Abstract
Rodents are recognized as significant reservoirs for a broad range of zoonotic pathogens, including bacteria, viruses, and parasites, many of which have substantial implications for human and animal health. The intensifying interaction between humans and rodent populations, fuelled by urbanization, climate change, and global trade, has amplified the risk of zoonotic disease transmission. This review compiles and examines current knowledge on key rodent-borne bacterial diseases, including leptospirosis, rat-bite fever, plague, salmonellosis, tularemia, Lyme disease, rickettsioses, Babesiosis, and associated parasitic infections such as toxoplasmosis and Chagas disease. Each disease is analyzed in terms of its etiology, transmission, clinical manifestations, diagnostic tools, and treatment options, with a particular focus on the impact of environmental changes. Emphasizing a One Health perspective, this work highlights the importance of interdisciplinary approaches to the surveillance, prevention, and control of rodent-borne zoonoses, particularly in the context of increasing climate variability and anthropogenic pressures.
1. Introduction
Rodents (order: Rodentia) are among the most prolific and adaptable mammalian species, thriving in nearly all environments, including densely populated urban areas. Their ubiquitous presence and close association with human settlements make them critical contributors to the ecology of zoonotic diseases. As reservoirs and vectors of a wide range of pathogens, including bacteria, viruses, and parasites, rodents pose a persistent and often underestimated threat to global health [1].
Rodent-borne bacterial infections, such as leptospirosis, rat-bite fever, plague, salmonellosis, tularemia, and Lyme disease, have re-emerged in recent years, driven by climatic fluctuations, ecological disruptions, and increased human encroachment into natural habitats [2]. These diseases vary widely in clinical presentation, ranging from mild flu-like symptoms to severe systemic conditions with high mortality rates. Given their dual role as ecological reservoirs and disease vectors, understanding the biology, behaviour, and epidemiological significance of rodents is vital for public health and biodiversity management [3].
Rodents, the most significant order of mammals, comprising over 2200 recognized species, account for approximately 42% of global mammalian diversity [4]. These mammals have a heterogeneous and cosmopolitan distribution, with their expansion closely linked to increasing human interaction. Beyond synanthropic species, other rodents inhabit wild, urban, and rural environments, further increasing contact with animals and humans [5,6]. These animals serve as natural reservoirs of infectious diseases, transmitting pathogens both directly and indirectly, as well as through vectors [3,7].
This review examines the epidemiology, transmission dynamics, diagnosis, and control of rodent-borne pathogens, providing a comprehensive synthesis of current knowledge on this topic. Special attention is given to the impact of climate change on pathogen prevalence and distribution, emphasizing the urgency of adopting integrated One Health strategies. Recognizing the hidden threat posed by rodent-borne zoonoses is essential for mitigating their impact and safeguarding both human and animal populations.
2. Leptospirosis
2.1. Etiology
Leptospirosis is a zoonotic bacterial disease with a worldwide distribution, affecting approximately 1 million people and causing around 60,000 deaths annually [8]. The highest estimates of disease morbidity and mortality were observed in the Global Burden of Disease (GBD) regions of South and Southeast Asia, Oceania, the Caribbean, Andean, Central, and Tropical Latin America, as well as East Sub-Saharan Africa [8].
Morphologically, leptospires are thin, flexible, Gram-negative, motile bacteria, with one or both ends shaped like a hook, giving them a characteristic question-mark appearance [9]. The 64 known species of Leptospira are grouped into two pathogenic subclades [P1 (Pathogenic species) and P2 (Intermediate pathogens)] and two saprophytic subclades (S1 and S2). Most Leptospira species responsible for infecting humans and animals result from infections by virulent P1 species, such as Leptospira interrogans, Leptospira kirschneri, Leptospira borgpetersenii, and Leptospira noguchii [10,11,12].
2.2. Epidemiology
Rodents are most frequently infected by Leptospira interrogans serovar Icterohaemorrhagiae. However, other species, such as L. borgpetersenii, L. kirschneri, L. broomii, and L. santarosai, have also been identified in wild Norway rats [13,14,15,16]. The first three species are also commonly found in other wild rodent populations [16]. Rats, along with other rodents, serve as the primary reservoir for Leptospira; however, not all species of Leptospira are present in rats [17].
Leptospira lives in the renal tubules of these animals, which act as reservoirs, playing a fundamental role in the epidemiology of the disease by contaminating environments through the excretion of Leptospira in their urine. This bacterium can survive in aquatic environments and soil for up to two months. Leptospires are typically transmitted to humans through direct contact with the urine of infected rodents, which usually remain asymptomatic, or indirectly via urine-contaminated water or soil [18]. The bacteria enter the human body through minor skin abrasions or mucous membranes of the eyes, mouth, or nose. Cases of transmission from pet rats to humans in close contact with them have been documented [19,20]. The highest prevalence rates remain in tropical, developing countries where leptospirosis cases are on the rise [21,22].
2.3. Clinical Signs
The incubation period for leptospirosis typically ranges from 7 to 14 days, though it can vary between 2 and 30 days. The majority (approximately 90%) of human infections are either asymptomatic or present with mild flu-like symptoms. However, severe forms can occur, potentially leading to life-threatening complications such as pulmonary hemorrhage, liver and kidney failure (Weil’s disease), as well as meningitis, pancreatitis, or encephalitis [18]. Transplacental transmission may also occur, resulting in abortion or stillbirth [18] (Figure 1). In animals, leptospirosis can cause a range of clinical manifestations and pathological changes. In calves, infection may result in hemolytic anemia and hemoglobinuria, with interstitial nephritis as a sequela, and late-term abortion in cows (combined for clarity). In dairy cows, mastitis syndrome and occasional abortions may occur. In pigs, the most significant losses arise from abortions, stillbirths, neonatal mortality, and interstitial nephritis (reworded for conciseness) [23]. In dogs, most infections are subclinical; however, symptomatic cases are characterized by lethargy, fever, anorexia, and polyuria/polydipsia, which may progress to multiple organ dysfunction, including acute kidney injury, cholestatic liver dysfunction, pancreatitis, varying degrees of pulmonary hemorrhage, myositis, and occasionally uveitis [24]. In horses, the disease may manifest as febrile illness, reproductive losses, and neonatal disease [24,25,26].
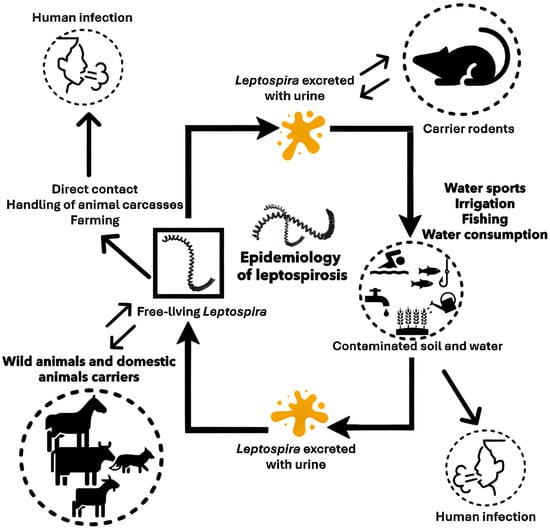
Figure 1.
Transmission of Leptospira to humans and the role of the rodents. Rodents, along with wild and domestic animals, serve as primary reservoirs of Leptospira, harboring the bacteria in their renal tubules and excreting them in urine. Human infection occurs through direct contact with infected urine or indirectly via contaminated water or soil, with bacteria entering through skin abrasions or mucous membranes.
2.4. Diagnosis
Diagnosis of leptospirosis can be made through direct pathogen detection, such as polymerase chain reaction (PCR)-based identification of pathogenic Leptospira DNA from blood, urine, cerebrospinal fluid, or tissue samples (e.g., kidney). Direct detection can also be performed through bacterial culture using the Ellinghausen-McCullough-Johnson-Harris (EMJH) medium, though due to the slow growth rate of Leptospira, this method is not suitable for rapid diagnosis [27]. In contrast, PCR testing provides faster results and allows for pathogen typing. Leptospires can also be detected through dark-field microscopy in blood or urine samples; however, this method has limited sensitivity and specificity. Alternative methods include silver staining techniques such as Steiner, Levaditi, Dieterle, and Warthin-Starry staining, as well as immunohistological examinations. Indirect detection relies on serological methods to identify antibodies against Leptospira. The microscopic agglutination test (MAT) is the gold standard for serological diagnosis, while enzyme-linked immunosorbent assays (ELISA) are also commonly used.
2.5. Treatment, Prevention, and Control
Symptomatic leptospirosis is treated with antibiotics as early as possible, as the bacteria generally respond well to antimicrobial therapy. Effective antibiotics include tetracyclines, β-lactams, and macrolides, particularly azalides [28].
Leptospirosis is a disease closely linked to the concept of One Health, so environmental balance is fundamental to understanding the disease dynamics. In this sense, reports indicate that climate change will lead to drastic changes in global ecosystems with unpredictable and incalculable impacts. Based on the disease dynamics, the intensification of events related to rainfall and floods is a critical point for the increase in leptospirosis cases [29]. Therefore, to achieve significant results in controlling leptospirosis, it must be addressed within the context of One Health.
Commercially available vaccines are composed of whole inactivated Leptospira and are approved for human use only in a few countries, such as Japan, Cuba, France, and China [30]. However, these vaccines induce only serovar-specific protection and short-term immunity, requiring annual immunization. Furthermore, serious side effects are reported after administration, limiting their use [31].
2.6. Recommendations
Given the complex epidemiology and environmental persistence of leptospirosis, a comprehensive, integrated approach (replaced “comprehensive and integrated” → “comprehensive, integrated” for conciseness) is essential for effective prevention and control. The author recommends implementing One Health strategies that acknowledge the interconnectedness of human, animal, and environmental health. The author recommends implementing One Health strategies that acknowledge the interconnectedness of human, animal, and environmental health. This includes controlling rodent populations, especially in urban and peri-urban areas, by reducing access to food sources, sealing entry points, and effectively managing waste and sanitation. Public education campaigns are crucial for raising awareness about risk factors, such as exposure to contaminated water or soil, and for promoting protective behaviors, like wearing boots and gloves in flood-prone or high-risk areas.
In endemic regions, early diagnosis using rapid molecular techniques, such as PCR, should be prioritized to improve clinical outcomes. Timely antibiotic treatment remains a key tool in managing infections. Where available, vaccines may be considered for high-risk groups; however, their limitations, including serovar-specific protection and potential side effects, must be taken into account. Finally, given the strong link between climate events (e.g., heavy rainfall and flooding) and outbreaks, climate adaptation strategies should be integrated into public health planning to anticipate and mitigate future spikes in leptospirosis incidence.
3. Rat-Bite Fever
3.1. Etiology
Rat-bite fever (RBF) is a systemic infectious disease primarily caused by two bacterial species: Streptobacillus moniliformis and Spirillum minus [32]. However, past case reports describing Spirillum minus are based solely on morphological observations made with dark-field microscopy, lacking molecular or phenotypic confirmation. S. moniliformis is now recognized as the primary causative agent of RBF, with wild rats serving as its primary reservoir [33,34]. Other Streptobacillus species of interest include S. notomytis, which can also cause RBF but is primarily transmitted by the black rat (Rattus rattus), and S. felis, which leads to a similar illness but originates from cats [35,36].
Rats harbor S. moniliformis in their oral cavity and throat, typically without exhibiting symptoms. The bacteria spread easily among rats through social interactions, although stressed or injured rats may develop abscesses or other bacterial infections [37]. House mice (Mus musculus) can contract S. moniliformis only through contact with infected rats, but they do not play a significant role in the epidemiology of human RBF. Susceptibility among mouse strains varies, with some developing fever, arthritis, abscesses, and septicemia upon infection [38].
3.2. Epidemiology
The prevalence of S. moniliformis in wild rat populations varies significantly, with reported rates ranging from 2% to 92%, depending on the location and the methods used in the studies [33,39]. Variations in detection techniques, such as antibody tests, genomic analysis, or bacterial cultures, as well as the specific rat populations studied (urban vs. rural, wild vs. pet store or feeder rats), contribute to these discrepancies. Although serological testing can be highly sensitive, no commercial diagnostic tests are currently available. Furthermore, the incidence of infection and the minimum infectious dose for humans remain unknown.
In Germany, it is estimated that between 30,000 and 50,000 bite wounds are treated each year, with bites from dogs and cats being the most common. Less than 10% of these injuries are caused by rodents such as rats, hamsters, and rabbits. Notably, 59% of the affected individuals are children or adolescents [40]. The overall risk of infection following a rat bite is estimated to be between 10% and 20%, with 30% to 60% of cases resulting in polymicrobial infections, which include both aerobic and anaerobic bacteria [40]. Pet stores and feeder rats are not routinely screened for S. moniliformis, meaning the bacterium is likely present in pet rats and can be transmitted to humans, primarily through close contact.
High-risk groups include veterinarians, animal caretakers, sewer workers, farmers, and homeless individuals. Additionally, due to the increasing popularity of pet rats, children are at an elevated risk [41,42]. Infections can also occur through indirect exposure to contaminated saliva, urine, feces, food, or materials from cages [43]. The bacteria can enter the body through direct contact, skin wounds, or mucous membranes, such as those found in the eyes, nose, or mouth [43] (Figure 2). Diagnostic samples can be collected from various sources, including blood, mucosal swabs, fine-needle aspirates, wound secretions, abscess fluid, synovial fluid, or cerebrospinal fluid [43]. Blood cultures should be taken repeatedly, as initial bacterial growth may not always be detected successfully [43].
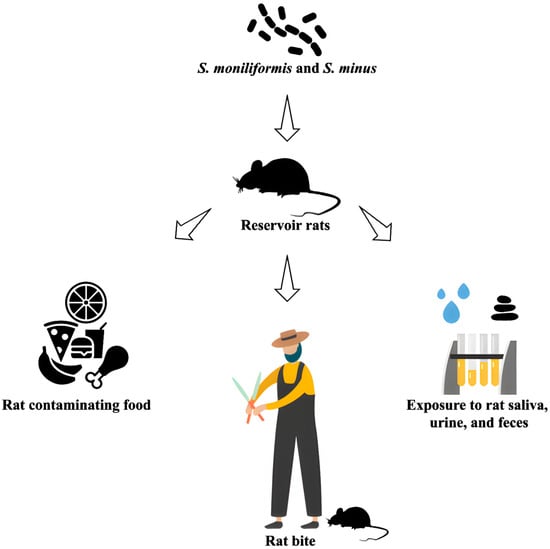
Figure 2.
Transmission of Rat-Bite Fever (RBF): Overview of how Streptobacillus moniliformis and S. minus are transmitted from rats to humans through bites, scratches, or contact with contaminated materials.
3.3. Clinical Aspects
The incubation period for S. moniliformis infection spans from 3 to 21 days [44,45]. Common symptoms include recurrent fever, migratory polyarthralgia (affecting approximately 50% of cases), and a rash on the hands and feet (observed in about 75% of cases) [44,45]. These key symptoms are often accompanied by general complaints, such as headaches, muscle pain, and elevated inflammatory markers, including neutrophilia, increased erythrocyte sedimentation rate, and high levels of C-reactive protein [31,32]. If left untreated, severe complications can arise, including abscesses, hepatitis, nephritis, pneumonia, meningitis, encephalitis, osteomyelitis, bacteremia, spondylodiscitis, and inflammation of the heart [44,45].
A foodborne variant known as Haverhill fever (Erythema arthriticum epidemicum) results from consuming contaminated food or water. This variant presents additional gastrointestinal symptoms, including pharyngitis, vomiting, and diarrhea. The estimated mortality rate for this condition is around 10%, although severe cases can have significantly higher fatality rates [46].
In laboratory mice infected with S. moniliformis, clinical signs ranging from septic lymphadenitis to polyarthritis and multiorgan microabscesses can be observed, leading to septicemia, cachexia, and death [47]. In nonhuman primates, rat-bite fever caused by S. moniliformis has been reported in a rhesus monkey (Macaca mullata) with valvular endocarditis and a titi monkey (Callicebus sp.) with septic arthritis [48].
3.4. Diagnosis
The PCR technique can be used to detect S. moniliformis in skin lesions [32]. Furthermore, the identification of S. moniliformis bacteria can be performed through culture from plasma or joint fluid. To do this, the sample must be injected into a culture medium free of sodium polyanethole sulfonate, an anticoagulant used in culture media because it inhibits the growth of the organism [49]. Other tests, such as gas–liquid chromatography and 16S rRNA sequencing, are more sensitive than culture [50,51]. S. minus cannot be cultured, and the only methods for identifying it are dark-ground microscopy from blood smears, lesions, or lymph nodes, and Giemsa staining [51].
