Mycobacterium marinum Immune Evasion in Zebrafish
Abstract
1. Introduction
1.1. Zebrafish as a Model Organism
1.2. Taxonomic Classification and Characteristics of M. marinum
1.3. Natural Habitat and Transmission of M. marinum
1.4. Host Range and Specificity of M. marinum
1.5. Genetic Similarities with M. tuberculosis and Other Atypical Mycobacteria
2. Host–Pathogen Interface in Zebrafish (Figure 1)
2.1. Zebrafish Immune System Overview

2.2. Cellular Components of Innate Immunity
2.3. Adaptive Immune Responses
2.4. Pattern Recognition Receptors and Signaling Pathways
3. M. marinum Virulence Mechanisms (Figure 2)
3.1. Cell Wall Components and Their Role in Pathogenesis
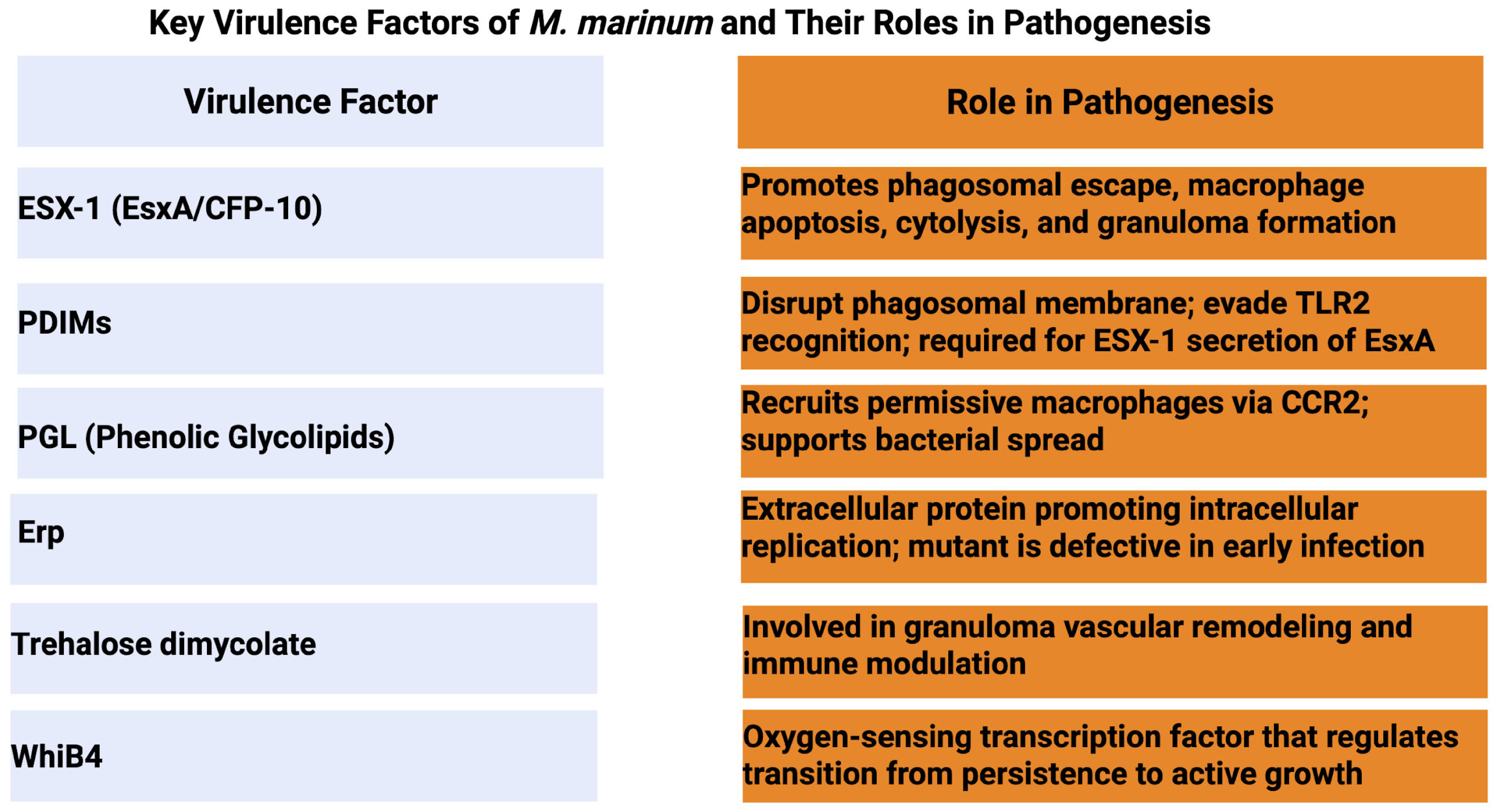
3.2. Secretion Systems (Especially ESX-1)
3.3. Virulence Factors and Effector Proteins
3.4. Metabolic Adaptations During Infection
3.5. Immune Evasion Strategies
3.6. Manipulation of Phagosome Maturation
3.7. Interference with Inflammatory Responses
3.8. Modulation of Cell Death Pathways
3.9. Evasion of Adaptive Immunity
3.10. Granuloma Formation and Maintenance
4. Disease Progression Patterns
4.1. Acute Infection Dynamics
4.1.1. Bacterial Replication Kinetics
4.1.2. Host Response Characteristics
4.1.3. Mortality Patterns
4.2. Chronic Infection Features
4.2.1. Granuloma Development Stages
4.2.2. Tissue-Specific Responses
4.2.3. Long-Term Survival Mechanisms
4.3. Host Immune Effector Responses
4.3.1. Innate Immune Mechanisms
4.3.2. Cytokine and Chemokine Profiles
4.3.3. Granuloma Structure and Function
4.3.4. Adaptive Immune Response Development
5. Clinical Implications and Applications
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Decostere, A.; Hermans, K.; Haesebrouck, F. Piscine mycobacteriosis: A literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 2004, 99, 159–166. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, L.; Yang, S.; Cai, S.; Jian, J.; Huang, Y. A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China. Fishes 2023, 8, 33. [Google Scholar] [CrossRef]
- Sanders, G.E.; Swaim, L.E. Atypical piscine mycobacteriosis in Japanese medaka (Oryzias latipes). Comp. Med. 2001, 51, 171–175. [Google Scholar] [PubMed]
