: https://virological.org/t/timing-and-molecular-characterisation-of-the-transmission-to-cattle-of-h5n1-influenza-a-virus-genotype-d1-1-clade-2-3-4-4b/991 (accessed on 30 June 2025).
- USDA’s Animal and Plant Health Inspection Service (APHIS). Available online: https://www.aphis.usda.gov/sites/default/files/small-ruminant-camelid-h5n1-info.pdf (accessed on 30 June 2025).
- Chrzastek, K.; Kapczynski, D.R. In Silico Genomic Analysis of Avian Influenza Viruses Isolated from Marine Seal Colonies. Pathogens 2024, 13, 1009. [Google Scholar] [CrossRef]
- UC Davis News. Available online: https://www.vetmed.ucdavis.edu/news/catastrophic-mortality-elephant-seals-argentina-identified-outbreak-avian-influenza (accessed on 10 March 2025).
- Gamarra-Toledo, V.; Plaza, P.I.; Gutiérrez, R.; Inga-Diaz, G.; Saravia-Guevara, P.; Pereyra-Meza, O.; Coronado-Flores, E.; Calderón-Cerrón, A.; Quiroz-Jiménez, G.; Martinez, P.; et al. Mass Mortality of Sea Lions Caused by Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg. Infect. Dis. 2023, 29, 2553–2556. [Google Scholar] [CrossRef]
- World Animal Health Information System (WOAH-WAHIS) Report. March 2023. Available online: https://www.woah.org/en/disease/avian-influenza/ (accessed on 10 March 2025).
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Munoz, G.; Navarro, C.; Avila, C.; Ulloa, M.; Reyes, R.; et al. Cross-species transmission and PB2 mammalian adaptations of highly pathogenic avian influenza A/H5N1 viruses in Chile. bioRxiv 2023. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill. 2023, 28, 2300001. [Google Scholar] [CrossRef] [PubMed]
- European Food and Safety Authority (EFSA). Avian Influenza Overview December 2020–February 2021. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6497 (accessed on 3 July 2025).
- Bussey, K.A.; Bousse, T.L.; Desmet, E.A.; Kim, B.; Takimoto, T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J. Virol. 2010, 84, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; Hesselink, H.; Lollinga, P.; Wesselman, R.; Prins, P.; Weesendorp, E.; Engelsma, M.; Heutink, R.; Harders, F.; Kik, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus in Wild Red Foxes, The Netherlands, 2021. Emerg. Infect. Dis. 2021, 27, 2960–2962. [Google Scholar] [CrossRef]
- Kareinen, L.; Tammiranta, N.; Kauppinen, A.; Zecchin, B.; Pastori, A.; Monne, I.; Terregino, C.; Giussani, E.; Kaarto, R.; Karkamo, V.; et al. Highly pathogenic avian influenza A(H5N1) virus infections on fur farms connected to mass mortalities of black-headed gulls, Finland, July to October 2023. Euro Surveill. 2024, 29, 2400063. [Google Scholar] [CrossRef]
- Lindh, E.; Lounela, H.; Ikonen, N.; Kantala, T.; Savolainen-Kopra, C.; Kauppinen, A.; Österlund, P.; Kareinen, L.; Katz, A.; Nokireki, T.; et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Euro Surveill. 2023, 28, 2300400. [Google Scholar] [CrossRef] [PubMed]
- Tammiranta, N.; Isomursu, M.; Fusaro, A.; Nylund, M.; Nokireki, T.; Giussani, E.; Zecchin, B.; Terregino, C.; Gadd, T. Highly pathogenic avian influenza A (H5N1) virus infections in wild carnivores connected to mass mortalities of pheasants in Finland. Infect. Genet. Evol. 2023, 111, 105423. [Google Scholar] [CrossRef]
- Chestakova, I.V.; van der Linden, A.; Bellido Martin, B.; Caliendo, V.; Vuong, O.; Thewessen, S.; Hartung, T.; Bestebroer, T.; Dekker, J.; Jonge Poerink, B.; et al. High number of HPAI H5 virus infections and antibodies in wild carnivores in the Netherlands, 2020–2022. Emerg. Microbes Infect. 2023, 12, 2270068. [Google Scholar] [CrossRef]
- Coleman, K.K.; Bemis, I.G. Avian Influenza Virus Infections in Felines: A Systematic Review of Two Decades of Literature. Open Forum Infect Dis. 2025, 12, ofaf261. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yeom, M.; Vu, T.T.H.; Do, H.Q.; Na, W.; Lee, M.; Jeong, D.G.; Cheon, D.S.; Song, D. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg. Microbes Infect. 2024, 13, 2290835. [Google Scholar] [CrossRef]
- European Food and Safety Authority (EFSA). Avian Influenza Overview April–June 2023. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8191 (accessed on 8 August 2025).