3.5. Treatment, Prevention, and Control
When diagnosed promptly, RBF can typically be treated without complications. The preferred antibiotics for this condition include penicillin, doxycycline, or ceftriaxone, which can be administered either orally or intravenously [52,53]. However, decolonizing infected rodents poses challenges, as these treatments do not guarantee long-term eradication of the bacteria [54]. Despite being treatable, Rat-Bite Fever (RBF) is often referred to as a “diagnostic dilemma” because its symptoms are nonspecific and resemble those of the flu. Additionally, there is a lack of systematic case reporting in both human and veterinary medicine [55]. The bacterium responsible for RBF grows slowly and requires microaerophilic conditions, along with specialized culture media, which complicates laboratory diagnosis. Moreover, routine molecular and serological diagnostic tools are not widely available [34].
3.6. Recommendations
To prevent Rat-Bite Fever, the author recommends raising awareness, particularly among high-risk groups, including veterinarians, pet owners, and individuals exposed to rodents. Individuals should avoid direct contact with rats’ saliva, urine, or feces and use protective gloves when handling rodents or cleaning cages. Children should be supervised around pet rats to prevent bites or close face contact. Pet stores and breeders should screen rats for Streptobacillus moniliformis to minimize the risk of transmission. In cases of rodent bites or symptoms such as fever and rash, clinicians should consider RBF and utilize appropriate diagnostic tools, including PCR or specialized cultures. Improved case reporting and access to reliable diagnostics are also essential for better monitoring and control.
4. Yersinia pestis
Human plague, caused by the bacterium Yersinia pestis, has resulted in three pandemics in history, including the first plague of Justinian (around 541 AD), the second Black Death (around 1347 AD), and the third plague (around 1880 AD) [56]. While often associated with historical events, plague is currently considered a re-emerging disease, with increasing human infection rates, particularly in Africa [57].
4.1. Etiology
Y. pestis is a Gram-negative bacterium belonging to the Enterobacteriaceae family, non-spore-forming, immobile coccobacillus cultured in broth, with bipolar staining using Giemsa or Wayson, with an optimal growth temperature between 26–28 °C, and an optimal pH of approximately 7.5 [58].
Indeed, Y. pestis is the most notorious species within the Yersinia genus, which includes two other species pathogenic to humans: Yersinia enterocolitica and Yersinia pseudotuberculosis [59]. Y. enterocolitica is an enteropathogenic bacterium primarily affecting the gastrointestinal tract. It is commonly found in soil environments and infects various mammalian and avian species, with an exceptionally high prevalence in domestic pig populations [60]. In humans, large outbreaks of Y. enterocolitica infections are typically associated with the consumption of contaminated food [61]. Y. pseudotuberculosis, like Y. enterocolitica, is also a gastrointestinal pathogen. It is widely distributed in the environment, particularly in soil and water, where it can persist for extended periods [62]. This bacterium has developed several adaptation mechanisms that enable it to survive under biotic and abiotic conditions in the soil, which differ significantly from those encountered during its host-associated life cycle [62]. Y. pseudotuberculosis primarily causes gastrointestinal infections following the ingestion of contaminated food.
4.2. Epidemiology
Plague has a global distribution, with the three most endemic countries being Madagascar, Congo, Uganda, Peru, Tanzania, and the United States [63]. Between 2010 and 2019, the World Health Organization received 4547 cases with a 17% fatality rate. Madagascar experienced four major outbreaks of primary pneumonic plague, affecting nearly 2000 people and causing 137 deaths, including one outbreak involving a streptomycin-resistant Yersinia pestis strain. The region with the highest concentration of diversity is China, which hosts a wide variety of hosts, including rodents [64,65,66]. Mammals are the most common host species, with about 351 species capable of acting as reservoirs. Among these species, 279 are rodents [66,67]. Additionally, the bacterium is maintained in flea populations that become infected by feeding on animals already infected with the bacterium [48,50,51]. Besides fleas, other blood-sucking arthropods, such as Argasidae, Gamasidae, Ixodidae, Anoplura, and Heteroptera, can harbor the bacterium, but they are not significant in epizootics and disease outbreaks [65] (Figure 3).
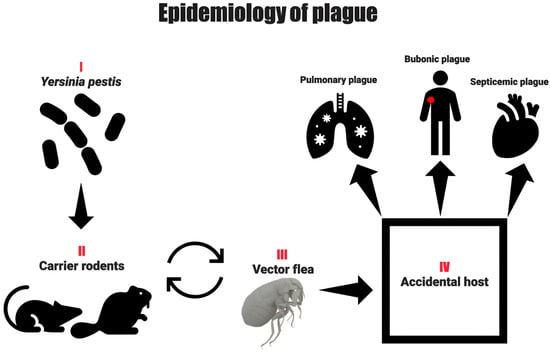
Figure 3.
Transmission routes of plague: Visual representation of plague transmission from infected rodents to humans primarily via flea bites, with possible secondary transmission through contact with infected animals or inhalation of infectious droplets in pneumonic plague cases.
By the end of 2021, there were 12 types of natural plague foci in the mainland of China, located in 322 county-level divisions of 19 provincial-level administrative divisions (PLADs), covering a total of 1,587,666.67 square kilometers. Between 2002 and 2021, plague epizootics or positive indications were identified for 12 types of natural plague foci in 196 county-level divisions of 16 PLADs [68]. An average of seven human plague cases are reported each year in the United States. Most plague cases occur in the western part of the country, mainly in northern New Mexico and Arizona [69].
The plague can be transmitted from a patient with pneumonic plague to other individuals through droplet transmission [70,71,72]. In addition to aerosol transmission, contact between susceptible individuals and infected tissue, whether of animal or human origin, poses a high risk of infection to humans. Contagion by direct contact could cause a contained spread to occur among relatives and close communities of people who take care of their dead [73]. In the environment, approximately 80 species of fleas can be infected with the bacterium [74], which is considered the most efficient method of transmission [75].
In the next century, the combination of climate change and anthropogenic activities is expected to result in a significant increase in pathogen spillover and the emergence of zoonotic diseases [76,77]. Following this line, plague cases are closely linked to climatic anomalies and the richness of rodent species [78]. Climate change can result in unfavorable situations, such as food shortages and famine, which can trigger migratory waves and, consequently, increased interaction between plague vectors [79,80]. Therefore, due to the acceleration of climate change, it is essential to maintain constant surveillance of Y. pestis, its hosts, and at-risk populations.
4.3. Clinical Signs
In 1940, G. P. Rudnev [81] proposed an epidemiological classification of plague that remains relevant to public health practice. He divided plague into three main categories based on the mode and extent of dissemination: (A) predominantly local forms, including cutaneous, bubonic, and cutaneous-bubonic types, which are typically peripheral and rarely spread externally; (B) internally disseminated or generalized forms, comprising primary and secondary septicemic plague; and (C) externally disseminated forms, which are often highly contagious and include primary pneumonic, secondary pneumonic, and intestinal plague. While this classification supports practical applications in disease control, subsequent research has noted that “pure” cutaneous and intestinal forms have not been observed in clinical practice [82].
- i.
- Septicemic Plague: Clinically, the septicemic form of plague, caused by Y. pestis, resembles septicemia caused by other Gram-negative bacteria. Affected individuals may present with hyperemia, chills, headache, apathy, and gastrointestinal disturbances. There is some evidence that patients with septicemic plague have a higher incidence of abdominal pain than patients with bubonic plague [83].
- ii.
- Pneumonic Plague: The pneumonic form has an incubation period of 1 to 3 days. Primary pneumonic plague is a rare but highly lethal form of the disease, mainly transmitted through droplets and aerosols via close contact (within 2 to 5 feet) with infected individuals. This form of the disease progresses rapidly from a febrile illness to severe pneumonia, producing cough and bloody sputum [84].
- iii.
- Bubonic Plague: Bubonic plague is the most frequent clinical form, occurring 2 to 10 days after inoculation with Y. pestis [85]. Clinical signs include hyperemia, myalgias, arthralgias, and apathy [74]. Another clinical sign is lymphadenomegaly, also known as “buboes,” with the femoral (~31%) and inguinal (~24%) lymph nodes being the most frequently affected, followed by the axillary (~22%) and cervical (~9%) lymph nodes [74,86].
In dogs, clinical signs may include fever, lethargy, anorexia, lymphadenopathy, vomiting, diarrhea, and abscesses [87]. Felids, including domestic cats, are highly susceptible, presenting with lymphadenopathy and unexplained high fever [88].
4.4. Diagnosis
The diagnosis of the disease requires laboratory confirmation, with the best method being a sample of pus from a bubo, blood, or sputum. Among the diagnostic methods, the rapid diagnostic test (RDT), which aims to detect the F1 antigen of Y. Pestis, is a practical tool that can be widely used, ensuring a wide range of tests and diagnostic coverage for the population, and can provide results in approximately 15 min [89].
The use of molecular tests, developed to ensure greater accuracy of results, such as conventional PCR, aims to amplify the genes pla, caf1, inv, and yopM [90,91], can ensure greater sensitivity in diagnoses, in addition to real-time PCR, which can be performed in just 2 h [92]. Other molecular tools, such as loop-mediated isothermal amplification (LAMP) technology, have also been developed [93].
The standard test for Y. pestis is bacterial isolation. Y. pestis grows in usual culture media; however, the use of selective agar supplemented with cefsulodin–irgasan–novobiocin (CIN) favors the isolation of the bacterium in polymicrobial samples, such as sputum. According to WHO recommendations, the patient should rinse their mouth with water before collecting the sample to reduce contamination by oral flora. After 2 or 3 days of incubation at 28 °C, suspected colonies on CIN agar can be identified by biochemical tests, PCR, and specific phage lysis for Y. pestis [94].
4.5. Treatment, Prevention, and Control
The recommended treatment includes the use of streptomycin or gentamicin in adult patients, including those who are immunocompromised and pregnant women. These drugs can be administered to children at a reduced dose. Alternatively, the combination of doxycycline, ciprofloxacin, and chloramphenicol can also be used for adults and children [95].
Preventive measures include informing people when zoonotic plague is present in their environment and advising them to take precautions against flea bites and avoid handling animal carcasses. Generally, people should be advised to avoid direct contact with infected body fluids and tissues. When handling potentially infected patients and collecting specimens, standard precautions should be applied. Surveillance and control require investigating the animal and flea species implicated in the plague cycle in the region and developing environmental management programs to understand the natural zoonotic cycle of the disease and limit its spread. Active long-term surveillance of animal foci, coupled with a rapid response during animal outbreaks, has successfully reduced the number of human plague outbreaks.
To effectively and efficiently manage plague outbreaks, it is crucial to have an informed and vigilant health care workforce (and community) to quickly diagnose and manage patients with infection, to identify risk factors, to conduct ongoing surveillance, to control vectors and hosts, to confirm diagnosis with laboratory tests, and to communicate findings with appropriate authorities [57].
Plague vaccines have been in existence for over a century. First-generation vaccines, while potentially effective, are limited by high rates of reactogenicity [96]. To date, there are no prequalified plague vaccines. The vaccine candidates in the most advanced stages are adjuvanted subunit vaccines based on the F1 and LcrV proteins [97,98,99,100]. All current vaccine candidates lack efficacy data in humans.
4.6. Recommendations
In areas with confirmed or suspected cases of zoonotic plague, it is essential to raise public awareness about the presence of the disease in the environment. People should avoid handling animal carcasses and any contact with potentially contaminated tissues or body fluids. When in or near forests or tall vegetation, individuals should minimize exposure by avoiding direct contact with the vegetation and wearing long-sleeved, light-colored clothing that fully covers the feet, ankles, and legs. The use of insect repellents containing DEET (N,N-diethyl-meta-toluamide) and thymol is recommended, as well as the use of citrus-scented essential oils around the home to help deter fleas. Pet care is also crucial—dogs should be taken to the vet regularly, and all pets should be kept up to date on deworming treatments. To reduce the rodent population, avoid accumulating garbage, especially materials like cardboard and old magazines. Lastly, avoid contact with wild rodents to further reduce the risk of exposure to the plague.
5. Salmonella
5.1. Etiology
Approximately 1 million people worldwide contract salmonellosis each year, resulting in 60,000 deaths [101]. Although the infection is commonly acquired through the ingestion of contaminated food, many cases are acquired through contact with animals [102,103]. It is a bacterium belonging to the Enterobacteriaceae family, rod-shaped, Gram-negative, facultative anaerobe, non-spore-forming, with an optimal growth temperature between 35 and 37 °C [104,105,106].
Salmonella can be divided into typhoidal and non-typhoidal serovars (NTS). Within the typhoidal serovar, we have Salmonella Typhi and Salmonella Paratyphi A, which cause typhoid and paratyphoid fever, respectively [107]. As for the non-typhoidal serovars of Salmonella, we have Typhimurium and Enteritidis [108]. The most prevalent species is S. enterica, with about 1500 serotypes, responsible for 99% of human and animal infections [109,110].
The genus Salmonella is divided into two species, S. enterica and S. bongori, with S. enterica being the most clinically relevant. It is further classified into six subspecies and over 2600 serotypes based on the Kauffmann–White scheme. Human-adapted typhoidal serovars include S. Typhi and S. Paratyphi A, B, and C, which are typically restricted to humans and cause enteric (typhoid) fever. In contrast, non-typhoidal Salmonella (NTS) serovars, such as S. Typhimurium and S. Enteritidis, have broad host ranges and are commonly acquired from animals or animal-derived food products. Other important NTS include S. Heidelberg, S. Newport, S. Dublin, S. Infantis, S. Agona, and S. Javiana, among others, which are also capable of zoonotic transmission. Rodents play a crucial role in maintaining and disseminating many non-typhoidal serovars across farms and food production systems, underscoring their importance as reservoirs [107,108,109,111].
5.2. Epidemiology
The main reservoir of Salmonella is warm-blooded animals, such as rodents [112]. On farms, rodents such as house mice are a significant source of infection. Among these species, the house mouse (Mus musculus) was identified as one of the rodents responsible for transmitting Salmonella Enteritidis infections among farm animals [113]. Other species, such as the roof rat (Rattus rattus), are also sources of S. Enteritidis infections [114,115]. The species Rattus rattus, Rattus norvegicus, and Mus musculus domesticus have been identified as sources of several Salmonella serotypes on poultry and swine farms [115,116,117,118].
The Typhimurium serotype is frequently isolated from captive and wild rodents, with intermittent fecal excretion lasting from weeks to months [119,120,121,122,123,124]. Pet rodents are an underrecognized source of human Salmonella infection [125]. In a Salmonella Typhimurium outbreak in Canada, rodents used to feed snakes supplied by a network of rodent breeders in Ontario were identified as the source of the outbreak. The cases likely acquired their illness through direct or indirect contact with these rodents [126]. In another study, Salmonella Typhimurium of the S. enterica serotype was isolated from 28 patients, 13 of whom reported exposure to hamsters, mice, or pet rats [125]. In Thailand, rats captured in eight traditional wet markets had a Salmonella prevalence of 49.10%, with 30% of these being Salmonella Typhimurium (30%) and 12.7% S. Weltevreden [127]. Of the 299 Salmonella isolates from rodents in several UK studies, S. Enteritidis and S. Typhimurium accounted for 58.5% and 28.4%, respectively [124].
Rodents can significantly amplify Salmonella contamination in the environment. As few as 15 Salmonella cells are sufficient to infect a rodent. Once colonized, a single fecal pellet from the infected animal can contain around 230,000 bacteria. Given that a rodent may excrete up to 100 pellets in a day, it can release over 23 million Salmonellae into its surroundings within 24 h. This substantial shedding can heavily contaminate barns and other environments, increasing the risk of food- or waterborne disease outbreaks (Figure 4).
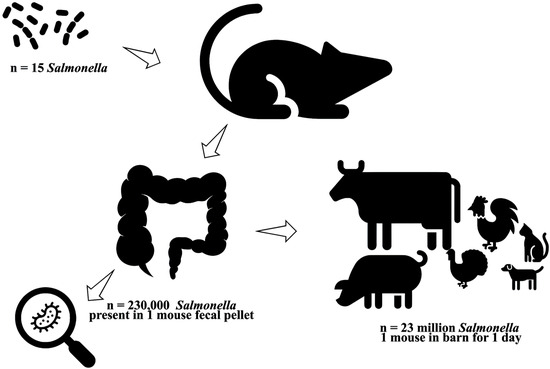
Figure 4.
Rodents as reservoirs, amplifiers, and vectors of Salmonella Infections. Rodents can significantly amplify environmental contamination: as few as 15 Salmonella cells can infect a rodent, and a single fecal pellet may contain approximately 230,000 bacteria. Given that a rodent may excrete up to 100 pellets per day, it can release over 23 million Salmonella cells into the environment within 24 h, heavily contaminating barns and other settings and increasing the risk of food- or waterborne outbreaks.