- Zanoni, R.; Florio, D.; Fioravanti, M.; Rossi, M.; Prearo, M. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J. Fish. Dis. 2008, 31, 433–441. [Google Scholar] [CrossRef]
- Li, B.; Zou, Y.; Wei, Q. Sturgeon aquaculture in China: Status of current difficulties as well as future strategies based on 2002–2006/2007 surveys in eleven provinces. J. Appl. Ichthyol. 2009, 25, 632–639. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, C.; Zhang, X.; Li, T.; Li, A.; Gong, X. Mixed mycobacterial infections in farmed sturgeons. Aquac. Res. 2015, 46, 1914–1923. [Google Scholar] [CrossRef]
- Gcebe, N.; Michel, A.L.; Hlokwe, T.M. Non-tuberculous Mycobacterium species causing mycobacteriosis in farmed aquatic animals of South Africa. BMC Microbiol. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Delghandi, M.R.; El-Matbouli, M.; Menanteau-Ledouble, S. Mycobacteriosis and Infections with Non-tuberculous Mycobacteria in Aquatic Organisms: A Review. Microorganisms 2020, 8, 1368. [Google Scholar] [CrossRef]
- Novotny, L.; Dvorska, L.; Lorencova, A.; Beran, V.; Pavlik, I. Fish: A potential source of bacterial pathogens for human beings. Vet. Med. 2004, 49, 343–358. [Google Scholar] [CrossRef]
- Rallis, E.; Koumantaki-Mathioudaki, E. Treatment of Mycobacterium marinum cutaneous infections. Expert. Opin. Pharmacother. 2007, 8, 2965–2978. [Google Scholar] [CrossRef]
- Amrani, M.E.; Adoui, M.; Patey, O.; Asselineau, A. Upper extremity Mycobacterium marinum infection. Orthop. Traumatol. Surg. Res. 2010, 96, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet. Q. 2018, 38, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Humbert, O.; Ramakrishnan, L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat. Immunol. 2004, 5, 828–835. [Google Scholar] [CrossRef]
- Mason, T.; Snell, K.; Mittge, E.; Melancon, E.; Montgomery, R.; McFadden, M.; Camoriano, J.; Kent, M.L.; Whipps, C.M.; Peirce, J. Strategies to Mitigate a Mycobacterium marinum Outbreak in a Zebrafish Research Facility. Zebrafish 2016, 13 (Suppl. S1), S-77–S-87. [Google Scholar] [CrossRef]