- Ly, H. Highly pathogenic avian influenza H5N1 virus infection of companion animals. Virulence 2024, 15, 2289780. [Google Scholar] [CrossRef]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- APHIS-USDA. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (accessed on 16 June 2025).
- Lee, S.H.; Kwon, J.H.; Youk, S.; Lee, S.W.; Lee, D.H.; Song, C.S. Epidemiology and pathobiology of H5Nx highly pathogenic avian influenza in South Korea (2003–2024): A comprehensive review. Vet. Q. 2025, 45, 23–38. [Google Scholar] [CrossRef]
- Cho, A.Y.; Si, Y.J.; Lee, D.Y.; Kim, D.J.; Kim, D.; Jeong, H.; Song, C.S.; Lee, D.H. Index case of H5N1 clade 2.3.4.4b highly pathogenic avian influenza virus in wild birds, South Korea, November 2023. Front. Vet. Sci. 2024, 11, 1366082. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Heo, G.B.; An, S.H.; Lee, H.; Park, E.; Cha, R.M.; Jang, Y.Y.; Sagong, M.; Kim, A.Y.; Kim, J.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in Cats, South Korea, 2023. Emerg. Infect. Dis. 2024, 30, 2510–2520. [Google Scholar] [CrossRef]
- Moreno, A.; Bonfante, F.; Bortolami, A.; Cassaniti, I.; Caruana, A.; Cottini, V.; Cereda, D.; Farioli, M.; Fusaro, A.; Lavazza, A.; et al. Asymptomatic infection with clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) in carnivore pets, Italy, April 2023. Euro Surveill. 2023, 28, 2300441. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.Y.; Seo, Y.; Song, C.S.; Lee, D.H. Immediate PB2-E627K amino acid substitution after single infection of highly pathogenic avian influenza H5N1 clade 2.3.4.4b in mice. Virol. J. 2025, 22, 183. [Google Scholar] [CrossRef]
- Brown, J.D.; Black, A.; Haman, K.H.; Diel, D.G.; Ramirez, V.E.; Ziejka, R.S.; Fenelon, H.T.; Rabinowitz, P.M.; Stevens, L.; Poulson, R.; et al. Antibodies to Influenza A(H5N1) Virus in Hunting Dogs Retrieving Wild Fowl, Washington, USA. Emerg. Infect. Dis. 2024, 30, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Thai dogs carry bird-flu virus, but will they spread it? Nature 2006, 439, 773. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Domestic Dog Tests Positive for Avian Influenza in Canada. Available online: https://www.canada.ca/en/food-inspection-agency/news/2023/04/domestic-dog-tests-positive-for-avian-influenza-in-canada.html (accessed on 16 June 2025).
- World Health Organization (WHO). Avian Influenza Weekly Update Number 1003. Available online: https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20250627.pdf (accessed on 8 September 2025).
- The Centers for Disease Control and Prevention (CDC). Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses; CDC: Atlanta, GA, USA, 29 December 2023. [Google Scholar]
- Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2023. Available online: https://cdn.who.int/media/docs/default-source/influenza/h5n1-human-case-cumulative-table/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2023071d9f0c-49c7-43ef-bf36-4ae01252b29a.pdf?sfvrsn=d9a96d9_3&download=true (accessed on 21 December 2023).
- Castillo, A.; Fasce, R.; Parra, B.; Andrade, W.; Covarrubias, P.; Hueche, A.; Campano, C.; Tambley, C.; Rojas, M.; Araya, M.; et al. The first case of human infection with H5N1 avian Influenza A virus in Chile. J. Travel Med. 2023, 30, taad083. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Disease Outbreak News. Avian Influenza A (H5N1)—Cambodia. 23 February 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON445 (accessed on 1 August 2025).