Salmonella is widely distributed in domestic and wild animals. They are prevalent in food animals, such as poultry, pigs, and cattle, as well as in pets, including cats, dogs, birds, and reptiles like turtles. Salmonella can circulate through the entire food chain from animal feed and primary production to households, food-service establishments, and institutions. In humans, Salmonella infections generally occur through the consumption of contaminated foods of animal origin (primarily eggs, meat, poultry, and milk). However, salmonellosis can also occur through the consumption of other foods, including green vegetables contaminated with manure. Person-to-person transmission can also occur through the faecal–oral route, which is of great epidemiological importance. Humans can also develop salmonellosis when individuals come into contact with infected animals, including pets. These infected animals often do not show signs of disease [128].
Like other zoonotic diseases, the increase in salmonellosis is related to rising temperatures [128,129]. Studies have shown that a 1 °C increase in temperature increases the estimated risk of salmonellosis by between 3% and 13% [130]. Climate change plays a significant role in the increase in Salmonella cases, leading to serious consequences for public health [131,132].
5.3. Clinical Signs
In humans, salmonellosis can cause symptoms such as fever, nausea, vomiting, abdominal pain, and headache [112]. Clinical signs typically begin 12 to 36 h after ingesting Salmonella, with an average illness duration of 2 to 7 days. Symptoms are mild, and the disease generally has a self-limiting course in affected individuals, who recover without specific treatment for salmonellosis [128]. However, in some cases, particularly in children and elderly patients, aggravating factors such as dehydration can be life-threatening. Although large Salmonella outbreaks usually attract media attention, the disease remains underreported, and the actual number of cases is unknown [128].
Non-typhoidal Salmonella (NTS) infections in humans are most frequently associated with self-limiting gastroenteritis, characterized by diarrhea, abdominal cramps, fever, and vomiting. However, in vulnerable populations such as young children, the elderly, and immunocompromised individuals, NTS can lead to invasive disease with bacteremia, focal infections (e.g., meningitis, osteomyelitis), and, rarely, septicemia [133,134]. Typhoidal Salmonella serovars, including S. Typhi and S. Paratyphi A, B, and C, cause enteric fever, a systemic illness characterized by prolonged fever, malaise, abdominal pain, hepatosplenomegaly, and, in severe cases, intestinal perforation. These invasive presentations underscore the importance of appropriate antimicrobial therapy in high-risk patients and those with enteric fever or bacteremia [133,135,136,137].
In cattle, Salmonella infections can result in clinical signs of enterocolitis, septicemia, and abortion [138]. Pneumonia is a relatively common clinical manifestation in calves [139,140]. Horses may present fever, diarrhea, or leukopenia [141]. Most Salmonella infections in dogs and cats are asymptomatic. In these animals, the most common clinical signs are acute enterocolitis and septicemia, with consequent endotoxemia. Rare syndromes include conjunctivitis in cats and in utero infections in dogs and cats, resulting in abortions, stillbirths, or the birth of weak kittens. Acute enterocolitis usually develops within 3 to 5 days of exposure and is limited to mucosal invasion. It manifests as watery or mucoid diarrhea, containing blood in severe cases, accompanied by vomiting, fever (40–41 °C), loss of appetite or anorexia, lethargy, abdominal pain, and progressive dehydration [142].
5.4. Diagnosis
One of the diagnostic methods used for Salmonella is microbial culture. Most diagnostic laboratories use a combination of selective enrichment broth followed by subculture to one or more selective agar plates, and then identify presumptive Salmonella colonies using biochemical techniques [143]. The use of molecular biology techniques, such as PCR, enables the detection of Salmonella in a wide range of samples, including water and human stool samples [144,145,146]. It is recommended that PCR after overnight enrichment in a nonselective broth be adopted as the gold standard, and that all positive PCR samples be cultured using selective enrichment [147,148].
5.5. Treatment, Prevention, and Control
In the setting of NTS bacteremia or disseminated disease, initial therapy should be with third-generation cephalosporins, such as ceftriaxone, for at least 7 to 10 days. Once bacterial susceptibilities are known, antibiotic treatment can be transitioned to azithromycin or a fluoroquinolone.
For enteric fever, the antibiotic treatment of choice is a fluoroquinolone. In cases of resistance, alternative antibiotics, such as third-generation cephalosporins and azithromycin, are recommended as an alternative. Fluoroquinolones are not used as frequently in children as they are in adults, and alternatives such as azithromycin are often preferred. The typical treatment duration is 10 to 14 days. In cases of severe enteric fever with symptoms of delirium, obtundation, stupor, or shock, additional treatment with corticosteroids may be considered. Dexamethasone at a dose of 3 mg/kg, followed by 1 mg/kg every 6 h for 48 h, has been shown to reduce mortality [149].
Prevention requires control measures at all stages of the food chain, from agricultural production to processing, manufacturing, and preparation of foods in both commercial establishments and at home. Preventive measures for Salmonella in the home are similar to those used against other foodborne bacterial diseases (see recommendations for food handlers below). The contact between infants/young children and pet animals that may be carrying Salmonella needs careful supervision [128].
Wild rodents can act as hosts for a variety of pathogens and transmit them to other farm animals through their feces, which can contaminate food and water throughout the farm. Therefore, frequent disinfection is required [150]. In addition, it is necessary to adopt measures such as the disposal of garbage and bedding, adequately filling any holes or openings to prevent access by mice, and storing supplies in a clean area to prevent access by rodents [151].
Only licensed vaccines are available against S. Typhi, the leading cause of typhoid fever. These include the orally administered live attenuated Ty21a vaccine, which protects against S. Typhi and offers some cross-protection against S. Paratyphi B, but not against S. Paratyphi A [152,153]. Additionally, there are injectable Vi capsular polysaccharide and conjugate vaccines, which are highly effective in protecting against S. Typhi. The Typbar-TCV vaccine, composed of S. Typhi Vi polysaccharide conjugated to tetanus toxoid, was over 80% effective in Phase 3 trials in children aged 9 months to 16 years [154].
5.6. Recommendations
To prevent rodent infestation and related health risks, it is crucial to eliminate factors that encourage their presence and reproduction. This includes maintaining clean and organized environments, avoiding the accumulation of garbage and debris, storing food in airtight containers, and sealing any cracks, holes, or other access points. Garbage should be collected regularly at the regional level, and food must be stored safely, with no leftovers left exposed. Areas that are not frequently visited should be cleaned routinely. Additionally, practicing proper hygiene is essential: wash hands thoroughly, especially before handling food and after using the bathroom, and ensure kitchen utensils are cleaned adequately before food preparation. Safe food and water practices must also be followed. Only drink filtered or boiled water. Avoid consuming raw or undercooked meat. Ensure eggs are well-cooked, and drink only pasteurized or boiled milk.
6. Francisella
Tularemia is a zoonosis bacteriosis caused by the bacterium Francisella tularensis. The disease occurs naturally in lagomorphs (rabbits and hares) and rodents, especially microtine rodents such as voles, vole rats, and muskrats, and also in beavers. Furthermore, several other mammals can be infected by the bacterium, and it has been isolated from birds, fish, amphibians, arthropods, and protozoa [155].
6.1. Etiology
Tularemia is a zoonotic bacterial disease caused by Francisella tularensis, a highly infectious, Gram-negative coccobacillus found throughout the Northern Hemisphere [156].
The bacterium is classified into two types: Type A (subsp. tularensis), associated with a terrestrial transmission cycle and found throughout North America, and Type B (Subsp. holarctica), associated with aquatic environments and found in North America, Australia, Japan, and Europe [157,158,159]. Francisella tularensis Subsp. Tularemia (Type A) is primarily associated with lagomorphs in North America and is typically transmitted by ticks, biting flies, or direct contact with infected animals. It is highly virulent for humans and domestic rabbits, and most isolates ferment glycerol. Francisella tularensis subsp. holarctica (Type B) occurs mainly in aquatic rodents (beavers, muskrats) and voles in North America, and lagomorphs (hares) and rodents in Eurasia. It is primarily transmitted through direct contact or by arthropods (primarily ticks and mosquitoes), but may also be transmitted via inhalation, contaminated water, or contaminated food. It is less virulent for humans and domestic rabbits, and does not ferment glycerol [155]. The species and subspecies of the genus Francisella are organized in the table below (https://lpsn.dsmz.de/search?word=Francisella), (accessed on 22 April 2025).
6.2. Epidemiology
Rodents and lagomorphs are maintenance hosts of F. tularensis, with small wild mammals being important reservoirs [160,161]. Due to their high reproduction rates and short lifespan, rodents are ideal hosts for F. tularensis [162]. In Finland, specific DNA of F. tularensis was detected in field voles (Microtus agrestis) [163]. The most common reservoirs are Arvicola terrestris (water vole) and Microtus arvalis (field vole), as well as Rattus rattus (black rat) in Europe, Arvicola terrestris, Mus musculus, and hares in the Lepus genus in Russia, and hares in the Sylvilagus genus in North America [164].
Rabbits, hares, and rodents are especially susceptible and often die in large numbers during outbreaks [154]. Transmission can occur through direct contact with infected animals, via ticks, mosquitoes, and fleas, or by ingesting contaminated water. People can become infected in several different ways, including bites from ticks and deer flies, as well as contact with infected animals (especially rodents, rabbits, and hares) [154]. In regions with Type B Tularemia, ingesting water from lakes and rivers, as well as consuming contaminated vegetables without proper hygiene, poses a significant health risk [165,166]. Additionally, transmission can occur by inhaling contaminated aerosols and through contact with the skin of infected animals (Figure 5).
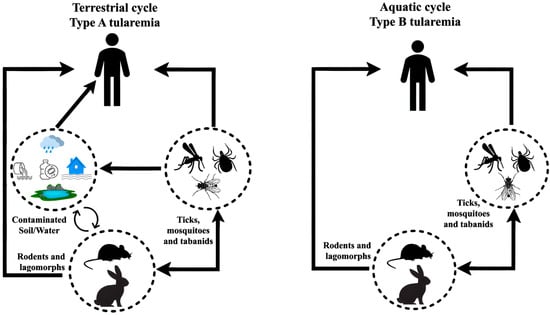
Figure 5.
Transmission of tularemia to humans by ticks and the life cycle of F. tularensis in nature. Two primary transmission cycles exist: the terrestrial cycle, involving wild rodents, lagomorphs, and arthropod vectors, and the aquatic cycle, in which contaminated water sources play a crucial role in human infections.
It is essential to strengthen scientific and research capacity on climate change and health to provide a more accurate assessment of the impact of climate change on vector- and rodent-borne infectious diseases, particularly in the context of One Health [167].
6.3. Clinical Signs
In sensitive animals, clinical signs of severe depression can be observed, followed by a fatal septicaemia. The disease lasts from 2–10 days, and affected animals die within a few days. Most domestic species do not usually manifest signs of tularemia infection, but they do develop specific antibodies against the bacteria.
Outbreaks with high mortality caused by the Type A organism have occurred in sheep. Among domestic pets, F. tularensis infection can result in clinical illness in cats, but it is less commonly seen in dogs. Both have been implicated in the transmission of the disease to humans, from cats to humans, most commonly via bites or scratches, and from dogs via close facial contact, ticks, and the retrieval of carcasses, as well as bites [155].
The first human case of tularemia was confirmed in 1914 in a patient presenting with ocular inflammation, characterized by ulcers and swelling of the eyelid, which progressed to enlarged lymph nodes, abscesses, and hyperemia [168].
In humans, clinical manifestations are divided into forms, including ulceroglandular, glandular, oculoglandular, oropharyngeal, respiratory, and typhoidal [157]. Ulceroglandular tularemia, the most common form of the disease, occurs after the introduction of bacteria into the skin through an arthropod bite or during the handling of infected carcasses, with a low mortality rate [169].
A prevalent form is oropharyngeal tularemia, resulting from the ingestion of contaminated water [156,166]. The respiratory form, resulting from the inhalation of aerosolized bacteria, can reach a mortality rate of up to 30% [157]. The oculoglandular form is rare and typically occurs through the manual transmission of bacteria to the eye or via contaminated droplets. Individuals affected by this form develop painful conjunctivitis and swelling around the eye, associated with edema of the lymph nodes located near the ear or neck [170]. Patients with glandular forms of tularemia do not have an identified ulcer. Approximately 30% of patients with adenopathy progress to lymph node abscesses that require surgical removal [171]. Patients presenting the typhoid or septicemic form may develop hyperemia, prostration, and, sometimes, neurological and/or digestive disorders, such as vomiting, diarrhea, and abdominal pain, without a detected entry point [170].
In dogs, clinical signs such as lethargy, pyrexia, anorexia, and lymphadenopathy may occur [172]. In young adult dogs with experimentally induced infection, the disease manifests similarly to that resulting from natural exposure, but puppies may be affected more severely [173]. In cats, the disease is associated with nonspecific clinical signs such as lethargy, lymphadenopathy, oral ulcers, pyrexia, vomiting, hepatomegaly, and icterus [174]. The localised form manifests with chronically draining subcutaneous abscesses [175].
6.4. Diagnosis
Diagnostic methods include serology, culture, and PCR from clinical samples [170,176,177]. Blood cultures can be used in patients with F. tularensis bacteremia or from other clinical specimens, such as conjunctival or pharyngeal exudates, lymph node biopsies or suppurations, sputum samples, and cerebrospinal fluid. PCR-based methods are helpful in localized forms of tularemia when exudates or tissue samples can be obtained. Due to the limitations of diagnosis by culture and PCR, serology is the diagnostic method of choice [170,176,177].
6.5. Treatment, Prevention, and Control
The treatment of choice for tularemia includes aminoglycosides, tetracyclines, and fluoroquinolones. However, this treatment was primarily developed to address emergencies in the context of bioterrorism. Intravenous (IV) gentamicin is recommended for 7 to 14 days, depending on the severity of the disease, as the most effective treatment [178,179,180,181,182].
To prevent tularemia, it is essential to minimize exposure to ticks, deer flies, and potentially infected animals. When spending time outdoors, such as hiking, camping, or working in nature, individuals should use insect repellents containing DEET, picaridin, IR3535, Oil of Lemon Eucalyptus (OLE), para-menthane-diol (PMD), or 2-undecanone. Wearing long pants, long sleeves, and high socks helps protect the skin from insect bites. Any attached ticks should be promptly removed using fine-tipped tweezers. Drinking untreated surface water should also be avoided. During mowing or landscaping, it is essential not to mow over sick or dead animals. Checking the area beforehand can help reduce this risk. Although not yet formally studied, wearing masks while mowing may lower the risk of inhaling bacteria. For those who hunt, trap, or handle animals, particularly rabbits, muskrats, prairie dogs, and other rodents, wearing gloves is recommended. Game meat should always be cooked thoroughly before consumption [183].
In the 1960s, a live vaccine strain (LVS) Francisella vaccine was developed in the Soviet Union, attenuating F. holarctica [184]. This vaccine is currently not licensed in the US due to its inability to provide complete protection against virulent human F. tularensis. It concerns that it may revert to a more virulent form of the bacterium [185]. Although protection is dose-dependent and route-dependent, this demonstrates that the vaccine is not sufficient to protect against virulent Francisella exposure in most cases fully [185].
6.6. Recommendations
In endemic areas, individuals should protect themselves by using insect repellents containing DEET, picaridin, IR3535, oil of lemon eucalyptus (OLE), para-menthane-diol (PMD), or 2-undecanone. When entering wildlife areas, it is essential to wear light-colored, long clothing that fully covers the legs and arms to reduce exposure to ticks and other vectors. After engaging in outdoor activities such as hiking, camping, or trekking, individuals should carefully inspect their bodies for ticks. Gardeners should wear appropriate personal protective equipment (PPE), and all individuals should avoid contact with animal carcasses. Garbage should be collected regularly, and food scraps should not be left exposed; food must be stored in safe places. Unfrequented areas should be cleaned routinely to prevent the accumulation of vegetation and debris that could provide shelter for animals such as rodents. Hunters, especially in endemic areas, must use PPE such as rubber gloves and face masks when handling rabbits, hares, and rodents, and should wash their hands thoroughly with soap and water afterward. Wild animal and game meat must be thoroughly cooked before consumption. Additionally, people should avoid drinking untreated water from lakes and rivers and must ensure vegetables are properly washed before eating.