- Rougeot, J.; Torraca, V.; Zakrzewska, A.; Kanwal, Z.; Jansen, H.J.; Sommer, F.; Spaink, H.P.; Meijer, A.H. RNAseq Profiling of Leukocyte Populations in Zebrafish Larvae Reveals a cxcl11 Chemokine Gene as a Marker of Macrophage Polarization During Mycobacterial Infection. Front. Immunol. 2019, 10, 832. [Google Scholar] [CrossRef]
- Swaim, L.; Connolly, L.; Volkman, H.; Humbert, O.; Born, D.; Ramakrishnan, L. Mycobacterium marinum infection of adult Zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 2006, 74, 6108–6117. [Google Scholar] [CrossRef]
- Meijer, A.H.; Spaink, H.P. Host-Pathogen Interactions Made Transparent with the Zebrafish Model. Curr. Drug Targets 2011, 12, 1000–1017. [Google Scholar] [CrossRef]
- Tsukamura, M. Numerical Classification of 280 Strains of Slowly Growing Mycobacteria. Microbiol. Immunol. 1983, 27, 315–334. [Google Scholar] [CrossRef]
- Daffé, M.; Draper, P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 1997, 39, 131–203. [Google Scholar]
- Kent, M.L.; Watral, V.; Wu, M.; Bermudez, L.E. In vivo and in vitro growth of Mycobacterium marinum at homoeothermic temperatures. FEMS Microbiol. Lett. 2006, 257, 69–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stinear, T.P.; Seemann, T.; Harrison, P.F.; Jenkin, G.A.; Davies, J.K.; Johnson, P.D.; Abdellah, Z.; Arrowsmith, C.; Chillingworth, T.; Churcher, C.; et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008, 18, 729–741. [Google Scholar] [CrossRef]
- Das, S.; Pettersson, B.M.F.; Behra, P.R.K.; Mallick, A.; Cheramie, M.; Ramesh, M.; Shirreff, L.; DuCote, T.; Dasgupta, S.; Ennis, D.G.; et al. Extensive genomic diversity among Mycobacterium marinum strains revealed by whole genome sequencing. Sci. Rep. 2018, 8, 12040. [Google Scholar] [CrossRef]
- Pedley, S.; Bartram, J.; Dufour, A.; Cotruvo, J. Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring, and Management; World Health Organization, Ed.; IWA Publishing: Colchester, UK, 2004; p. 237. Available online: https://www.who.int/publications/i/item/9241562595 (accessed on 8 August 2025).
