Bioactive Compounds from Tithonia diversifolia Aerial Parts Against Eggs and Infective Larvae of the Parasitic Nematode Haemonchus contortus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Obtaining the Hydroalcoholic Extract (HA-E)
2.3. Liquid–Liquid Separation of HA-E
2.4. Chemical Fractionation of EtOAc-F
2.5. Compound Identification Through HPLC
2.6. Obtaining Biological Material
2.7. Haemonchus contortus Eggs Recovery
2.8. Haemonchus contortus Infective Larvae (L3) Recovery
2.9. Egg Hatch Inhibition (EHI) Assay
2.10. Larval Mortality Assay
2.11. Statistical Analysis
3. Results
3.1. Hydroalcoholic Extract, Fractions, and Subfractions Yields
3.2. Chemical Identification of Tithonia diversifolia HA-E and Fractions
3.3. EHI of Tithonia diversifolia HA-E and Fractions
3.4. Larval Mortality of Tithonia diversifolia EtOAc-F and Its Subfractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naeem, M.; Iqbal, Z.; Roohi, N. Ovine haemonchosis: A review. Trop. Anim. Health Prod. 2021, 53, 19. [Google Scholar] [CrossRef]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef]
- Qamar, M.F.; Maqbool, A.; Ahmad, N. Economic losses due to haemonchosis in sheep and goats. Sci. Intern. 2011, 23, 321–324. [Google Scholar]
- Almeida, F.A.; Garcia, K.C.O.D.; Torgerson, P.R.; Amarante, A.F.T.D. Multiple resistance to anthelmintics by Haemonchus contortus and Trichostrongylus colubriformis in sheep in Brazil. Parasitol. Int. 2010, 59, 622–625. [Google Scholar] [CrossRef]
- Falzon, L.C.; O’Neill, T.J.; Menzies, P.I.; Peregrine, A.S.; Jones-Bitton, A.; vanLeeuwen, J.; Mederos, A. A systematic review and meta-analysis of factors associated with anthelmintic resistance in sheep. Prev. Vet. Med. 2014, 117, 388–402. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Jiménez-Jacinto, V.; Alonso-Morales, R.A.; Alonso-Díaz, M.Á.; Maza-Lopez, J.; Camas-Pereyra, R.; Olmedo-Juárez, A.; Higuera-Piedrahita, R.I.; López-Arellano, M.E. Assembly and Analysis of Haemonchus contortus transcriptome as a tool for the knowledge of ivermectin resistance mechanisms. Pathogens 2023, 12, 499. [Google Scholar] [CrossRef]
- Arowolo, M.A.; He, J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: A review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, Z.M.; Tsukahara, Y.; Amadeu, R.R.; Goetsch, A.L.; Gipson, T.A.; Sahlu, T.; Puchala, R.; Wang, Z.; Hart, S.P.; Mateescu, R.G. Signatures of selection for resistance to Haemonchus contortus in sheep and goats. BMC Genom. 2019, 20, 735. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.; Sacarrão-Birrento, L.; Almeida, M.; Ribeiro, D.M.; Guedes, C.; González Montaña, J.R.; Pereira, A.F.; Zaralis, K.; Geraldo, A.; Tzamaloukas, O.; et al. Extensive sheep and goat production: The role of novel technologies towards sustainability and animal welfare. Animals 2022, 12, 885. [Google Scholar] [CrossRef]
- Pérez, A.; Montejo, I.; Iglesias, J.M.; López, O.; Martín, G.J.; García, D.E.; Millián, I.; Hernández, A. Tithonia diversifolia (Hemsl.) A. Gray. Past. Forr. 2009, 32, 1–15. [Google Scholar]
- Ajao, A.; Moteetee, A. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. S. Afr. J. Bot. 2017, 113, 396–403. [Google Scholar] [CrossRef]
- Thongsom, M.; Chunglok, W.; Kuanchuea, R. Antioxidant and Hypoglycemic Effects of Tithonia diversifolia Aqueous Leaves Extract in Alloxan-induced Diabetic Mice. Adv. Environ. Biol. 2013, 7, 2116–2125. [Google Scholar]
- Ferreira Farias, A.L.; Lobato Rodrigues, A.B.; Lopes Martins, R.; de Menezes Rabelo, É.; Ferreira Farias, C.W.; Moreira da Silva de Almeida, S.S. Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals 2019, 12, 34. [Google Scholar] [CrossRef]
- Nguepi, I.S.T.; Ngueguim, F.T.; Gounoue, R.K.; Mbatchou, A.; Dimo, T. Curative effects of the aqueous extract of Tithonia diversifolia (Hemsl.) A. gray (Asteraceae) against ethanol induced-hepatotoxicity in rats. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1137–1143. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Calsavara, L.H.F.; Ribeiro, R.S.; Pereira, L.G.R.; de Freitas, D.S.; Paciullo, D.S.; Barahona, R.; Rivera, J.E.; Chará, J.; Murgueitio, E. Feed ruminants using Tithonia diversifolia as a forage. J. Dairy Vet. Anim. Res. 2017, 5, 117–120. [Google Scholar] [CrossRef]