7. Borrelia
Lyme disease is a bacterial illness transmitted to humans through the bite of infected ticks. The disease is caused by bacteria in the family Borreliaceae, particularly Borrelia (Borreliella) burgdorferi s.l. Ticks become infected by feeding on animals that carry the bacteria in their blood. The bacteria are only transmitted by the bites of the ticks Ixodes ricinus and I. persulcatus (the former is common in most of Europe, while the latter is found in the Baltic countries and Finland). In the most affected regions, tick infection rates may exceed 10%. These areas are mainly located in central Europe; however, in recent years, there has been a spread of infected ticks toward northern latitudes (i.e., Scandinavia) [186].
7.1. Etiology
Lyme borreliosis (LB) is the most common tick-borne disease in the Northern Hemisphere [187,188]. It is a zoonotic disease caused by the Gram-negative spirochete bacteria belonging to the family Spirochaetaceae, specifically the Borrelia burgdorferi sensu lato complex (B. burgdorferi s.l.). The complex is divided into genospecies, including B. afzelii, B. burgdorferi sensu stricto, B. garinii, and possibly B. valaisiana [189,190,191].
7.2. Epidemiology
Approximately 476,000 cases are reported annually in the United States due to B. burgdorferi [192], and around 200,000 in Europe [187]. The disease is transmitted by Ixodes ticks, with rodents being the primary hosts of the bacteria [193]. In Europe, Ixodes ricinus is the primary vector of B. burgdorferi s.l. [194]. In eastern and central North America, the black-legged tick Ixodes scapularis is the primary vector [195,196]. Other species, such as Ixodes persulcatus and Ixodes pacificus, also contribute to transmission [197]. Among the reservoirs, the white-footed mouse (Peromyscus leucopus), native to eastern North America [198]; it is highly susceptible to infection [199,200].
Ticks carrying pathogenic strains of B. burgdorferi transmit infection while feeding on vertebrate hosts, such as humans [201,202,203]. Transmission occurs 48 to 72 h after the tick first attaches to the host [202]. Infected ticks are unlikely to transmit the organisms within the first few hours after feeding; however, the risk increases gradually with the duration of the blood meal [204] (Figure 6).
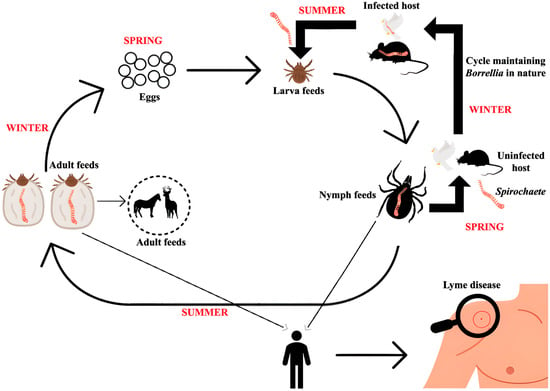
Figure 6.
Transmission cycle of Borrelia species. Rodents act as reservoir hosts, maintaining Borrelia in nature and supporting its persistence. Larval and nymphal ticks acquire the bacteria when feeding on infected rodents. Infected nymphs and adult ticks can then transmit Borrelia to vertebrate hosts, including humans, during blood meals. This cycle, involving rodents and ticks at different life stages, facilitates the continued circulation and environmental spread of Borrelia.
The enzootic cycle of Lyme borreliosis involves Ixodes ticks transitioning through larval, nymph, and adult stages. During their larval stage, ticks become infected with Borrelia burgdorferi sensu lato by feeding on infected reservoir hosts, primarily small mammals. Migratory birds play a dual role by spreading the spirochetes geographically and acting as reservoir hosts themselves. Humans, however, are incidental hosts who do not contribute to the transmission cycle.
Notably, climate change is expected to accelerate the spread of LB, making it crucial to implement preventive measures, including epidemiological and molecular surveillance, as well as promoting health education to the general public [205].
7.3. Clinical Signs
In humans, the disease has an incubation period of 3 to 30 days [206]. LB can manifest clinical signs such as fatigue, hyperemia, myalgia, erythema migrans, and cardiac and neurological signs [207]. In Europe, neuroborreliosis is the most frequently observed neurological sign in the early phase of LB [208].
In animals, clinical signs are barely noticeable [209,210]. In white-footed mice (Peromyscus leucopus), no effect of the infection on animal survival was observed [210,211].
In dogs, lameness may be observed, often with associated hyperemia and anorexia. Arthritis commonly occurs in a single joint, most often in the carpus or tarsus [212]. Clinical signs observed in horses include lethargy, low-grade fever, and stiffness and swelling of the distal appendicular joints [213].
7.4. Diagnosis
According to CDC guidelines, a two-step testing process should be adopted for serological testing for Lyme disease. Both steps are required and can be done using the same blood sample. If this first step is negative, no further testing is necessary. However, if the first step yields a positive or indeterminate result (sometimes referred to as “equivocal”), the second step should be performed. The final result is only positive when the first test is positive (or equivocal) and the second test is positive (or, for some tests, equivocal). Standard two-tier testing (STTT) employs enzyme immunoassay (EIA) as the initial step and Western blotting (WB) as the second step. Increasingly, laboratories are using modified two-tier testing (MTTT) in which both assays are EIAs [214].
7.5. Treatment, Prevention, and Control
Prevention of Lyme borreliosis following a tick bite has been reported using a single dose of doxycycline [215]. The use of doxycycline, amoxicillin, and cefuroxime axetil for 14 days in patients with advanced clinical symptoms has also demonstrated efficacy [216].
The primary methods of preventing infection are avoiding tick bites and promptly removing attached ticks. The most effective tick-bite avoidance strategies include wearing protective clothing, like long trousers and long-sleeved shirts, and using tick repellents. The skin should be checked periodically for attached ticks, which should be removed using tweezers or fine-pointed forceps. For safe removal, grasp the tick as closely as possible to the skin, pulling gently upwards and trying not to break off the mouthparts. The risk of borrelial infection is not increased if the tick’s mouth parts are left behind. It is recommended to use a skin disinfectant after tick removal to prevent pyogenic infection. When searching for attached ticks, pay particular attention to skin folds, the groin area, armpits, under the breasts, the waistband area, and the backs of the knees, as ticks tend to seek out more humid areas for attachment. In children, the head, including the scalp, and neck should be carefully checked, as tick bites are relatively more common in these areas in this age group. An effective preventive measure for individuals with intense tick exposure, such as forestry workers, rural workers, or military personnel on active duty, is the use of permethrin-impregnated clothing [204].
Targeting ticks on mice with acaricides has also been used, either by using bait boxes that coat mice as they enter a feeder, nesting material impregnated with an acaricide that transfers the agent to the fur of mice in the nest, or oral feeding with baits containing acaricides [217].
Currently, there is no vaccine available for humans against Lyme disease, also known as Lyme borreliosis [218]. However, an alternative vaccine is the OspA-based vaccine (LYMErix). Outer surface protein A (OspA) has been the basis for at least two different vaccines, LYMErix (SmithKline Beecham) and ImuLyme (Pasteur-Mé-rieux-Connaught). However, only LYMErix was licensed and available to consumers from 1998 until 2002, when it was voluntarily withdrawn from the market [219].
7.6. Recommendations
To prevent exposure to rodents and ticks, it is essential to implement preventive rodent control measures, such as avoiding the accumulation of garbage and debris, storing food in tightly sealed containers, and installing metal screens on air and sewage inlets. People should avoid areas where ticks may be present, such as lawns, forests, and wildlife habitats. The use of effective tick repellents is highly recommended. In endemic regions, individuals should apply insect repellents containing DEET, picaridin, IR3535, oil of lemon eucalyptus (OLE), para-menthane-diol (PMD), or 2-undecanone, and wear light-colored, long clothing that fully covers the legs and arms when entering wildlife areas. After potential exposure to ticks, such as visiting tick-infested environments, individuals should promptly remove their clothing, wash it, take a shower, and carefully inspect their body, especially hairy areas, for ticks. If a tick is found, it should be removed using fine-tipped tweezers, gripping it at the head or mouth parts close to the skin and pulling it out slowly. Ticks should never be handled with bare or unprotected hands. When hiking or trekking, it is also advised to avoid sitting on the ground, vegetation, or rocks, and to stay on designated paths and trails to minimize contact with ticks.
8. Rickettsia
Rickettsiae are intracellular bacteria responsible for causing vector-borne zoonotic diseases worldwide [220,221]. Rickettsiae can be transmitted by ticks, fleas, lice, and mites, infecting domestic animals, wild animals, and humans, thus representing diseases of direct implication to human health [222].
8.1. Etiology
Rickettsioses (Rickettsiales: Rickettsiaceae) are diseases that severely impact public health, caused by intracellular, Gram-negative bacteria transmitted by ticks, performing enzootic or epizootic cycles in wild vertebrate hosts [223]. These diseases have gained greater notoriety in the medical and scientific communities in recent years [224]. In recent years, several species of bacteria have been incriminated as pathogens in humans [220]. This bacterial genus can be divided into the spotted fever group (SFG) and the typhus group (TG) [225], with the majority belonging to the central group of classical spotted fever [226].
8.2. Epidemiology
Regardless of the length of travel (short- or long-term), all age groups are at risk for rickettsial infections during visits to endemic areas. Transmission risk increases with the time spent participating in outdoor activities, particularly during seasons when the vector is at its peak in terms of feeding and lifecycle activity. In many parts of the world, however, rickettsial infections occur year-round. The most commonly diagnosed rickettsial diseases in travelers belong to the spotted fever or typhus groups; notably, rickettsial infections can also be caused by emerging and newly recognized species [227]. Numerous species of Rickettsia are associated with diseases in humans, as well as various vectors (Table 1).

Table 1.
Main features of rickettsiosis.
Most rickettsial pathogens are transmitted directly to humans by infected arthropod vectors (i.e., fleas, lice, mites, or ticks) during feeding. In addition, pathogens can also be transmitted when a person accidentally inoculates Rickettsiae into the arthropod bite wound (or other breaks in the skin); this transmission can occur by scratching skin contaminated with infectious arthropod fluids or feces, or by crushing the arthropod vector at the bite site. Another possible transmission route is through inhalation of Rickettsiae or inoculation of the conjunctiva with infectious material. While possible, transmission of some rickettsiae through transfusion of infected blood products or organ transplants is less common, especially Anaplasma and Ehrlichia species [227] (Figure 7).
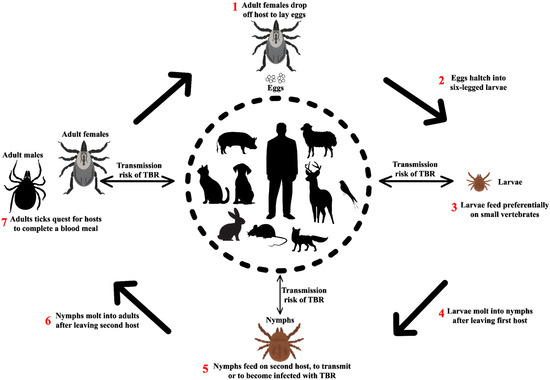
Figure 7.
Transmission of Rickettsia to humans by ticks and its natural life cycle. Rodents act as reservoir hosts, perpetuating Rickettsia in nature and maintaining the epidemiological cycle. Larval and nymphal ticks acquire the bacteria when feeding on infected rodents. Infected nymphs and adult ticks can then transmit Rickettsia to vertebrate hosts, including humans, during blood meals.
The impact of climate change on vector-borne and rodent-borne infectious diseases is essential [167]. The increase in rickettsial infections is catalyzed by several factors, including climate change [220,238,239].
8.3. Clinical Signs
Several Rickettsia species are responsible for distinct illnesses within the spotted fever and typhus group, each with characteristic clinical presentations. R. conorii causes Mediterranean spotted fever (MSF), marked by fever, headache, a maculopapular rash affecting the palms and soles, and a black eschar (“tache noire”) at the tick bite site [240]. R. rickettsii leads to Rocky Mountain spotted fever (RMSF), presenting with fever, headache, muscle pain, and a maculopapular rash that may become purpuric in severe cases [231]. Japanese spotted fever (JSF), caused by Rickettsia japonica, typically presents with symptoms including fever, asthenia, myalgia, rash, and anorexia [234]. R. africae is responsible for African tick-bite fever (ATBF), characterized by fever, skin lesions (eschar), rash, lymphangitis, headache, myalgia, and lymphadenopathy [241]. R. conorii can develop Mediterranean spotted fever (MSF), resulting in clinical symptoms such as fever, headache, maculopapular rash affecting the palms of the hands and soles of the feet, and the presence of an eschar, the “tache noire”, at the site of the tick bite [240].
In a study evaluating clinically ill cats for evidence of rickettsial infection, no association was found between antibody positivity and fever, and no febrile cats tested positive for R. felis or R. rickettsii [242]. In an experimental infection of dogs with a Brazilian strain of Rickettsia rickettsi fever, it was possible to observe lethargy, anorexia, ocular lesions, thrombocytopenia, and anemia [243]. In dogs, RMSF manifests with fever, lethargy, decreased appetite, tremors, scleral injection, a maculopapular rash on the ears and exposed skin, and petechial lesions on the mucous membranes [243,244,245].
8.4. Diagnosis
Methods for diagnosing rickettsial infections include serology, such as indirect immunofluorescence assay and enzyme-linked immunosorbent assay (ELISA) for IgM or IgG [246]. Molecular tests, such as PCR, are capable of detecting rickettsiae in the acute phase of the disease [247].
8.5. Treatment, Prevention, and Control
In infections caused by Rickettsia conorii, the gold standard treatment is doxycycline at a dose of 200 mg per day [240]. Doxycycline is also the treatment of choice in cases of infection by Rickettsia rickettsii [248], Rickettsia japonica [249], Rickettsia typhi [250], Rickettsia prowazekii [251], Rickettsia akari [248], and Rickettsia africae [252].
There is no vaccine available to prevent rickettsial infections. Antibiotic prophylaxis is not recommended for rickettsiae, and antimicrobial agents should not be given to asymptomatic individuals. Travelers to areas endemic for rickettsiae should seek to minimize their exposure to infectious arthropods (including fleas, lice, mites, and ticks) and avoid animal reservoirs (particularly dogs and rats) [227].
It is not necessary to exclude individuals with rickettsial infections from daycare, preschool, school, or the workplace. It is recommended that long-sleeved protective clothing and a wide-brimmed hat be worn to reduce the risk of infection when engaging in activities where human contact with ticks, lice, mites, or fleas may occur, such as hiking and camping in areas where these pests are present. It is also recommended that you use insect repellent containing DEET or picaridin and examine your skin for possible bites (especially behind the ears, on the back of the head, in the groin, armpits, and behind the knees) [253].
The first whole-cell antigen (WCA) vaccines against R. prowazekii and R. rickettsii were produced in the 1920s. This vaccine was used on German soldiers during World War II [254]. A similar vaccine was developed by the US military, which helped alleviate the disease [255]. Another vaccine option against epidemic typhus was produced by isolating R. prowazekii from the lungs of infected rabbits (Castaneda vaccine) [255] or from the vaginal tunica and peritoneum of infected rats (Zinsser-Castaneda vaccine) [256]. Similarly, in the 1970s, formalin-inactivated R. rickettsii in chicken embryonic fibroblasts [257,258] protected monkeys [259,260]. This vaccine ameliorated the disease in humans but did not prevent infection [261]. One alternative is the use of avirulent or attenuated bacteria, such as low-virulence strains of O. tsutsugamushi, which effectively induce the human immune system [262]. New strategies and vaccines with good immune induction, prolonged protection, and large-scale production capacity are needed.
8.6. Recommendations
In endemic areas, integrated pest control is crucial for disease prevention. It should involve a thorough environmental assessment, the use of physical barriers, controlled chemical applications, continuous monitoring, and community-based environmental education. Keeping pets dewormed is an essential preventive measure. Individuals in these regions should use insect repellents containing DEET, picaridin, IR3535, oil of lemon eucalyptus (OLE), para-menthane-diol (PMD), or 2-undecanone, especially when entering areas with wildlife. It is also advisable to wear light-colored, long-sleeved clothing that covers the legs and arms. During outdoor activities such as hiking or camping, individuals should stay on safe trails and avoid contact with potentially infested environments. Hunters, particularly in endemic regions, must use personal protective equipment (PPE), such as rubber gloves and face masks, when handling animals like rabbits, hares, and rodents. They should then wash their hands thoroughly with soap and water to minimize the risk of infection.
9. Future Research
We must understand that rodent-borne diseases are fully embedded in the context of One Health (Figure 8). Therefore, broader and more in-depth research is needed on the epidemiological role of rodents as pathogen reservoirs and their relationship within the One Health context [4]. It is crucial to study how deforestation, urbanization, and climate change can alter rodent behavior and distribution, influencing the risk of pathogen transmission [263]. At the same time, rodent genomic and microbiota analysis must be expanded to identify genes associated with resistance or susceptibility to pathogens. The use of artificial intelligence must be explored and utilized rationally and positively, such as in the development of mathematical and computational models that connect ecological, climatic, and population information and data to predict outbreaks of rodent-borne diseases. At the same time, we must seek innovative and sustainable methods of rodent control.