- Broussard, G.W.; Ennis, D.G. Mycobacterium marinum produces long-term chronic infections in medaka: A new animal model for studying human tuberculosis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 45–54. [Google Scholar] [CrossRef]
- Chang, C.T.; Lewis, J.; Whipps, C.M. Source or Sink: Examining the Role of Biofilms in Transmission of Mycobacterium spp. in Laboratory Zebrafish. Zebrafish 2019, 16, 197–206. [Google Scholar] [CrossRef]
- Peterson, T.; Ferguson, J.; Watral, V.; Mutoji, K.; Ennis, D.; Kent, M. Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and M. chelonae in zebrafish Danio rerio. Dis. Aquat. Org. 2013, 106, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Ramos, B.; Reis, A.C.; Cunha, M.V. Non-Tuberculous Mycobacteria: Molecular and Physiological Bases of Virulence and Adaptation to Ecological Niches. Microorganisms 2020, 8, 1380. [Google Scholar] [CrossRef]
- Greub, G.; Raoult, D. Microorganisms Resistant to Free-Living Amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Ostland, V.; Watral, V.; Whipps, C.; Austin, F.; St-Hilaire, S.; Westerman, M.; Kent, M. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops × M. saxatilis and zebrafish Danio rerio. Dis. Aquat. Org. 2008, 79, 107–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gauthier, D.; Rhodes, M. Mycobacteriosis in fishes: A review. Vet. J. 2009, 180, 33–47. [Google Scholar] [CrossRef]
- Strike, T.; Feltrer, Y.; Flach, E.; Macgregor, S.; Guillaume, S. Investigation and management of an outbreak of multispecies mycobacteriosis in Australian lungfish (Neoceratodus fosteri) including the use of triple antibiotic treatment. J. Fish. Dis. 2016, 40, 557–570. [Google Scholar] [CrossRef]
- Whipps, C.M.; Gauthier, D.T.; Kent, M.L. Climate Change and Infectious Fish Diseases-Fish Mycobacteriosis; CABI: Egham, UK, 2020; pp. 265–279. [Google Scholar]
- Sette, C.S.; Wachholz, P.A.; Masuda, P.Y.; Figueira RBFda, C.; Mattar FRde, O.; Ura, D.G. Mycobacterium marinum infection: A case report. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 7. [Google Scholar] [CrossRef]
- Aubry, A.; Chosidow, O.; Caumes, E.; Robert, J.; Cambau, E. Sixty-three Cases of Mycobacterium marinum Infection: Clinical Features, Treatment, and Antibiotic Susceptibility of Causative Isolates. Arch. Intern. Med. 2002, 162, 1746–1752. [Google Scholar]
- Canetti, D.; Riccardi, N.; Antonello, R.M.; Nozza, S.; Sotgiu, G. Mycobacterium marinum: A brief update for clinical purposes. Eur. J. Intern. Med. 2022, 105, 15–19. [Google Scholar] [CrossRef]
- Jernigan, J.; Farr, B. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: Case report and review of the literature. Clin. Infect. Dis. 2000, 31, 439–443. [Google Scholar] [CrossRef]
- Tobin, D.; Ramakrishnan, L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol. 2008, 10, 1027–1039. [Google Scholar]
- Lienard, J.; Carlsson, F. Bacterial Pathogenesis, Methods and Protocols. Methods Mol. Biol. 2016, 1535, 301–315. [Google Scholar]
- Menon, A.R.; Prest, R.J.; Tobin, D.M.; Champion, P.A. Mycobacterium marinum as a model for understanding principles of mycobacterial pathogenesis. J. Bacteriol. 2025, 207, e00047-25. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Vedithi, S.C.; Blundell, T.L. Decoding the similarities and differences among mycobacterial species. PLoS Neglected Trop. Dis. 2017, 11, e0005883. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Mougari, F.; Reibel, F.; Cambau, E. Mycobacterium marinum. Microbiol. Spectr. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.R. Species and genetic diversity of nontuberculous mycobacteria in suspected tuberculosis cases in East Azerbaijan, Iran: A cross-sectional analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1477015. [Google Scholar] [CrossRef]