- World Health Organization (WHO). Influenza at the Human-Animal Interface Summary and Assessment. 3 March 2023. Available online: https://www.who.int/publications/m/item/influenza-at-the-human-animal-interface-summary-and-assessment-3-march-2023 (accessed on 1 August 2025).
- World Health Organization (WHO). Influenza at the Human-Animal Interface Summary and Assessment. 1 November 2023. Available online: https://www.who.int/publications/m/item/influenza-at-the-human-animal-interface-summary-and-assessment-1-november-2023 (accessed on 1 August 2025).
- Pan American Health Organization/World Organization. Epidemiological Update: Avian Influenza A(H5N1) in the Americas Region; PAHO/WHO: Washington, DC, USA, 2025; Available online: https://www.paho.org/sites/default/files/2025-03/2025-mar-4-phe-epidupdate-avianinfluenza-eng-final.pdf (accessed on 4 March 2025).
- The Centers for Disease Control and Prevention (CDC). Global Summary of Recent Human Cases of H5N1 Bird Flu. Available online: https://www.cdc.gov/bird-flu/spotlights/h5n1-summary-08042025.html (accessed on 8 September 2025).
- World Health Organization (WHO). Influenza at the Human-Animal Interface Summary and Risk Assessment, from 20 March to 22 April 2025. Available online: https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/influenza-at-the-human-animal-interface-summary-and-assessment--from-20-march-to-22-april-2025.pdf?sfvrsn=94dbbd93_3&download=true (accessed on 22 April 2025).
- The Centers for Disease Control and Prevention (CDC). CDC Reports Fourth Human Case of H5 Bird Flu Tied to Dairy Cow Outbreak. Available online: https://www.cdc.gov/media/releases/2024/p-0703-4th-human-case-h5.html (accessed on 3 July 2024).
- Uyeki, T.M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N. Engl. J. Med. 2024, 390, 2028–2029. [Google Scholar] [CrossRef]
- Ison, M.G.; Marrazzo, J. The emerging threat of H5N1 to Human health. N. Engl. J. Med. 2024, 392, 916–918. [Google Scholar] [CrossRef]
- Jassem, A.N.; Roberts, A.; Tyson, J.; Zlosnik, J.E.; Russell, S.L.; Caleta, J.M.; Eckbo, E.J.; Gao, R.; Chestley, T.; Grant, J.; et al. Critical illness in an adolescent with influenza A(H5N1) virus infection. N. Engl. J. Med. 2024, 392, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shi, Y.; Ge, H.; Wang, Y.; Lu, L.; Jiang, S.; Wang, Q. Genomic signatures and host adaptation of H5N1 clade 2.3.4.4b: A call for global surveillance and multi-target antiviral strategies. Curr. Res. Microb. Sci. 2025, 8, 100377. [Google Scholar] [CrossRef]
- Dyer, O. Bird flu: Canadian teenager is critically ill with new genotype. BMJ 2024, 387, q2529. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzastek, K.; Lieber, C.M.; Plemper, R.K. H5N1 Clade 2.3.4.4b: Evolution, Global Spread, and Host Range Expansion. Pathogens 2025, 14, 929. https://doi.org/10.3390/pathogens14090929
Chrzastek K, Lieber CM, Plemper RK. H5N1 Clade 2.3.4.4b: Evolution, Global Spread, and Host Range Expansion. Pathogens. 2025; 14(9):929. https://doi.org/10.3390/pathogens14090929
Chicago/Turabian StyleChrzastek, Klaudia, Carolin M. Lieber, and Richard K. Plemper. 2025. "H5N1 Clade 2.3.4.4b: Evolution, Global Spread, and Host Range Expansion" Pathogens 14, no. 9: 929. https://doi.org/10.3390/pathogens14090929
APA StyleChrzastek, K., Lieber, C. M., & Plemper, R. K. (2025). H5N1 Clade 2.3.4.4b: Evolution, Global Spread, and Host Range Expansion. Pathogens, 14(9), 929. https://doi.org/10.3390/pathogens14090929