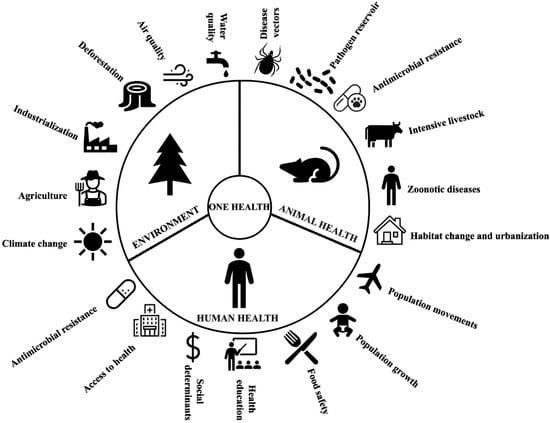
Figure 8.
The role of rodents in the One Health context: integrating environmental, animal, and human health to understand and prevent rodent-borne diseases.
10. General Recommendations
To efficiently manage rodent-borne zoonotic illnesses, the following priorities are recommended:
- 1.
- Public Health and Prevention
- −
- Awareness Campaigns: Educate communities endemic for rodents and rodent-borne diseases on rodent-borne illnesses, transmission, and preventive measures.
- −
- Personal Protection: Promote the utilization of protective equipment, insect repellents (e.g., DEET, picaridin), and proper hygiene measures.
- −
- Rodent Control: Employ integrated pest management (IPM) practices, such as sanitation, rodent-proofing buildings, and secure waste disposal.
- −
- Vector Control: Regulate tick and flea populations through environmental management, acaricides, and treatment of pets.
- 2.
- Health care and Diagnostics
- −
- Early Diagnosis: Enhance laboratory infrastructure to achieve early diagnosis of zoonotic pathogens using molecular (PCR) and serological diagnostic methods.
- −
- Antibiotic Stewardship: Support proper use of antimicrobials to prevent resistance, particularly in leptospirosis and rat-bite fever.
- −
- Vaccination Research: Develop vaccine research for high-mortality diseases like plague and tularemia.
- 3.
- Environmental and One Health Strategies
- −
- Climate Adaptation: Monitor climate-driven fluctuations in rodent and vector populations for predicting disease outbreaks.
- −
- Ecosystem Management: Re-establish ecosystems to minimize human-rodent interaction and maintain biodiversity.
- −
- Wildlife Surveillance: Regularly monitor rodent and arthropod populations in high-risk zones.
- 4.
- Policy and Global Coordination
- −
- International Reporting: Improve international surveillance networks for prompt reporting of zoonotic outbreaks.
- −
- Regulatory Measures: Enact stricter rodent trade, pet keeping, and food hygiene regulations to minimize risks of transmission.
- −
- One Health Frameworks: Promote interspecies collaboration among veterinarians, ecologists, and public health professionals to respond to zoonotic threats integratively.
11. Conclusions
Rodents play a significant but often underrecognized role in the emergence and transmission of zoonotic bacterial infections. Due to their ability to adapt to diverse habitats, including cities, they coexist closely with human and domestic animal populations. This review highlights the major public health threats posed by rodent-borne pathogens, including Leptospira, Streptobacillus moniliformis, Yersinia pestis, Salmonella, Francisella tularensis, Borrelia burgdorferi, and Rickettsia spp. These agents cause diseases that range across the spectrum from mild febrile illnesses to life-threatening illnesses, with transmission occurring through direct contact, a contaminated environment, or an arthropod vector. Climate change, urbanization, and habitat encroachment facilitate the transmission of these diseases by altering rodent population dynamics, vector distribution, and increasing the risk of exposure to humans. The One Health approach, which synthesizes human-animal-environment interactions, is essential in managing these risks. Increased surveillance, diagnostic capabilities, and interdisciplinary coordination are the building blocks for preventing outbreaks and reducing disease burden. Rodents are sneaky carriers of deadly pathogens that necessitate concerted and preventive measures to stop their impact. Through a synergistic approach combining scientific research, public health interventions, and environmental conservation, it is possible to reduce the prevalence of rodent-borne diseases and safeguard global health in an era of environmental and climatic change.
Author Contributions
Conceptualization, S.B., P.M.D., A.A.S. and A.J.R.-M.; methodology, S.B., P.M.D., A.A.S. and A.J.R.-M.; resources, P.M.D. and A.J.R.-M.; writing—original draft preparation, S.B., P.M.D., A.A.S. and A.J.R.-M.; writing—review and editing, S.B., P.M.D., A.A.S. and A.J.R.-M.; supervision, S.B., P.M.D., A.A.S. and A.J.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This article has been registered in the Research Proposal Registration of the Coordination of Scientific Integrity and Surveillance of Universidad Cientifica del Sur, Lima, Peru, under the number PI-50-2025-0792.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Keesing, F.; Ostfeld, R.S. Emerging Patterns in Rodent-Borne Zoonotic Diseases. Science 2024, 385, 1305–1310. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-Borne Diseases and Their Risks for Public Health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Shehata, A.A.; Parvin, R.; Tasnim, S.; Duarte, P.M.; Basiouni, S. Rodent-Borne Parasites and Human Disease: A Growing Public Health Concern. Animals 2025, 15, 2681. [Google Scholar] [CrossRef]
- Shehata, A.A.; Parvin, R.; Tasnim, S.; Duarte, P.M.; Rodriguez-Morales, A.J.; Basiouni, S. The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health. Viruses 2025, 17, 809. [Google Scholar] [CrossRef]
- Cavia, R.; Cueto, G.R.; Suárez, O.V. Techniques to Estimate Abundance and Monitoring Rodent Pests in Urban Environments. In Integrated Pest Management and Pest Control—Current and Future Tactics; Soloneski, S., Larramendy, M.L., Eds.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0050-8. [Google Scholar][Green Version]
- Garden, J.; Mcalpine, C.; Peterson, A.; Jones, D.; Possingham, H. Review of the Ecology of Australian Urban Fauna: A Focus on Spatially Explicit Processes. Austral Ecol. 2006, 31, 126–148. [Google Scholar] [CrossRef]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of Zoonotic Pathogens and Characterization of Novel Viruses Carried by Commensal Rattus Norvegicus in New York City. mBio 2014, 5, e01933-01914. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.M. Environmental and Biological Determinants for the Prevalence of Leptospirosis among Wild Small Mammal Hosts, Island of Hawaii. Int. J. Zoonoses 1984, 11, 173–188. [Google Scholar] [PubMed]
- Levett, P.N.; Morey, R.E.; Galloway, R.L.; Steigerwalt, A.G. Leptospira broomii sp. nov., Isolated from Humans with Leptospirosis. Int. J. Syst. Evol. Microbiol. 2006, 56, 671–673. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the Taxonomy and Evolution of Pathogenicity of the Genus Leptospira through the Prism of Genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Abd Rahman, A.N.; Hasnul Hadi, N.H.; Sun, Z.; Thilakavathy, K.; Joseph, N. Regional Prevalence of Intermediate Leptospira spp. in Humans: A Meta-Analysis. Pathogens 2021, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Amaddeo, D.; Ieradi, L.A.; Autorino, G.L.; Perrella, D. Leptospirosis in Wild Rodents Living in Urban Areas (Rome—Italy). In Proceedings of the 1st European Congress of Mammalogy, Lisboa, Portugal, 18–23 March 1996; pp. 105–114. [Google Scholar]
- Strand, T.M.; Löhmus, M.; Persson Vinnersten, T.; Råsbäck, T.; Sundström, K.; Bergström, T.; Lundkvist, Å. Highly Pathogenic Leptospira Found in Urban Brown Rats (Rattus norvegicus) in the Largest Cities of Sweden. Vector Borne Zoonotic Dis. 2015, 15, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Desvars-Larrive, A.; Pascal, M.; Gasqui, P.; Cosson, J.-F.; Benoît, E.; Lattard, V.; Crespin, L.; Lorvelec, O.; Pisanu, B.; Teynié, A.; et al. Population Genetics, Community of Parasites, and Resistance to Rodenticides in an Urban Brown Rat (Rattus norvegicus) Population. PLoS ONE 2017, 12, e0184015. [Google Scholar] [CrossRef]
- Fischer, S.; Mayer-Scholl, A.; Imholt, C.; Spierling, N.G.; Heuser, E.; Schmidt, S.; Reil, D.; Rosenfeld, U.M.; Jacob, J.; Nöckler, K.; et al. Leptospira Genomospecies and Sequence Type Prevalence in Small Mammal Populations in Germany. Vector Borne Zoonotic Dis. 2018, 18, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira Infection in Rats: A Literature Review of Global Prevalence and Distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in Humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef]
- Mori, M.; Bourhy, P.; Le Guyader, M.; Van Esbroeck, M.; Djelouadji, Z.; Septfons, A.; Kodjo, A.; Picardeau, M. Pet Rodents as Possible Risk for Leptospirosis, Belgium and France, 2009 to 2016. Euro Surveill. 2017, 22, 16-00792. [Google Scholar] [CrossRef]
- Kraijenhoff, G.H.P.S.; van Zoest, J.K.G.C.M.; van den Brand, J.; De Schryver, E.L.L.M. [Severe paediatric leptospirosis caused by a pet rat]. Ned. Tijdschr. Geneeskd. 2022, 166, D6017. [Google Scholar]
- Gonçalves, A.J.; de Carvalho, J.E.; Guedes e Silva, J.B.; Rozembaum, R.; Vieira, A.R. Hemoptysis and the adult respiratory distress syndrome as the causes of death in leptospirosis. Changes in the clinical and anatomicopathological patterns. Rev. Soc. Bras. Med. Trop. 1992, 25, 261–270. [Google Scholar] [CrossRef]
- Kuriakose, M.; Eapen, C.K.; Punnoose, E.; Koshi, G. Leptospirosis—Clinical Spectrum and Correlation with Seven Simple Laboratory Tests for Early Diagnosis in the Third World. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 419–421. [Google Scholar] [CrossRef]
- Sullivan, N.D. Leptospirosis in animals and man. Aust. Vet. J. 1974, 50, 216–223. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM Small Animal Consensus Statement on Leptospirosis: Diagnosis, Epidemiology, Treatment, and Prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Divers, T.J.; Chang, Y.-F.; Irby, N.L.; Smith, J.L.; Carter, C.N. Leptospirosis: An Important Infectious Disease in North American Horses. Equine Vet. J. 2019, 51, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Fouché, N.; Graubner, C.; Lanz, S.; Schweighauser, A.; Francey, T.; Gerber, V. Acute kidney injury due to Leptospira interrogans in 4 foals and use of renal replacement therapy with intermittent hemodiafiltration in 1 foal. Vet. Intern. Med. 2020, 34, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.V.; Senthilkumar, K.; Rai, P.; Kabekkodu, S.P.; Karunasagar, I.; Kumar, B.K. Current Methods for the Diagnosis of Leptospirosis: Issues and Challenges. J. Microbiol. Methods 2022, 195, 106438. [Google Scholar] [CrossRef]
- Petakh, P.; Behzadi, P.; Oksenych, V.; Kamyshnyi, O. Current Treatment Options for Leptospirosis: A Mini-Review. Front. Microbiol. 2024, 15, 1403765. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Xu, Y.; Ye, Q. Human Leptospirosis Vaccines in China. Hum. Vaccin. Immunother. 2018, 14, 984–993. [Google Scholar] [CrossRef]
- Silveira, M.M.; Oliveira, T.L.; Schuch, R.A.; McBride, A.J.A.; Dellagostin, O.A.; Hartwig, D.D. DNA Vaccines against Leptospirosis: A Literature Review. Vaccine 2017, 35, 5559–5567. [Google Scholar] [CrossRef]
- Kämmerer, T.; Lesmeister, T.; Wollenberg, A.; French, L.E.; Strobel, E.; Reinholz, M. Rat Bite Fever, a Diagnostic Challenge: Case Report and Review of 29 Cases. J. Dtsch. Dermatol. Ges. 2021, 19, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Julius, R.S.; Brettschneider, H.; Chimimba, C.T.; Bastos, A.D.S. Prevalence and Diversity of the Streptobacillus Rat-Bite Fever Agent, in Three Invasive, Commensal Rattus Species from South Africa. Yale J. Biol. Med. 2021, 94, 217–226. [Google Scholar]
- Fawzy, A.; Giel, A.-S.; Fenske, L.; Bach, A.; Herden, C.; Engel, K.; Heuser, E.; Boelhauve, M.; Ulrich, R.G.; Vogel, K.; et al. Development and Validation of a Triplex Real-Time qPCR for Sensitive Detection and Quantification of Major Rat Bite Fever Pathogen Streptobacillus moniliformis. J. Microbiol. Methods 2022, 199, 106525. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kasahara, K.; Lee, S.-T.; Ito, T.; Hasegawa, H.; Hirose, S.; Santo, S.; Yoshida, A.; Nakano, R.; Yano, H.; et al. Rat-Bite Fever in Human with Streptobacillus notomytis Infection, Japan. Emerg. Infect. Dis. 2018, 24, 1377–1379. [Google Scholar] [CrossRef]
- Matt, U.; Schmiedel, J.; Fawzy, A.; Trauth, J.; Schmidt, K.; Vogel, K.; Herold, S.; Karrasch, T.; Imirzalioglu, C.; Eisenberg, T. Infection in a Young Immunocompetent Male Caused by Streptobacillus felis, a Putative Zoonotic Microorganism Transmitted by Cats. Clin. Infect. Dis. 2021, 72, 1826–1829. [Google Scholar] [CrossRef]
- Michel, V.; Ulber, C.; Pöhle, D.; Köpke, B.; Engel, K.; Kaim, U.; Fawzy, A.; Funk, S.; Fornefett, J.; Baums, C.G.; et al. Clinical Infection in House Rats (Rattus rattus) Caused by Streptobacillus notomytis. Antonie Van Leeuwenhoek 2018, 111, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Fornefett, J.; Krause, J.; Klose, K.; Fingas, F.; Hassert, R.; Eisenberg, T.; Schrödl, W.; Grunwald, T.; Müller, U.; Baums, C.G. Comparative Analysis of Clinics, Pathologies and Immune Responses in BALB/c and C57BL/6 Mice Infected with Streptobacillus moniliformis. Microbes Infect. 2018, 20, 101–110. [Google Scholar] [CrossRef] [PubMed]
- De Cock, M.P.; De Vries, A.; Fonville, M.; Esser, H.J.; Mehl, C.; Ulrich, R.G.; Joeres, M.; Hoffmann, D.; Eisenberg, T.; Schmidt, K.; et al. Increased Rat-Borne Zoonotic Disease Hazard in Greener Urban Areas. Sci. Total Environ. 2023, 896, 165069. [Google Scholar] [CrossRef]
- Rothe, K.; Tsokos, M.; Handrick, W. Animal and Human Bite Wounds. Dtsch. Ärzteblatt Int. 2015, 112, 433–443. [Google Scholar] [CrossRef]
- Kache, P.A.; Person, M.K.; Seeman, S.M.; McQuiston, J.R.; McCollum, J.; Traxler, R.M. Rat-Bite Fever in the United States: An Analysis Using Multiple National Data Sources, 2001–2015. Open Forum Infect. Dis. 2020, 7, ofaa197. [Google Scholar] [CrossRef]
- Hadvani, T.; Vallejo, J.G.; Dutta, A. Rat Bite Fever: Variability in Clinical Presentation and Management in Children. Pediatr. Infect. Dis. J. 2021, 40, e439–e442. [Google Scholar] [CrossRef]
- Costa-Pinto, J.; Morley, C.; Hauser, S. A Case of Rat Bite Fever in a 12-year-old Boy. J. Paediatr. Child. Health 2017, 53, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.; Jordão, S.; Simões, M.J.; Oleastro, M.; Neves, I. A Rare Cause of Vertebral Osteomyelitis: The First Case Report of Rat-Bite Fever in Portugal. Rev. Soc. Bras. Med. Trop. 2019, 53, e20190328. [Google Scholar] [CrossRef]
- Crofton, K.R.; Ye, J.; Lesho, E.P. Severe Recurrent Streptobacillus moniliformis Endocarditis in a Pregnant Woman, and Review of the Literature. Antimicrob. Resist. Infect. Control 2020, 9. [Google Scholar] [CrossRef]
- Eisenberg, T.; Poignant, S.; Jouan, Y.; Fawzy, A.; Nicklas, W.; Ewers, C.; Mereghetti, L.; Guillon, A. Acute Tetraplegia Caused by Rat Bite Fever in Snake Keeper and Transmission of Streptobacillus moniliformis. Emerg. Infect. Dis. 2017, 23, 719–721. [Google Scholar] [CrossRef]
- Holden, F.A.; Mackay, J.C. Rat-Bite Fever—An Occupational Hazard. Can. Med. Assoc. J. 1964, 91, 78–81. [Google Scholar]
- Valverde, C.R.; Lowenstine, L.J.; Young, C.E.; Tarara, R.P.; Roberts, J.A. Spontaneous Rat Bite Fever in Non-human Primates: A Review of Two Cases. J. Med. Primatol. 2002, 31, 345–349. [Google Scholar] [CrossRef]
- Mahmoodi, E.; Grainge, C.; Erdstein, A.; Okane, G. Septic arthritis caused by pet rodents: A diagnostic dilemma. AMJ 2016, 09. [Google Scholar] [CrossRef]
- Kelly, A.J.; Ivey, M.L.; Gulvik, C.A.; Humrighouse, B.W.; McQuiston, J.R. A Real-Time Multiplex PCR Assay for detection of the causative agents of rat bite fever, Streptobacillus moniliformis and zoonotic Streptobacillus species. Diagn. Microbiol. Infect. Dis. 2021, 100, 115335. [Google Scholar] [CrossRef]
- Pal, M.; Paulos Gutama, K. Rat Bite Fever: An Infectious Under Reported Bacterial Zoonotic Disease. Am. J. Public Health 2023, 11, 84–87. [Google Scholar] [CrossRef]
- Hryciw, B.; Wright, C.; Tan, K. Rat Bite Fever on Vancouver Island: 2010-2016. Can. Commun. Dis. Rep. 2018, 44, 215–219. [Google Scholar] [CrossRef]
- Mathé, P.; Schmidt, K.; Schindler, V.; Fawzy, A.; Schultze, T.; Voll, R.E.; Pauli, D.; Popova, M.; Schauer, F.; Eisenberg, T. Streptobacillus moniliformis and IgM and IgG Immune Response in Patient with Endocarditis1. Emerg. Infect. Dis. 2024, 30, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Wullenweber, M.; Kaspareit-Rittinghausen, J.; Farouq, M. Streptobacillus moniliformis Epizootic in Barrier-Maintained C57BL/6J Mice and Susceptibility to Infection of Different Strains of Mice. Lab. Anim. Sci. 1990, 40, 608–612. [Google Scholar]
- Eisenberg, T.; Ewers, C.; Rau, J.; Akimkin, V.; Nicklas, W. Approved and Novel Strategies in Diagnostics of Rat Bite Fever and Other Streptobacillus Infections in Humans and Animals. Virulence 2016, 7, 630–648. [Google Scholar] [CrossRef]
- Yang, R.; Butler, T. Discovery of the Plague Pathogen: Lessons Learned. In Yersinia pestis: Retrospective and Perspective; Yang, R., Anisimov, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2016; Volume 918, pp. 27–33. ISBN 978-94-024-0888-1. [Google Scholar]
- World Health Organization (WHO). Plague. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/plague (accessed on 22 April 2025).