- Veyrier, F.J.; Dufort, A.; Behr, M.A. The rise and fall of the Mycobacterium tuberculosis genome. Trends Microbiol. 2011, 19, 156–161. [Google Scholar] [CrossRef]
- Fedrizzi, T.; Meehan, C.J.; Grottola, A.; Giacobazzi, E.; Serpini, G.F.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; Bertorelli, R.; De Sanctis, V.; et al. Genomic characterization of Nontuberculous Mycobacteria. Sci. Rep. 2017, 7, 45258. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M. The New Paradigm of Immunity to Tuberculosis; Springer: New York, NY, USA, 2013. [Google Scholar]
- Franza, M.; Varricchio, R.; Alloisio, G.; De Simone, G.; Di Bella, S.; Ascenzi, P.; di Masi, A. Zebrafish (Danio rerio) as a Model System to Investigate the Role of the Innate Immune Response in Human Infectious Diseases. Int. J. Mol. Sci. 2024, 25, 12008. [Google Scholar] [CrossRef]
- Varela, M.; Meijer, A.H. A fresh look at mycobacterial pathogenicity with the zebrafish host model. Mol. Microbiol. 2022, 117, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Stamm, L.M.; Morisaki, J.H.; Gao, L.-Y.; Jeng, R.L.; McDonald, K.L.; Roth, R.; Takeshita, S.; Heuser, J.; Welch, M.D.; Brown, E.J. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 2003, 198, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L. Looking Within the Zebrafish to Understand the Tuberculous Granuloma. Adv. Exp. Med. Biol. 2013, 783, 251–266. [Google Scholar] [CrossRef]
- Clay, H.; Davis, J.M.; Beery, D.; Huttenlocher, A.; Lyons, S.E.; Ramakrishnan, L. Dichotomous Role of the Macrophage in Early Mycobacterium marinum Infection of the Zebrafish. Cell Host Microbe 2007, 2, 29–39. [Google Scholar] [CrossRef]
- Cosma, C.L.; Humbert, O.; Sherman, D.R.; Ramakrishnan, L. Trafficking of Superinfecting Mycobacterium Organisms into Established Granulomas Occurs in Mammals and Is Independent of the Erp and ESX-1 Mycobacterial Virulence Loci. J. Infect. Dis. 2008, 198, 1851–1855. [Google Scholar] [CrossRef]
- Harjula, S.-K.E.; Saralahti, A.K.; Ojanen, M.J.; Rantapero, T.; Uusi-Mäkelä, M.I.; Nykter, M.; Lohi, O.; Parikka, M.; Rämet, M. Characterization of immune response against Mycobacterium marinum infection in the main hematopoietic organ of adult zebrafish (Danio rerio). Dev. Comp. Immunol. 2020, 103, 103523. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef]
- Reed, M.B.; Domenech, P.; Manca, C.; Su, H.; Barczak, A.K.; Kreiswirth, B.N.; Kaplan, G.; Barry, C.E. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 2004, 431, 84–87. [Google Scholar] [CrossRef]
- Hammarén, M.M.; Oksanen, K.E.; Nisula, H.M.; Luukinen, B.V.; Pesu, M.; Rämet, M.; Parikka, M.; Ramakrishnan, L. Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish. PLoS Pathog. 2014, 10, e1004190. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Peng, S.; Zhang, Y.; Zhao, J.; Zhao, Q.; Wu, X.; Shen, F.; Sun, K.; Yu, L.; Cen, S. Identification of immune-associated genes involved in latent Mycobacterium marinum infection. Microbes Infect. 2025, 27, 105407. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.M.; Pagán, A.J.; Shanahan, J.K.; Ramakrishnan, L. Mycobacterium marinum phthiocerol dimycocerosates enhance macrophage phagosomal permeabilization and membrane damage. PLoS ONE 2020, 15, e0233252. [Google Scholar] [CrossRef]