- Tagne, A.M.; Marino, F.; Cosentino, M. Tithonia diversifolia (Hemsl.) A. Gray as a medicinal plant: A comprehensive review of its ethnopharmacology, phytochemistry, pharmacotoxicology and clinical relevance. J. Ethnopharmacol. 2018, 220, 94–116. [Google Scholar] [CrossRef]
- Pérez-Márquez, S.; Ovani, V.S.; Lima, P.d.M.T.; Lana, Â.M.Q.; Louvandini, H.; Abdalla, A.L.; Maurício, R.M. Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production. Agronomy 2023, 13, 2768. [Google Scholar] [CrossRef]
- Krüger, A.M.; Lima, P.d.M.T.; Ovani, V.; Pérez-Marquéz, S.; Louvandini, H.; Abdalla, A.L. Ruminant Grazing Lands in the Tropics: Silvopastoral Systems and Tithonia diversifolia as Tools with Potential to Promote Sustainability. Agronomy 2024, 14, 1386. [Google Scholar] [CrossRef]
- Lezcano-Más, Y.; Soca-Pérez, M.; Roque-López, E.; Ojeda-García, F.; Machado-Castro, R.; Fontes-Marrero, D. Forraje de Tithonia diversifolia para el control de estrongílidos gastrointestinales en bovinos jóvenes. Past. Forr. 2016, 39, 133–138. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2001. [Google Scholar]
- Cortes-Morales, J.A.; Salinas-Sánchez, D.O.; de Jesús Perea-Flores, M.; González-Cortazar, M.; Tapia-Maruri, D.; López-Arellano, M.E.; Rivas-González, J.M.; Zamilpa, A.; Olmedo-Juárez, A. In vitro anthelmintic activity and colocalization analysis of hydroxycinnamic acids obtained from Chamaecrista nictitans against two Haemonchus contortus isolates. Vet. Parasitol. 2024, 331, 110282. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Cedillo-Borda, M.; Alonso-Morales, R.A.; Alonso-Díaz, M.A.; Olmedo-Juárez, A.; Mendoza-de Gives, P.; López-Arellano, M.E. Comparative study of transcription profiles of the P-glycoprotein transporters of two Haemonchus contortus isolates: Susceptible and resistant to ivermectin. Mol. Biochem. Parasitol. 2020, 238, 111281. [Google Scholar] [CrossRef]
- Coles, G.C.; Bauer, C.; Borgsteede, F.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World association for advancement in veterinary parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–43. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Zamilpa, A.; Mendoza-de Gives, P.; Arece-García, J.; López-Arellano, M.E.; von Son-de Fernex, E. In vitro larvicidal effect of a hydroalcoholic extract from Acacia cochliacantha leaf against ruminant parasitic nematodes. Vet. Res. Commun. 2017, 41, 227–232. [Google Scholar] [CrossRef]
- Coles, G.; Jackson, F.; Pomroy, W.; Prichard, R.; Von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Duarte, E.R.; David, P.P.D.; Oliveria, K.B.A.; Lima, M.D.; dos Santos Mangaço, F.; Costa, F.M. Efficacy of Tithonia diversifolia (Hemsl) A. Gray on the inhibition of larval development of Haemonchus contortus. Acta Vet. Bras. 2020, 14, 191–195. [Google Scholar] [CrossRef]
- Buyi, L.; Owoyesigire, B.B.; Idibu, J.; Odoch, T.; Owere, L. Effects of Ethanolic Extracts of Tithonia diversifolia and Azadirachta indica on Haemonchus contortus in Goats. World Vet. J. 2024, 14, 611–616. [Google Scholar] [CrossRef]
- Jasso-Díaz, G.; Torres-Hernández, G.; Zamilpa, A.; Becerril-Pérez, C.M.; Ramírez-Bribiesca, J.E.; Hernández-Mendo, O.; Sánchez-Arroyo, H.; González-Cortazar, M.; Mendoza-de Gives, P. In vitro assessment of Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia against Haemonchus contortus nematode eggs and infective (L3) larvae. Microb. Pathog. 2017, 109, 162–168. [Google Scholar] [CrossRef]
- Cortes-Morales, J.A.; Olmedo-Juárez, A.; Trejo-Tapia, G.; González-Cortazar, M.; Domínguez-Mendoza, B.E.; Mendoza-de Gives, P.; Zamilpa, A. In vitro ovicidal activity of Baccharis conferta Kunth against Haemonchus contortus. Exp. Parasitol. 2019, 197, 20–28. [Google Scholar] [CrossRef] [PubMed]
- von Son-de Fernex, E.; Alonso-Díaz, M.A.; Valles-de la Mora, B.; Mendoza-de Gives, P.; González-Cortazar, M.; Zamilpa, A. Anthelmintic effect of 2H-chromen-2-one isolated from Gliricidia sepium against Cooperia punctate. Exp. Parasitol. 2017, 178, 1–6. [Google Scholar] [CrossRef]