- Brubaker, R.R. The Genus Yersinia: Biochemistry and Genetics of Virulence With 3 Figures. In Modern Aspects of Electrochemistry; White, R.E., Bockris, J.O., Conway, B.E., Eds.; Modern Aspects of Electrochemistry; Springer: Boston, MA, USA, 1972; Volume 18, pp. 111–158. ISBN 978-1-4612-9003-2. [Google Scholar]
- Tan, S.Y.; Dutta, A.; Jakubovics, N.S.; Ang, M.Y.; Siow, C.C.; Mutha, N.V.; Heydari, H.; Wee, W.Y.; Wong, G.J.; Choo, S.W. YersiniaBase: A Genomic Resource and Analysis Platform for Comparative Analysis of Yersinia. BMC Bioinform. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Signoli, M.; Chevé, D.; Costedoat, C.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. Yersinia Pestis: The Natural History of Plague. Clin. Microbiol. Rev. 2020, 34, e00044-19. [Google Scholar] [CrossRef]
- Bari, M.d.L.; Hossain, M.A.; Isshiki, K.; Ukuku, D. Behavior of Yersinia enterocolitica in Foods. J. Pathog. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Erickson, D.L. Yersinia Pestis Biofilm in the Flea Vector and Its Role in the Transmission of Plague. In Bacterial Biofilms; Romeo, T., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 322, pp. 229–248. ISBN 978-3-540-75417-6. [Google Scholar]
- Butler, T. Plague Gives Surprises in the Second Decade of the Twenty-First Century. Am. J. Trop. Med. Hyg. 2023, 109, 985–988. [Google Scholar] [CrossRef]
- Wong, D.; Wild, M.A.; Walburger, M.A.; Higgins, C.L.; Callahan, M.; Czarnecki, L.A.; Lawaczeck, E.W.; Levy, C.E.; Patterson, J.G.; Sunenshine, R.; et al. Primary Pneumonic Plague Contracted from a Mountain Lion Carcass. Clin. Infect. Dis. 2009, 49, e33–e38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dubyanskiy, V.M.; Yeszhanov, A.B. Ecology of Yersinia Pestis and the Epidemiology of Plague. Adv. Exp. Med. Biol. 2016, 918, 101–170. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Kryštufek, B.; Sludsky, A.; Schmid, B.V.; DEAlmeida, A.M.P.; Lei, X.; Ramasindrazana, B.; Bertherat, E.; Yeszhanov, A.; Stenseth, N.C.; et al. Plague Reservoir Species throughout the World. Integr. Zool. 2021, 16, 820–833. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent Reservoirs of Future Zoonotic Diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Li, H.; Yao, X.; Xu, C.; Cong, X.; Shao, K.; Ju, C. Plague Risk Assessment—China, 2022. China CDC Wkly. 2022, 4, 417–420. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Maps and Statistics. 2025. Available online: https://www.cdc.gov/plague/maps-statistics/index.html (accessed on 22 April 2025).
- Chernin, E. Richard Pearson Strong and the Manchurian Epidemic of Pneumoic Plague, 1910–1911. J. Hist. Med. Allied Sci. 1989, 44, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Gamsa, M. The Epidemic of Pneumonic Plague in Manchuria 1910–1911. Past Present 2006, 190, 147–183. [Google Scholar] [CrossRef]
- Nishiura, H. Epidemiology of a Primary Pneumonic Plague in Kantoshu, Manchuria, from 1910 to 1911: Statistical Analysis of Individual Records Collected by the Japanese Empire. Int. J. Epidemiol. 2006, 35, 1059–1065. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Persson, B. Pestens Gåta: Farsoter i Det Tidiga 1700-Talets Skåne; Lund University: Lund, Sweden, 2001; Volume 5. [Google Scholar][Green Version]
- Pollitzer, R.; World Health Organization. Plague; World Health Organization: Geneva, Switzerland, 1954. [Google Scholar][Green Version]
- Hinnebusch, B.J. The Evolution of Flea-Borne Transmission in Yersinia Pestis. Curr. Issues Mol. Biol. 2005, 7, 197–212. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; De La Fuente, J. Effects of Environmental Change on Zoonotic Disease Risk: An Ecological Primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef]
- Carlson, C.J. embarcadero: Species Distribution Modelling with Bayesian Additive Regression Trees in r. Methods Ecol. Evol. 2020, 11, 850–858. [Google Scholar] [CrossRef]
- Carlson, C.J.; Bevins, S.N.; Schmid, B.V. Plague Risk in the Western United States over Seven Decades of Environmental Change. Glob. Change Biol. 2022, 28, 753–769. [Google Scholar] [CrossRef]
- Kausrud, K.L.; Begon, M.; Ari, T.B.; Viljugrein, H.; Esper, J.; Büntgen, U.; Leirs, H.; Junge, C.; Yang, B.; Yang, M.; et al. Modeling the Epidemiological History of Plague in Central Asia: Palaeoclimatic Forcing on a Disease System over the Past Millennium. BMC Biol. 2010, 8, 112. [Google Scholar] [CrossRef]
- Yue, R.P.H.; Lee, H.F. The Delayed Effect of Cooling Reinforced the NAO-Plague Connection in Pre-Industrial Europe. Sci. Total Environ. 2021, 762, 143122. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, G.P. Clinical Picture of Plague; Medgiz: Moscow, Russia, 1940. [Google Scholar]
- Yang, R.; Anisimov, A. (Eds.) Yersinia pestis: Retrospective and Perspective; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2016; ISBN 978-94-024-0888-1. [Google Scholar]
- Hull, H.F.; Montes, J.M.; Mann, J.M. Septicemic Plague in New Mexico. J. Infect. Dis. 1987, 155, 113–118. [Google Scholar] [CrossRef]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis—Etiologic Agent of Plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Butler, T. Plague into the 21st Century. Clin. Infect. Dis. 2009, 49, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Yersin, A. La peste bubonique à Hong Kong. Ann. Inst. Pasteur. 1894, 8, 662–667. [Google Scholar]
- Nichols, M.C.; Ettestad, P.J.; VinHatton, E.S.; Melman, S.D.; Onischuk, L.; Pierce, E.A.; Aragon, A.S. Yersinia pestis Infection in Dogs: 62 Cases (2003–2011). J. Am. Vet. Med. Assoc. 2014, 244, 1176–1180. [Google Scholar] [CrossRef]
- Weller, C.; Malmlov, A.; Daniels, J.; Schaffer, P.; Pabilonia, K. Detection of Yersinia Pestis in Canine and Feline Companion Animals in the United States. Int. J. Infect. Dis. 2025, 152, 107546. [Google Scholar] [CrossRef]
- Chanteau, S.; Rahalison, L.; Ralafiarisoa, L.; Foulon, J.; Ratsitorahina, M.; Ratsifasoamanana, L.; Carniel, E.; Nato, F. Development and Testing of a Rapid Diagnostic Test for Bubonic and Pneumonic Plague. Lancet 2003, 361, 211–216. [Google Scholar] [CrossRef]
- Hinnebusch, J.; Schwan, T.G. New Method for Plague Surveillance Using Polymerase Chain Reaction to Detect Yersinia Pestis in Fleas. J. Clin. Microbiol. 1993, 31, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, H.; Itoh, K.; Suzuki, S.; Watanabe, H. Detection and Identification of Yersinia Pestis by Polymerase Chain Reaction (PCR) Using Multiplex Primers. Microbiol. Immunol. 1996, 40, 773–775. [Google Scholar] [CrossRef]
- Parkhill, J.; Wren, B.W.; Thomson, N.R.; Titball, R.W.; Holden, M.T.; Prentice, M.B.; Sebaihia, M.; James, K.D.; Churcher, C.; Mungall, K.L.; et al. Genome Sequence of Yersinia Pestis, the Causative Agent of Plague. Nature 2001, 413, 523–527. [Google Scholar] [CrossRef]
- Feng, N.; Zhou, Y.; Fan, Y.; Bi, Y.; Yang, R.; Zhou, Y.; Wang, X. Yersinia Pestis Detection by Loop-Mediated Isothermal Amplification Combined with Magnetic Bead Capture of DNA. Braz. J. Microbiol. 2018, 49, 128–137. [Google Scholar] [CrossRef]
- World Health Organization (WHO). How to Safely Collect Sputum Samples from Patients Suspected to Be Infected with Pneumonic Plague; Technical Guidance; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Yang, R. Plague: Recognition, Treatment, and Prevention. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Meyer, K.F. Effectiveness of Live or Killed Plague Vaccines in Man. Bull. World Health Organ. 1970, 42, 653–666. [Google Scholar] [PubMed]
- Quenee, L.E.; Schneewind, O. Plague Vaccines and the Molecular Basis of Immunity against Yersinia Pestis. Hum. Vaccin. 2009, 5, 817–823. [Google Scholar] [CrossRef]
- Heath, D.G.; Anderson, G.W.; Mauro, J.M.; Welkos, S.L.; Andrews, G.P.; Adamovicz, J.; Friedlander, A.M. Protection against Experimental Bubonic and Pneumonic Plague by a Recombinant Capsular F1-V Antigen Fusion Protein Vaccine. Vaccine 1998, 16, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, D.; Qu, Z.; Sun, L.; Miao, Y.; Yang, Y.; Lu, J.; Du, W.; Wang, B.; Li, B. A Safety and Immunogenicity Study of a Novel Subunit Plague Vaccine in Cynomolgus Macaques. J. Appl. Toxicol. 2018, 38, 408–417. [Google Scholar] [CrossRef]
- Hu, J.; Jiao, L.; Hu, Y.; Chu, K.; Li, J.; Zhu, F.; Li, T.; Wu, Z.; Wei, D.; Meng, F.; et al. One Year Immunogenicity and Safety of Subunit Plague Vaccine in Chinese Healthy Adults: An Extended Open-Label Study. Hum. Vaccin. Immunother. 2018, 14, 2701–2705. [Google Scholar] [CrossRef]
- Bourhy, P.; Picardeau, M.; Septfons, A.; Trombert, S.; Cart-tanneur, E. Émergence de la leptospirose humaine en France métropolitaine ? Actualités sur la surveillance. Méd. Mal. Infect. 2017, 47, S150. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-Related Illness and Death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Voetsch, A.C.; Van Gilder, T.J.; Angulo, F.J.; Farley, M.M.; Shallow, S.; Marcus, R.; Cieslak, P.R.; Deneen, V.C.; Tauxe, R.V.; The Emerging Infections Program FoodNet Working Group. FoodNet Estimate of the Burden of Illness Caused by Nontyphoidal Salmonella Infections in the United States. Clin. Infect. Dis. 2004, 38, S127–S134. [Google Scholar] [CrossRef]
- Agbaje, M.; Begum, R.H.; Oyekunle, M.A.; Ojo, O.E.; Adenubi, O.T. Evolution of Salmonella Nomenclature: A Critical Note. Folia Microbiol. 2011, 56, 497–503. [Google Scholar] [CrossRef]
- Keerthirathne, T.; Ross, K.; Fallowfield, H.; Whiley, H. A Review of Temperature, pH, and Other Factors That Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products. Pathogens 2016, 5, 63. [Google Scholar] [CrossRef]
- Waldman, J.; Souza, M.N.; Fonseca, A.S.K.; Ikuta, N.; Lunge, V.R. Direct Detection of Salmonella from Poultry Samples by DNA Isothermal Amplification. Br. Poult. Sci. 2020, 61, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Mylona, E.; Frankel, G. Typhoidal Salmonella: Distinctive Virulence Factors and Pathogenesis. Cell Microbiol. 2018, 20, e12939. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, Colonization, and Virulence Differences among Serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, H.J.; Cho, W.H.; Kim, J.H.; Oh, M.H.; Kim, S.H.; Lee, B.K.; Ricke, S.C.; Kim, H.Y. Identification of Salmonella Enterica Subspecies I, Salmonella Enterica Serovars Typhimurium, Enteritidis and Typhi Using Multiplex PCR. FEMS Microbiol. Lett. 2009, 301, 137–146. [Google Scholar] [CrossRef]
- Guibourdenche, M.; Roggentin, P.; Mikoleit, M.; Fields, P.I.; Bockemühl, J.; Grimont, P.A.D.; Weill, F.-X. Supplement 2003–2007 (No. 47) to the White-Kauffmann-Le Minor Scheme. Res. Microbiol. 2010, 161, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Beltran, P.; Musser, J.M.; Helmuth, R.; Farmer, J.J.; Frerichs, W.M.; Wachsmuth, I.K.; Ferris, K.; McWhorter, A.C.; Wells, J.G.; Cravioto, A. Toward a Population Genetic Analysis of Salmonella: Genetic Diversity and Relationships among Strains of Serotypes S. Choleraesuis, S. Derby, S. Dublin, S. Enteritidis, S. Heidelberg, S. Infantis, S. Newport, and S. Typhimurium. Proc. Natl. Acad. Sci. USA 1988, 85, 7753–7757. [Google Scholar] [CrossRef]
- Dietrich, J.; Hammerl, J.-A.; Johne, A.; Kappenstein, O.; Loeffler, C.; Nöckler, K.; Rosner, B.; Spielmeyer, A.; Szabo, I.; Richter, M.H. Impact of Climate Change on Foodborne Infections and Intoxications. J. Health Monit. 2023, 8 (Suppl. S3), 78. [Google Scholar] [CrossRef]
- Henzler, D.J.; Opitz, H.M. The Role of Mice in the Epizootiology of Salmonella Enteritidis Infection on Chicken Layer Farms. Avian Dis. 1992, 36, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Wray, C. Mice as Carriers of Salmonella Enteritidis on Persistently Infected Poultry Units. Vet. Rec. 1995, 137, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lapuz, R.; Tani, H.; Sasai, K.; Shirota, K.; Katoh, H.; Baba, E. The Role of Roof Rats (Rattus rattus) in the Spread of Salmonella Enteritidis and S. Infantis contamination in layer farms in Eastern Japan. Epidemiol. Infect. 2008, 136, 1235–1243. [Google Scholar] [CrossRef]
- Le Moine, V.; Vannier, P.; Jestin, A. Microbiological Studies of Wild Rodents in Farms as Carriers of Pig Infectious Agents. Prev. Vet. Med. 1987, 4, 399–408. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Searle, J.B.; Betts, W.B.; White, P.C.L. Patterns of Infection by Salmonella and Yersinia spp. in Commensal House Mouse (Mus musculus domesticus) Populations. J. Appl. Microbiol. 2001, 90, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Jacobs-Reitsma, W.F.; Wagenaar, J.A.; Kijlstra, A. Presence of Salmonella and Campylobacter spp. in Wild Small Mammals on Organic Farms. Appl. Environ. Microbiol. 2006, 72, 960–962. [Google Scholar] [CrossRef]
- Petrie, G.F.; O’Brien, E.A. The Experimental Production of the Carrier-State by Feeding. Proc. R. Soc. Med. 1911, 4, 70–72. [Google Scholar] [CrossRef]
- Bartram, M.T.; Welch, H.; Ostrolenk, M. Incidence of Members of the Salmonella Group in Rats. J. Infect. Dis. 1940, 67, 222–226. [Google Scholar] [CrossRef]
- Habermann, R.T.; Williams, F.P. Salmonellosis in Laboratory Animals. J. Natl. Cancer Inst. 1958, 20, 933–947. [Google Scholar]
- Sparrow, S. Diseases of Pet Rodents. J. Small Anim. Pract. 1980, 21, 1–16. [Google Scholar] [CrossRef]
- Steffen, E.K.; Wagner, J.E. Salmonella Enteriditis Serotype Amsterdam in a Commercial Rat Colony. Lab. Anim. Sci. 1983, 33, 454–456. [Google Scholar]
- Healing, T.D. Salmonella in Rodents: A Risk to Man? CDR (Lond. Engl. Rev.) 1991, 1, R114–R116. [Google Scholar]
- Swanson, S.J.; Snider, C.; Braden, C.R.; Boxrud, D.; Wünschmann, A.; Rudroff, J.A.; Lockett, J.; Smith, K.E. Multidrug-Resistant Salmonella enterica Serotype Typhimurium Associated with Pet Rodents. N. Engl. J. Med. 2007, 356, 21–28. [Google Scholar] [CrossRef]
- Sivaramalingam, T.; Pearl, D.L.; McEwen, S.A.; Ojkic, D.; Guerin, M.T. A Temporal Study of Salmonella Serovars from Fluff Samples from Poultry Breeder Hatcheries in Ontario between 1998 and 2008. Can. J. Vet. Res. 2013, 77, 12–23. [Google Scholar]
- Ribas, A.; Saijuntha, W.; Agatsuma, T.; Prantlová, V.; Poonlaphdecha, S. Rodents as a Source of Salmonella Contamination in Wet Markets in Thailand. Vector-Borne Zoonotic Dis. 2016, 16, 537–540. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Salmonella (Non-Typhoidal). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 22 April 2025).