- Walton, E.M.; Cronan, M.R.; Cambier, C.; Rossi, A.; Marass, M.; Foglia, M.D.; Brewer, W.J.; Poss, K.D.; Stainier, D.Y.; Bertozzi, C.R.; et al. Cyclopropane Modification of Trehalose Dimycolate Drives Granuloma Angiogenesis and Mycobacterial Growth through Vegf Signaling. Cell Host Microbe 2018, 24, 514–525.e6. [Google Scholar] [CrossRef]
- Cronin, R.M.; Ferrell, M.J.; Cahir, C.W.; Champion, M.M.; Champion, P.A. Proteo-genetic analysis reveals clear hierarchy of ESX-1 secretion in Mycobacterium marinum. Proc. Natl. Acad. Sci. USA 2022, 119, e2123100119. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.Y.; Lin, T.L.; Chen, Y.Y.; Hsieh, P.F.; Wang, J.T. Role of the Mycobacterium marinum ESX-1 Secretion System in Sliding Motility and Biofilm Formation. Front. Microbiol. 2018, 9, 1160. [Google Scholar] [CrossRef]
- Chirakos, A.E.; Balaram, A.; Conrad, W.; Champion, P.A. Modeling Tubercular ESX-1 Secretion Using Mycobacterium marinum. Microbiol. Mol. Biol. Rev. 2020, 84, 23. [Google Scholar] [CrossRef]
- Ates, L.S.; Brosch, R. Discovery of the type VII ESX-1 secretion needle? Mol. Microbiol. 2017, 103, 7–12. [Google Scholar] [CrossRef]
- Yu, J.; Tran, V.; Li, M.; Huang, X.; Niu, C.; Wang, D.; Zhu, J.; Wang, J.; Gao, Q.; Liu, J.; et al. Both Phthiocerol Dimycocerosates and Phenolic Glycolipids Are Required for Virulence of Mycobacterium marinum. Infect. Immun. 2012, 80, 1381–1389. [Google Scholar] [CrossRef]
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host Evasion and Exploitation Schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509. [Google Scholar] [CrossRef]
- Cambier, C.J.; O’Leary, S.M.; O’Sullivan, M.P.; Keane, J.; Ramakrishnan, L. Phenolic Glycolipid Facilitates Mycobacterial Escape from Microbicidal Tissue-Resident Macrophages. Immunity 2017, 47, 552–565.e4. [Google Scholar] [CrossRef]
- de Mendonça-Lima, L.; Picardeau, M.; Raynaud, C.; Rauzier, J.; de la Salmonière, Y.-O.G.; Barker, L.; Bigi, F.; Cataldi, A.; Gicquel, B.; Reyrat, J.-M. Erp, an extracellular protein family specific to mycobacteria. Microbiology 2001, 147, 2315–2320. [Google Scholar] [CrossRef]
- Cosma, C.L.; Klein, K.; Kim, R.; Beery, D.; Ramakrishnan, L. Mycobacterium marinum Erp Is a Virulence Determinant Required for Cell Wall Integrity and Intracellular Survival. Infect. Immun. 2006, 74, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tang, Y.; Wang, Y.; Zhang, J.; Li, Y.; Xu, S.; Xia, B.; Zhai, Q.; Li, Y.; Zhang, L.; et al. WhiB4 Is Required for the Reactivation of Persistent Infection of Mycobacterium marinum in Zebrafish. Microbiol. Spectr. 2022, 10, e00443-21. [Google Scholar] [CrossRef]
- Bouz, G.; Hasawi, N.A. The zebrafish model of tuberculosis—No lungs needed. Crit. Rev. Microbiol. 2018, 44, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Lienard, J.; Nobs, E.; Lovins, V.; Movert, E.; Valfridsson, C.; Carlsson, F. The Mycobacterium marinum ESX-1 system mediates phagosomal permeabilization and type I interferon production via separable mechanisms. Proc. Natl. Acad. Sci. USA 2020, 117, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.Y.; Joshi, S.A.; Ball, D.A.; Leggett, H.; Park, S.; Kim, J.; Austin, C.D.; Paler-Martinez, A.; Xu, M.; Downing, K.H.; et al. Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor alpha in vivo. Infect. Immun. 2012, 80, 3512–3520. [Google Scholar] [CrossRef]
- Sullivan, J.; Young, E.; McCann, J.; Braunstein, M. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect. Immun. 2012, 80, 996–1006. [Google Scholar] [CrossRef]
- Xu, J.; Ma, S.; Huang, Y.; Zhang, Q.; Huang, L.; Xu, H.; Suleiman, I.M.; Li, P.; Wang, Z.; Xie, J. Mycobacterium marinum MMAR_0267-regulated copper utilization facilitates bacterial escape from phagolysosome. Commun. Biol. 2024, 7, 1180. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Dirks, R.P.; Ordas, A.; Jong-Raadsen, S.; Brittijn, S.A.; Haks, M.C.; Henkel, C.V.; Oravcova, K.; Racz, P.I.; Tuinhof-Koelma, N.; Wiweger, M.I.K.N.; et al. The Human Pathogen Mycobacterium tuberculosis and the Fish Pathogen Mycobacterium marinum Trigger a Core Set of Late Innate Immune Response Genes in Zebrafish Larvae. Biology 2024, 13, 688. [Google Scholar] [CrossRef]
- Helguera-Repetto, C.; Cox, R.A.; Muñoz-Sànchez, J.L.; Gonzalez-y-Merchand, J.A. The pathogen Mycobacterium marinum, a faster growing close relative of Mycobacterium tuberculosis, has a single rRNA operon per genome. FEMS Microbiol. Lett. 2004, 235, 281–288. [Google Scholar] [CrossRef]
- Chan, K.; Knaak, T.; Satkamp, L.; Humbert, O.; Falkow, S.; Ramakrishnan, L. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc. Natl. Acad. Sci. USA 2002, 99, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Prouty, M.; Correa, N.; Barker, L.; Jagadeeswaran, P.; Klose, K. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol. Lett. 2003, 225, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Cambier, C.J.; Davis, J.M.; Hall, C.J.; Crosier, P.S.; Ramakrishnan, L. Neutrophils Exert Protection in the Early Tuberculous Granuloma by Oxidative Killing of Mycobacteria Phagocytosed from Infected Macrophages. Cell Host Microbe 2012, 12, 301–312. [Google Scholar] [CrossRef]
- Takaki, K.; Ramakrishnan, L.; Basu, S. A zebrafish model for ocular tuberculosis. PLoS ONE 2018, 13, e0194982. [Google Scholar] [CrossRef]
- Antuofermo, E.; Pais, A.; Polinas, M.; Cubeddu, T.; Righetti, M.; Sanna, M.A.; Prearo, M. Mycobacteriosis caused by Mycobacterium marinum in reared mullets: First evidence from Sardinia (Italy). J. Fish. Dis. 2017, 40, 327–337. [Google Scholar] [CrossRef]
- Volkman, H.E.; Pozos, T.C.; Zheng, J.; Davis, J.M.; Rawls, J.F.; Ramakrishnan, L. Tuberculous Granuloma Induction via Interaction of a Bacterial Secreted Protein with Host Epithelium. Science 2010, 327, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Sar Avan der Abdallah, A.; Sparrius, M.; Reinders, E.; Vandenbroucke-Grauls, C.; Bitter, W. Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 2004, 72, 6306–6312. [Google Scholar] [CrossRef]
- Clay, H.; Volkman, H.E.; Ramakrishnan, L. Tumor Necrosis Factor Signaling Mediates Resistance to Mycobacteria by Inhibiting Bacterial Growth and Macrophage Death. Immunity 2008, 29, 283–294. [Google Scholar] [CrossRef]
- Aguiló, N.; Marinova, D.; Martín, C.; Pardo, J. ESX-1-induced apoptosis during mycobacterial infection: To be or not to be, that is the question. Front. Cell. Infect. Microbiol. 2013, 3, 88. [Google Scholar] [CrossRef]
- Ruley, K.; Ansede, J.; Pritchett, C.; Talaat, A.; Reimschuessel, R.; Trucksis, M. Identification of Mycobacterium marinum virulence genes using signature-tagged mutagenesis and the goldfish model of mycobacterial pathogenesis. FEMS Microbiol. Lett. 2004, 232, 75–81. [Google Scholar] [CrossRef]
- Talaat, A.M.; Reimschuessel, R.; Wasserman, S.S.; Trucksis, M. Goldfish, Carassius auratus, a Novel Animal Model for the Study of Mycobacterium marinum Pathogenesis. Infect. Immun. 1998, 66, 2938–2942. [Google Scholar] [CrossRef]