- Akil, L.; Ahmad, H.A.; Reddy, R.S. Effects of Climate Change on Salmonella Infections. Foodborne Pathog. Dis. 2014, 11, 974–980. [Google Scholar] [CrossRef]
- Chua, P.L.C.; Ng, C.F.S.; Tobias, A.; Seposo, X.T.; Hashizume, M. Associations between Ambient Temperature and Enteric Infections by Pathogen: A Systematic Review and Meta-Analysis. Lancet Planet. Health 2022, 6, e202–e218. [Google Scholar] [CrossRef]
- Sabeq, I.; Awad, D.; Hamad, A.; Nabil, M.; Aboubakr, M.; Abaza, M.; Fouad, M.; Hussein, A.; Shama, S.; Ramadan, H.; et al. Prevalence and Molecular Characterization of Foodborne and Human-derived Salmonella Strains for Resistance to Critically Important Antibiotics. Transbounding Emerg. Dis. 2022, 69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Zhang, R.; Chen, L.; Zhang, H.; Qi, X.; Chen, J. Epidemiology of Foodborne Diseases Caused by Salmonella in Zhejiang Province, China, between 2010 and 2021. Front. Public Health 2023, 11, 1127925. [Google Scholar] [CrossRef]
- Smith, R.; Takkinen, J.; Editorial Team, C. Lyme Borreliosis: Europe-Wide Coordinated Surveillance and Action Needed? Euro Surveill. 2006, 11, E060622.1. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Lynne, A.M. Food Animal-Associated Salmonella Challenges: Pathogenicity and Antimicrobial Resistance. J. Anim. Sci. 2008, 86, E173–E187. [Google Scholar] [CrossRef]
- Meltzer, E.; Sadik, C.; Schwartz, E. Enteric Fever in Israeli Travelers: A Nationwide Study. J. Travel Med. 2005, 12, 275–281. [Google Scholar] [CrossRef][Green Version]
- Patel, T.A.; Armstrong, M.; Morris-Jones, S.D.; Wright, S.G.; Doherty, T. Imported Enteric Fever: Case Series from the Hospital for Tropical Diseases, London, United Kingdom. Am. Soc. Trop. Med. Hyg. 2010, 82, 1121–1126. [Google Scholar] [CrossRef]
- Olsen, S.J.; Bleasdale, S.C.; Magnano, A.R.; Landrigan, C.; Holland, B.H.; Tauxe, R.V.; Mintz, E.D.; Luby, S. Outbreaks of Typhoid Fever in the United States, 1960–99. Epidemiol. Infect. 2003, 130, 13–21. [Google Scholar] [CrossRef]
- Peek, S.F.; Mcguirk, S.M.; Sweeney, R.W.; Cummings, K.J. Infectious Diseases of the Gastrointestinal Tract. In Rebhun’s Diseases of Dairy Cattle; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–356. ISBN 978-0-323-39055-2. [Google Scholar]
- Pecoraro, H.L.; Thompson, B.; Duhamel, G.E. Histopathology Case Definition of Naturally Acquired Salmonella enterica Serovar Dublin Infection in Young Holstein Cattle in the Northeastern United States. J. Vet. Diagn. Investig. 2017, 29, 860–864. [Google Scholar] [CrossRef]
- Nielsen, L.R. Review of Pathogenesis and Diagnostic Methods of Immediate Relevance for Epidemiology and Control of Salmonella Dublin in Cattle. Vet. Microbiol. 2013, 162, 1–9. [Google Scholar] [CrossRef]
- Burgess, B.A. Salmonella in Horses. Vet. Clin. N. Am. Equine Pract. 2023, 39, 25–35. [Google Scholar] [CrossRef]
- Wray, C.; Wray, A. Salmonella Infections in Dogs and Cats. In Salmonella in Domestic Animals; CABI: London, UK, 2013; 238 AD. [Google Scholar]
- Marks, S.L.; Rankin, S.C.; Byrne, B.A.; Weese, J.S. Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control. Vet. Intern. Med. 2011, 25, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, J.L.E.; Anderson, J.L.; Carlson, S.A.; Sharma, V.K. Twelve Hour Real-Time PCR Technique for the Sensitive and Specific Detection of Salmonella in Raw and Ready-to-Eat Meat Products. Mol. Cell Probes 2004, 18, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Asako, N.T.; Aihara, M.; Hayashi, K. Improvement in the Detection Rate of Diarrhoeagenic Bacteria in Human Stool Specimens by a Rapid Real-Time PCR Assay. J. Med. Microbiol. 2004, 53, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Liming, S.H.; Bhagwat, A.A. Application of a Molecular Beacon-Real-Time PCR Technology to Detect Salmonella Species Contaminating Fruits and Vegetables. Int. J. Food Microbiol. 2004, 95, 177–187. [Google Scholar] [CrossRef]
- Ward, M.P.; Alinovi, C.A.; Couëtil, L.L.; Wu, C.C. Evaluation of a PCR to Detect Salmonella in Fecal Samples of Horses Admitted to a Veterinary Teaching Hospital. J. Vet. Diagn. Investig. 2005, 17, 118–123. [Google Scholar] [CrossRef]
- Bohaychuk, V.M.; Gensler, G.E.; McFall, M.E.; King, R.K.; Renter, D.G. A Real-Time PCR Assay for the Detection of Salmonella in a Wide Variety of Food and Food-Animal Matricest. J. Food Prot. 2007, 70, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, A.; Shabbir, N. Salmonella. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Meerburg, B.G.; Kijlstra, A. Role of Rodents in Transmission of Salmonella and Campylobacter. J. Sci. Food Agric. 2007, 87, 2774–2781. [Google Scholar] [CrossRef]
- Zamora-Sanabria, R.; Alvarado, A.M. Preharvest Salmonella Risk Contamination and the Control Strategies. In Current Topics in Salmonella and Salmonellosis; Mares, M., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3065-9. [Google Scholar]
- Simanjuntak, C.H.; Paleologo, F.P.; Punjabi, N.H.; Darmowigoto, R.; Soeprawoto, n.u.l.l.; Totosudirjo, H.; Haryanto, P.; Suprijanto, E.; Witham, N.D.; Hoffman, S.L. Oral Immunisation against Typhoid Fever in Indonesia with Ty21a Vaccine. Lancet 1991, 338, 1055–1059. [Google Scholar] [CrossRef]
- Levine, M.M.; Ferreccio, C.; Black, R.E.; Lagos, R.; San Martin, O.; Blackwelder, W.C. Ty21a Live Oral Typhoid Vaccine and Prevention of Paratyphoid Fever Caused by Salmonella enterica Serovar Paratyphi B. Clin. Infect. Dis. 2007, 45 (Suppl. S1), S24–S28. [Google Scholar] [CrossRef]
- Shakya, M.; Colin-Jones, R.; Theiss-Nyland, K.; Voysey, M.; Pant, D.; Smith, N.; Liu, X.; Tonks, S.; Mazur, O.; Farooq, Y.G.; et al. Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. N. Engl. J. Med. 2019, 381, 2209–2218. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH). Tularemia. 2016. Available online: https://www.woah.org/en/disease/tularemia/ (accessed on 22 April 2025).
- Petersen, J.M.; Mead, P.S.; Schriefer, M.E. Francisella tularensis: An Arthropod-Borne Pathogen. Vet. Res. 2009, 40, 7. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, A. Tularemia: History, Epidemiology, Pathogen Physiology, and Clinical Manifestations. Ann. N. Y. Acad. Sci. 2007, 1105, 1–29. [Google Scholar] [CrossRef]
- Jackson, J.; McGregor, A.; Cooley, L.; Ng, J.; Brown, M.; Ong, C.W.; Darcy, C.; Sintchenko, V. Francisella tularensis Subspecies holarctica, Tasmania, Australia, 2011. Emerg. Infect. Dis. 2012, 18, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M.; Dresvyannikova, S. Tularemia in the Arctic. In Arctic One Health. Challenges for Northern Animals and People; Tryland, M., Ed.; Springer: Cham, Switzerland, 2022; pp. 377–392. [Google Scholar]
- Kaysser, P.; Seibold, E.; Mätz-Rensing, K.; Pfeffer, M.; Essbauer, S.; Splettstoesser, W.D. Re-Emergence of Tularemia in Germany: Presence of Francisella tularensis in Different Rodent Species in Endemic Areas. BMC Infect. Dis. 2008, 8, 157. [Google Scholar] [CrossRef]
- Gurcan, S. Epidemiology of Tularemia. Balk. Med. J. 2014, 33, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wobeser, G.A. Essentials of Diseases in Wild Animals, 1st ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006. [Google Scholar]
- Rossow, H.; Sissonen, S.; Koskela, K.A.; Kinnunen, P.M.; Hemmilä, H.; Niemimaa, J.; Huitu, O.; Kuusi, M.; Vapalahti, O.; Henttonen, H.; et al. Detection of Francisella tularensis in Voles in Finland. Vector-Borne Zoonotic Dis. 2014, 14, 193–198. [Google Scholar] [CrossRef]
- Şahin, M. Francisella tularensis’ in Vektörleri ve Doğal Rezervuarları. In Francisella tularensis ve Tularemi; Gürcan, Ş., Ed.; Nobel Tıp Kitabevleri: İstanbul, Türkiye, 2009; pp. 139–160. [Google Scholar]
- Akalın, H.; Helvacı, S.; Gedikoğlu, S. Re-Emergence of tularemia in Turkey. Int. J. Infect. Dis. 2009, 13, 547–551. [Google Scholar] [CrossRef]
- Hennebique, A.; Boisset, S.; Maurin, M. Tularemia as a Waterborne Disease: A Review. Emerg. Microbes Infect. 2019, 8, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Beermann, S.; Dobler, G.; Faber, M.; Frank, C.; Habedank, B.; Hagedorn, P.; Kampen, H.; Kuhn, C.; Nygren, T.; Schmidt-Chanasit, J.; et al. Impact of Climate Change on Vector- and Rodent-Borne Infectious Diseases. J. Health Monit. 2023, 8 (Suppl. S3), 33. [Google Scholar] [CrossRef]
- Wherry, W.B.; Lamb, B.H. Infection of Man with Bacterium Tularense. J. Infect. Dis. 1914, 15, 331–340. [Google Scholar] [CrossRef]
- Snowden, J.; Simonsen, K.A. Tularemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Maurin, M.; Gyuranecz, M. Tularaemia: Clinical Aspects in Europe. Lancet Infect. Dis. 2016, 16, 113–124. [Google Scholar] [CrossRef]
- Maurin, M.; Pelloux, I.; Brion, J.P.; Del Banõ, J.-N.; Picard, A. Human Tularemia in France, 2006-2010. Clin. Infect. Dis. 2011, 53, e133–e141. [Google Scholar] [CrossRef]
- Kwit, N.A.; Middaugh, N.A.; VinHatton, E.S.; Melman, S.D.; Onischuk, L.; Aragon, A.S.; Nelson, C.A.; Mead, P.S.; Ettestad, P.J. Francisella Tularensis Infection in Dogs: 88 Cases (2014–2016). J. Am. Vet. Med. Assoc. 2020, 256, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.N. Natural Occurrence of Tularemia in Dogs Used as a Source of Canine Distemper Virus. J. Lab. Clin. Med. 1944, 29, 906–915. [Google Scholar]
- Woods, J.P.; Crystal, M.A.; Morton, R.J.; Panciera, R.J. Tularemia in Two Cats. J. Am. Vet. Med. Assoc. 1998, 212, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Valentine, B.A.; DeBey, B.M.; Sonn, R.J.; Stauffer, L.R.; Pielstick, L.G. Localized Cutaneous Infection with Francisella tularensis Resembling Ulceroglandular Tularemia in a Cat. J. Vet. Diagn. Investig. 2004, 16, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Tärnvik, A.; Chu, M.C. New Approaches to Diagnosis and Therapy of Tularemia. Ann. N. Y. Acad. Sci. 2007, 1105, 378–404. [Google Scholar] [CrossRef]
- Hepburn, M.J.; Simpson, A.J. Tularemia: Current Diagnosis and Treatment Options. Expert. Rev. Anti-Infect. Ther. 2008, 6, 231–240. [Google Scholar] [CrossRef]
- Dennis, D.T.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Friedlander, A.M.; Hauer, J.; Layton, M.; et al. Tularemia as a Biological Weapon: Medical and Public Health Management. JAMA 2001, 285, 2763. [Google Scholar] [CrossRef]
- Bossi, P.; Tegnell, A.; Baka, A.; Van Loock, F.; Hendriks, J.; Werner, A.; Maidhof, H.; Gouvras, G. Bichat Guidelines for the Clinical Management of Tularaemia and Bioterrorism-Related Tularaemia. Euro Surveill. 2004, 9, 27–28. [Google Scholar] [CrossRef]
- Balestra, A.; Bytyci, H.; Guillod, C.; Braghetti, A.; Elzi, L. A Case of Ulceroglandular Tularemia Presenting with Lymphadenopathy and an Ulcer on a Linear Morphoea Lesion Surrounded by Erysipelas. Int. Med. Case Rep. J. 2018, 11, 313–318. [Google Scholar] [CrossRef]
- Donate-Pérez-Molino, P.; Castelló-Abietar, C.; Fernández-Suárez, J.; de Vicente, J.C. Tularemia: Diagnosis of an Unexpected Oculoglandular Case in a Non-Endemic Area by Universal PCR. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2019, 37, 620–621. [Google Scholar] [CrossRef]
- Kwit, N.A.; Schwartz, A.; Kugeler, K.J.; Mead, P.S.; Nelson, C.A. Human Tularaemia Associated with Exposure to Domestic Dogs-United States, 2006–2016. Zoonoses Public Health 2019, 66, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Tularemia. 2024. Available online: https://www.cdc.gov/tularemia/about/index.html (accessed on 22 April 2025).
- Eigelsbach, H.T.; Downs, C.M. Prophylactic Effectiveness of Live and Killed Tularemia Vaccines. J. Immunol. 1961, 87, 415–425. [Google Scholar] [CrossRef]
- Elkins, K.L.; Kurtz, S.L.; De Pascalis, R. Progress, Challenges, and Opportunities in Francisella Vaccine Development. Expert Rev. Vaccines 2016, 15, 1183–1196. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Borreliosis (Lyme Disease). 2025. Available online: https://www.ecdc.europa.eu/en/borreliosis-lyme-disease (accessed on 22 April 2025).
- Sykes, R.A.; Makiello, P. An Estimate of Lyme Borreliosis Incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004-2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef]
- Gylfe, A.; Bergström, S.; Lundström, J.; Olsen, B. Reactivation of Borrelia Infection in Birds. Nature 2000, 403, 724–725. [Google Scholar] [CrossRef]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schäfer, S.M.; Sewell, H.-S.; Brade, V.; Kraiczy, P. Host Association of Borrelia burgdorferi Sensu Lato—The Key Role of Host Complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef]
- Gernat, A.A.; Santos, F.B.O.; Grimes, J.L. Alternative Antimicrobials in the Turkey Industry: Challenges and Perspectives. Ger. J. Vet. Res. 2021, 1, 37–47. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Kugeler, K.J.; Nelson, C.A.; Marx, G.E.; Hinckley, A.F. Use of Commercial Claims Data for Evaluating Trends in Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hanincová, K.; Schäfer, S.M.; Etti, S.; Sewell, H.S.; Taragelová, V.; Ziak, D.; Labuda, M.; Kurtenbach, K. Association of Borrelia afzelii with Rodents in Europe. Parasitology 2003, 126, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Barbour, A.G.; Fish, D. The Biological and Social Phenomenon of Lyme Disease. Science 1993, 260, 1610–1616. [Google Scholar] [CrossRef]
- Ostfeld, R.S. Lyme Disease: The Ecology of a Complex System; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Eisen, L. Vector Competence Studies with Hard Ticks and Borrelia burgdorferi Sensu Lato Spirochetes: A Review. Ticks Tick-Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
- Desrosiers, N.; Morin, R.; Jutras, J. Atlas Des Micromammifères Du Québec; Société de La Faune et Des Parcs Du Québec, Direction Du Développement de La Faune: Québec City, QC, Canada, 2002. [Google Scholar]
- Shaw, M.T.; Keesing, F.; McGrail, R.; Ostfeld, R.S. Factors Influencing the Distribution of Larval Blacklegged Ticks on Rodent Hosts. Am. J. Trop. Med. Hyg. 2003, 68, 447–452. [Google Scholar] [CrossRef]
- Eisen, R.J.; Piesman, J.; Zielinski-Gutierrez, E.; Eisen, L. What Do We Need to Know About Disease Ecology to Prevent Lyme Disease in the Northeastern United States? J. Med. Entomol. 2012, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. Lyme Arthritis: An Epidemic of Oligoarticular Arthritis in Children and Adults in Three Connecticut Communities. Arthritis Rheum. 1977, 20, 7–17. [Google Scholar] [CrossRef]
- Steere, A.C.; Coburn, J.; Glickstein, L. The Emergence of Lyme Disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus Macaques Following Antibiotic Treatment of Disseminated Infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Factsheet about Borreliosis. 2016. Available online: https://www.ecdc.europa.eu/en/borreliosis/facts/factsheet (accessed on 22 April 2025).
- Lindgren, E.; Jaenson, G.T.T. Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures; WHO: Geneva, Switzerland, 2006; Available online: https://iris.who.int/bitstream/handle/10665/107800/E89522.pdf?sequence=1&isAllowed=y (accessed on 22 April 2025).
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010-2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme Borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Halperin, J.J. Diagnosis and Management of Lyme Neuroborreliosis. Expert Rev. Anti-Infect. Ther. 2018, 16, 5–11. [Google Scholar] [CrossRef]
- Cayol, C.; Giermek, A.; Gomez-Chamorro, A.; Hytönen, J.; Kallio, E.R.; Mappes, T.; Salo, J.; Voordouw, M.J.; Koskela, E. Borrelia afzelii Alters Reproductive Success in a Rodent Host. Proc. Biol. Sci. 2018, 285, 20181056. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Brisson, D.; Oggenfuss, K.; Devine, J.; Levy, M.Z.; Keesing, F. Effects of a Zoonotic Pathogen, Borrelia burgdorferi, on the Behavior of a Key Reservoir Host. Ecol. Evol. 2018, 8, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Voordouw, M.J.; Lachish, S.; Dolan, M.C. The Lyme Disease Pathogen Has No Effect on the Survival of Its Rodent Reservoir Host. PLoS ONE 2015, 10, e0118265. [Google Scholar] [CrossRef]
- Appel, M.J.; Allan, S.; Jacobson, R.H.; Lauderdale, T.L.; Chang, Y.F.; Shin, S.J.; Thomford, J.W.; Todhunter, R.J.; Summers, B.A. Experimental Lyme Disease in Dogs Produces Arthritis and Persistent Infection. J. Infect. Dis. 1993, 167, 651–664. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Anderson, J.F.; Shaw, E.; Post, J.E.; Palka, F.C. Borreliosis in Equids in Northeastern United States. Am. J. Vet. Res. 1988, 49, 359–362. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Testing and Diagnosis for Lyme Disease. 2024. Available online: https://www.cdc.gov/lyme/diagnosis-testing/index.html (accessed on 22 April 2025).
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Dumler, J.S.; O’Connell, S.; Radolf, J.D.; Nadelman, R.B. Single-Dose Prophylaxis against Lyme Disease. Lancet Infect. Dis. 2007, 7, 371–373. [Google Scholar] [CrossRef]
- Wormser, G.P. Early Lyme Disease. N. Engl. J. Med. 2006, 354, 2794–2801. [Google Scholar] [CrossRef]
- White, A.; Gaff, H. Review: Application of Tick Control Technologies for Blacklegged, Lone Star, and American Dog Ticks. J. Integr. Pest. Manag. 2018, 9. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Hovius, J.W.; Van Der Poll, T.; Van Dam, A.P.; Fikrig, E. Lyme Borreliosis Vaccination: The Facts, the Challenge, the Future. Trends Parasitol. 2011, 27, 40–47. [Google Scholar] [CrossRef]
- Comstedt, P.; Hanner, M.; Schüler, W.; Meinke, A.; Lundberg, U. Design and Development of a Novel Vaccine for Protection against Lyme Borreliosis. PLoS ONE 2014, 9, e113294. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Abdad, M.Y.; Abou Abdallah, R.; Fournier, P.-E.; Stenos, J.; Vasoo, S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018, 56, e01728-17. [Google Scholar] [CrossRef]
- Adem, P.V. Emerging and Re-Emerging Rickettsial Infections. Semin. Diagn. Pathol. 2019, 36, 146–151. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V. Rickettsioses as Paradigms of New or Emerging Infectious Diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Lippi, C.A.; Ryan, S.J.; White, A.L.; Gaff, H.D.; Carlson, C.J. Trends and Opportunities in Tick-Borne Disease Geography. J. Med. Entomol. 2021, 58, 2021–2029. [Google Scholar] [CrossRef]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Williams, K.; Shukla, M.; Snyder, E.E.; Nordberg, E.K.; Ceraul, S.M.; Dharmanolla, C.; Rainey, D.; Soneja, J.; Shallom, J.M.; et al. Rickettsia Phylogenomics: Unwinding the Intricacies of Obligate Intracellular Life. PLoS ONE 2008, 3, e2018. [Google Scholar] [CrossRef]
- McCormick, D.W.; Nicholson, W.L. Rickettsial Diseases. In CDC Yellow Book; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Zanetti, G.; Francioli, P.; Tagan, D.; Paddock, C.D.; Zaki, S.R. Imported Epidemic Typhus. Lancet 1998, 352, 1709. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Pachón, J.; Alarcón, A.; López-Cortés, L.F.; Viciana, P.; Jiménez-Mejías, M.E.; Villanueva, J.L.; Torronteras, R.; Caballero-Granado, F.J. Murine Typhus as a Common Cause of Fever of Intermediate Duration: A 17-Year Study in the South of Spain. Arch. Intern. Med. 1999, 159, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Bitam, I.; Raoult, D.; Parola, P. Transmission of Rickettsia conorii conorii in Naturally Infected Rhipicephalus sanguineus. Clin. Microbiol. Infect. 2009, 15, 319–321. [Google Scholar] [CrossRef]
- Sexton, D.J.; Walker, D.H. Spotted Fever Group Rickettsioses. In Tropical Infectious Diseases: Principles, Pathogens and Practice; Elsevier: Amsterdam, The Netherlands, 2011; pp. 323–328. ISBN 978-0-7020-3935-5. [Google Scholar]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States: A Practical Guide for Health Care and Public Health Professionals. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Fujita, H.; Takada, N.; Raoult, D. Genetic Identification of Rickettsiae Isolated from Ticks in Japan. J. Clin. Microbiol. 2002, 40, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, P.-H.; Du, J.; Yang, Z.-D.; Cui, N.; Xing, B.; Zhang, X.-A.; Liu, W. Rickettsia japonica Infections in Humans, Xinyang, China, 2014–2017. Emerg. Infect. Dis. 2019, 25, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Pillay, A.; Manyangadze, T.; Mukaratirwa, S. Prevalence of Rickettsia africae in Tick Vectors Collected from Mammalian Hosts in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Ticks Tick-Borne Dis. 2022, 13, 101960. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, S.; Feng, H.M.; Morovic, M.; Djelalija, B.; Popov, V.; Crocquet-Valdes, P.; Walker, D.H. Isolation of Rickettsia akari from a Patient in a Region Where Mediterranean Spotted Fever Is Endemic. Clin. Infect. Dis. 1996, 22, 216–220. [Google Scholar] [CrossRef]
- Renvoisé, A.; van’t Wout, J.W.; van der Schroeff, J.-G.; Beersma, M.F.; Raoult, D. A Case of Rickettsialpox in Northern Europe. Int. J. Infect. Dis. 2012, 16, e221–e222. [Google Scholar] [CrossRef]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Epidemiology and Statistics. Rocky Mountain Spotted Fever (RMSF). 2020. Available online: https://www.cdc.gov/rocky-mountain-spotted-fever/about/index.html (accessed on 22 April 2025).
- Botelho-Nevers, E.; Rovery, C.; Richet, H.; Raoult, D. Analysis of Risk Factors for Malignant Mediterranean Spotted Fever Indicates That Fluoroquinolone Treatment Has a Deleterious Effect. J. Antimicrob. Chemother. 2011, 66, 1821–1830. [Google Scholar] [CrossRef]
- Silva-Ramos, C.R.; Faccini-Martínez, Á.A. Clinical, Epidemiological, and Laboratory Features of Rickettsia africae Infection, African Tick-Bite Fever: A Systematic Review. Infez. Med. 2021, 29, 366–377. [Google Scholar] [CrossRef]
- Bayliss, D.B.; Morris, A.K.; Horta, M.C.; Labruna, M.B.; Radecki, S.V.; Hawley, J.R.; Brewer, M.M.; Lappin, M.R. Prevalence of Rickettsia Species Antibodies and Rickettsia Species DNA in the Blood of Cats with and without Fever. J. Feline Med. Surg. 2009, 11, 266–270. [Google Scholar] [CrossRef]
- Piranda, E.M.; Faccini, J.L.H.; Pinter, A.; Saito, T.B.; Pacheco, R.C.; Hagiwara, M.K.; Labruna, M.B. Experimental Infection of Dogs with a Brazilian Strain of Rickettsia rickettsii: Clinical and Laboratory Findings. Mem. Inst. Oswaldo Cruz 2008, 103, 696–701. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Meuten, D.J.; Walker, D.H.; Levy, M.; Kennedy, K.; King, M.; Curtis, B. Canine Rocky Mountain Spotted Fever: A Kennel Epizootic. Am. J. Vet. Res. 1985, 46, 2124–2128. [Google Scholar] [CrossRef]
- Levin, M.L.; Killmaster, L.F.; Zemtsova, G.E.; Ritter, J.M.; Langham, G. Clinical Presentation, Convalescence, and Relapse of Rocky Mountain Spotted Fever in Dogs Experimentally Infected via Tick Bite. PLoS ONE 2014, 9, e115105. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. In Vitro Susceptibilities of Spotted Fever Group Rickettsiae and Coxiella burnetii to Clarithromycin. Antimicrob. Agents Chemother. 1993, 37, 2633–2637. [Google Scholar] [CrossRef]
- Ives, T.J.; Manzewitsch, P.; Regnery, R.L.; Butts, J.D.; Kebede, M. In Vitro Susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari, and R. prowazekii to Macrolide Antibiotics as Determined by Immunofluorescent-Antibody Analysis of Infected Vero Cell Monolayers. Antimicrob. Agents Chemother. 1997, 41, 578–582. [Google Scholar] [CrossRef]
- Rolain, J.M.; Maurin, M.; Vestris, G.; Raoult, D. In Vitro Susceptibilities of 27 Rickettsiae to 13 Antimicrobials. Antimicrob. Agents Chemother. 1998, 42, 1537–1541. [Google Scholar] [CrossRef]
- Nakamura, T.; Takagaki, K.; Matsubara, Y.; Kikuchi, K. Predictive Values of Clinical Parameters for Severe Japanese Spotted Fever. J. Infect. Chemother. 2011, 17, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Raoult, D. Typhus Group Rickettsioses. In Tropical Infectious Diseases: Principles, Pathogens, and Practice; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; Elsevier: New York, NY, USA, 2011; pp. 329–334. [Google Scholar]
- Huys, J.; Kayhigi, J.; Freyens, P.; Berghe, G.V. Single-Dose Treatment of Epidemic Typhus with Doxycyline. Chemotherapy 1973, 18, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-Borne Rickettsioses around the World: Emerging Diseases Challenging Old Concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Iyakaremye, V. Epidemiology and Tropical Diseases. Int. J. Emerg. Trends Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Weigl, R. Immunization against Typhus Fever in Poland during World War II. Med. Dent. Bull. 1947, 19, 106. [Google Scholar] [PubMed]
- Walker, D.H. The Realities of Biodefense Vaccines against Rickettsia. Vaccine 2009, 27, D52–D55. [Google Scholar] [CrossRef]
- Zinsser, H.; Castaneda, M.R. Studies on Typhus Fever: Vii. Active Immunization against Mexican Typhus Fever with Dead Virus. J. Exp. Med. 1931, 53, 493–497. [Google Scholar] [CrossRef][Green Version]
- Kenyon, R.H.; Pedersen, C.E. Preparation of Rocky Mountain Spotted Fever Vaccine Suitable for Human Immunization. J. Clin. Microbiol. 1975, 1, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, R.H.; Sammons, L.S.; Pedersen, C.E. Comparison of Three Rocky Mountain Spotted Fever Vaccines. J. Clin. Microbiol. 1975, 2, 300–304. [Google Scholar] [CrossRef]
- Gonder, J.C.; Kenyon, R.H.; Pedersen, C.E. Evaluation of a Killed Rocky Mountain Spotted Fever Vaccine in Cynomolgus Monkeys. J. Clin. Microbiol. 1979, 10, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Maugh, T.H. Rickettsiae: A New Vaccine for Rocky Mountain Spotted Fever. Science 1978, 201, 604. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.L.; Wisseman, C.L.; Woodward, T.E.; Fiset, P.; Dumler, J.S.; McNamee, W.; Black, R.E.; Rooney, J.; Hughes, T.P.; Levine, M.M. Reactogenicity, Immunogenicity, and Efficacy of a Chick Embryo Cell-Derived Vaccine for Rocky Mountain Spotted Fever. J. Infect. Dis. 1983, 148, 922–930. [Google Scholar] [CrossRef]
- Kawamura, R.; Kasahar, S.; Toyama, T.; Nishinarita, F.; Tsubaki, S. On the Prevention of Tsutsugamushi. Results of Preventive Inoculations for People in the Endemic Region, and Laboratory Tests with the Pescadores Strain. Trop. Dis. Bull. 1940, 37, 269–270. [Google Scholar]
- Prins, C.; Sreekumar, D.; Sejian, V. Climate Change and Wildlife Biodiversity: Impact and Mitigation Strategies. Arch. Life Sci. Res. 2025, 1, 6–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).