- Chang, C.T.; Benedict, S.; Whipps, C.M. Transmission of Mycobacterium chelonae and Mycobacterium marinum in laboratory zebrafish through live feeds. J. Fish. Dis. 2019, 42, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Bucsan, A.N.; Mehra, S.; Khader, S.A.; Kaushal, D. The current state of animal models and genomic approaches towards identifying and validating molecular determinants of Mycobacterium tuberculosis infection and tuberculosis disease. Pathog. Dis. 2019, 77, ftz037. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.H. Protection and pathology in TB: Learning from the zebrafish model. Semin. Immunopathol. 2016, 38, 261–273. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, R.C.; Hu, W.; Dijkema, S.M.; van den Berg, D.; Liu, J.; Bahi, R.; Verbeek, F.J.; Simonsson, U.S.H.; Spaink, H.P.; van der Graaf, P.H.; et al. Anti-tuberculosis effect of isoniazid scales accurately from zebrafish to humans. Br. J. Pharmacol. 2020, 177, 5518–5533. [Google Scholar] [CrossRef]
- Antunes, S.S.; Forn-Cuní, G.; Romeiro, N.C.; Spaink, H.P.; Verbeek, F.J.; Muzitano, M.F. Embryonic and larval zebrafish models for the discovery of new bioactive compounds against tuberculosis. Drug Discov. Today 2024, 29, 104163. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Cronan, M.R.; Scott, N.R.; Thomas, M.I.; Okuda, K.S.; Walton, E.M.; Beerman, R.W.; Crosier, P.S.; Tobin, D.M. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015, 517, 612–615. [Google Scholar] [CrossRef]
- Habjan, E.; Ho, V.Q.T.; Gallant, J.; van Stempvoort, G.; Jim, K.K.; Kuijl, C.; Geerke, D.P.; Bitter, W.; Speer, A. Anti-tuberculosis Compound Screen using a Zebrafish Infection Model identifies an Aspartyl-tRNA Synthetase Inhibitor. Dis. Model. Mech. 2021, 14, dmm049145. [Google Scholar] [CrossRef]
- Niskanen, M.; Myllymäki, H.; Rämet, M. DNA vaccination with the Mycobacterium marinum MMAR_4110 antigen inhibits reactivation of a latent mycobacterial infection in the adult Zebrafish. Vaccine 2020, 38, 5685–5694. [Google Scholar] [CrossRef]
- Chen, D.; Huang, W.; Shen, L.; Zhang, J.; Pan, Z.; Zhang, C.; Tang, Y.; Zhou, Z.; Tao, J.; Luo, G.; et al. An mRNA vaccine induces antimycobacterial immunity by activating DNA damage repair and autophagy. Mol. Ther. Nucleic Acids 2025, 36, 102402. [Google Scholar] [CrossRef]
- Myllymäki, H.; Niskanen, M.; Luukinen, H.; Parikka, M.; Rämet, M. Identification of protective postexposure mycobacterial vaccine antigens using an immunosuppression-based reactivation model in the zebrafish. Dis. Model. Mech. 2018, 11, dmm033175. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.; Hooley, G.; Champion, M.; Medie, F.M.; Champion, P. A novel ESX-1 locus reveals that surface-associated ESX-1 substrates mediate virulence in Mycobacterium marinum. J. Bacteriol. 2014, 196, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.R.; Cronin, R.M.; Prest, R.J.; Menon, A.R.; Yang, Y.; Jennisch, M.K.; Champion, M.M.; Tobin, D.M.; Champion, P.A.; Ehrt, S. The antagonistic transcription factors, EspM and EspN, regulate the ESX-1 secretion system in M. marinum. mBio 2024, 15, e03357-23. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sassetti, C. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Cameron, J.; Saviola, B.; Venketaraman, V. Mycobacterium marinum Immune Evasion in Zebrafish. Pathogens 2025, 14, 908. https://doi.org/10.3390/pathogens14090908
Kumar P, Cameron J, Saviola B, Venketaraman V. Mycobacterium marinum Immune Evasion in Zebrafish. Pathogens. 2025; 14(9):908. https://doi.org/10.3390/pathogens14090908
Chicago/Turabian StyleKumar, Priyank, Joshua Cameron, Beatrice Saviola, and Vishwanath Venketaraman. 2025. "Mycobacterium marinum Immune Evasion in Zebrafish" Pathogens 14, no. 9: 908. https://doi.org/10.3390/pathogens14090908
APA StyleKumar, P., Cameron, J., Saviola, B., & Venketaraman, V. (2025). Mycobacterium marinum Immune Evasion in Zebrafish. Pathogens, 14(9), 908. https://doi.org/10.3390/pathogens14090908