- Escareño-Díaz, S.; Alonso-Díaz, M.A.; Mendoza-de Gives, P.; Castillo-Gallegos, E.; von Son-de Fernex, E. Anthelmintic-like activity of polyphenolic compounds and their interactions against the cattle nematode Cooperia punctata. Vet. Parasitol. 2019, 274, 108909. [Google Scholar] [CrossRef]
- Cortes-Morales, J.A.; Olmedo-Juárez, A.; González-Cortazar, M.; Zamilpa, A.; López- Arellano, M.A.; Ble-González, E.A.; Tapia-Maruri, D.; Flores-Franco, G.; Salinas- Sánchez, D.O. In vitro ovicidal activity of Brongniartia montalvoana against small ruminant gastrointestinal nematodes. Exp. Parasitol. 2022, 240, 108336. [Google Scholar] [CrossRef]
- Castillo-Mitre, G.F.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Mendoza-de Gives, P.; Vázquez-Armijo, J.F.; Zamilpa, A.; Lee-Rangel, H.A.; Avendaño-Reyes, L.; Macias-Cruz, U. In vivo anthelmintic activity of Acacia cochliacantha leaves against Haemonchus contortus in Boer goat kids. Rev. Mex. Cien. Pec. 2021, 12, 138–150. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Borges-Argáez, R.; Cáceres-Farfán, M.; Mancilla-Montelongo, G.; Mathieu, C. Bio-guided fractionation to identify Senegalia gaumeri leaf extract compounds with anthelmintic activity against Haemonchus contortus eggs and larvae. Vet. Parasitol. 2019, 270, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Pereira, M.H.; Gainza, Y.A.; Hoste, H.; Regasini, L.O.; de Souza Chagas, A.C. Anthelmintic effect of Pterogyne nitens (Fabaceae) on eggs and larvae of Haemonchus contortus: Analyses of structure-activity relationships based on phenolic compounds. Ind. Crops Prod. 2021, 164, 113348. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Jimenez-Chino, A.L.; Bugarin, A.; Zamilpa, A.; Gives, P.M.D.; Villa-Mancera, A.; López-Arellano, M.A.; Olivares-Pérez, J.; Delgado-Núñez, E.J.; González-Cortazar, M. Phenolic acids and flavonoids from Pithecellobium dulce (Robx.) benth leaves exhibit ovicidal activity against Haemonchus contortus. Plants 2022, 11, 2555. [Google Scholar] [CrossRef]
- Auniq, R.B.J.; Chy, M.N.U.; Adnan, M.; Roy, A.; Islam, M.A.; Khan, T.N.; Hasan, M.d.Z.; Ahmed, S.; Khan, M.F.; Islam, N.; et al. Assessment of anti-nociceptive and anthelmintic activities of Vitex Peduncularis Wall. leaves and in silico molecular docking, ADME/T, and PASS prediction studies of its isolated compounds. J. Complement. Med. Res. 2019, 10, 170–185. [Google Scholar] [CrossRef]
- García-Hernández, C.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Zamilpa, A.; Mendoza de Gives, P.; Antonio-Romo, I.A.; Aguilar-Marcelino, L.; Arece-García, J.; Tapia-Maruri, D.; González-Cortazar, M. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp. Parasitol. 2019, 200, 16–23. [Google Scholar] [CrossRef]
- Zarza-Albarrán, M.A.; Olmedo-Juárez, A.; Rojo-Rubio, R.; Mendoza-de Gives, P.; González-Cortazar, M.; Tapia-Maruri, D.; Mondragon-Ancelmo, J.; García- Hernández, C.; Blé-González, E.A.; Zamilpa, A. Galloyl flavonoids from Acacia farnesiana pods possess potent anthelmintic activity against Haemonchus contortus eggs and infective larvae. J. Ethnopharmacol. 2020, 249, 112402. [Google Scholar] [CrossRef]
- Pavičić, A.; Zajíčková, M.; Šadibolová, M.; Svobodová, G.; Matoušková, P.; Szotáková, B.; Langhansová, L.; Maršík, P.; Skálová, L. Anthelmintic activity of European fern extracts against Haemonchus contortus. Vet. Res. 2023, 54, 59. [Google Scholar] [CrossRef]
- De Jesús-Martínez, X.; Olmedo-Juárez, A.; Olivares-Pérez, J.; Rivero-Pérez, N.; González-Cortazar, M.; Zaragoza-Bastida, A.; Villa-Mancera, A.; López-Arellano, M.E.; Gives, P.M.-D.; Cortes-Morales, J. Secondary compounds from Cyrtocarpa procera bark inhibits the biological cycle of Haemonchus contortus: In vitro ovicidal and larvicidal studies. Ind. Crops Prod. 2024, 222, 119477. [Google Scholar] [CrossRef]
- Becerril-Gil, M.M.N.; Estrada-Flores, J.G.; González-Cortazar, M.; Zamilpa, A.; Endara-Agramont, Á.R.; Mendoza-de Gives, P.; López-Arellano, M.E.; Olmedo-Juárez, A. Bioactive compounds from the parasitic plant Arceuthobium vaginatum inhibit Haemonchus contortus egg hatching. Braz. J. Vet. Parasitol. 2023, 33, e013223. [Google Scholar] [CrossRef] [PubMed]
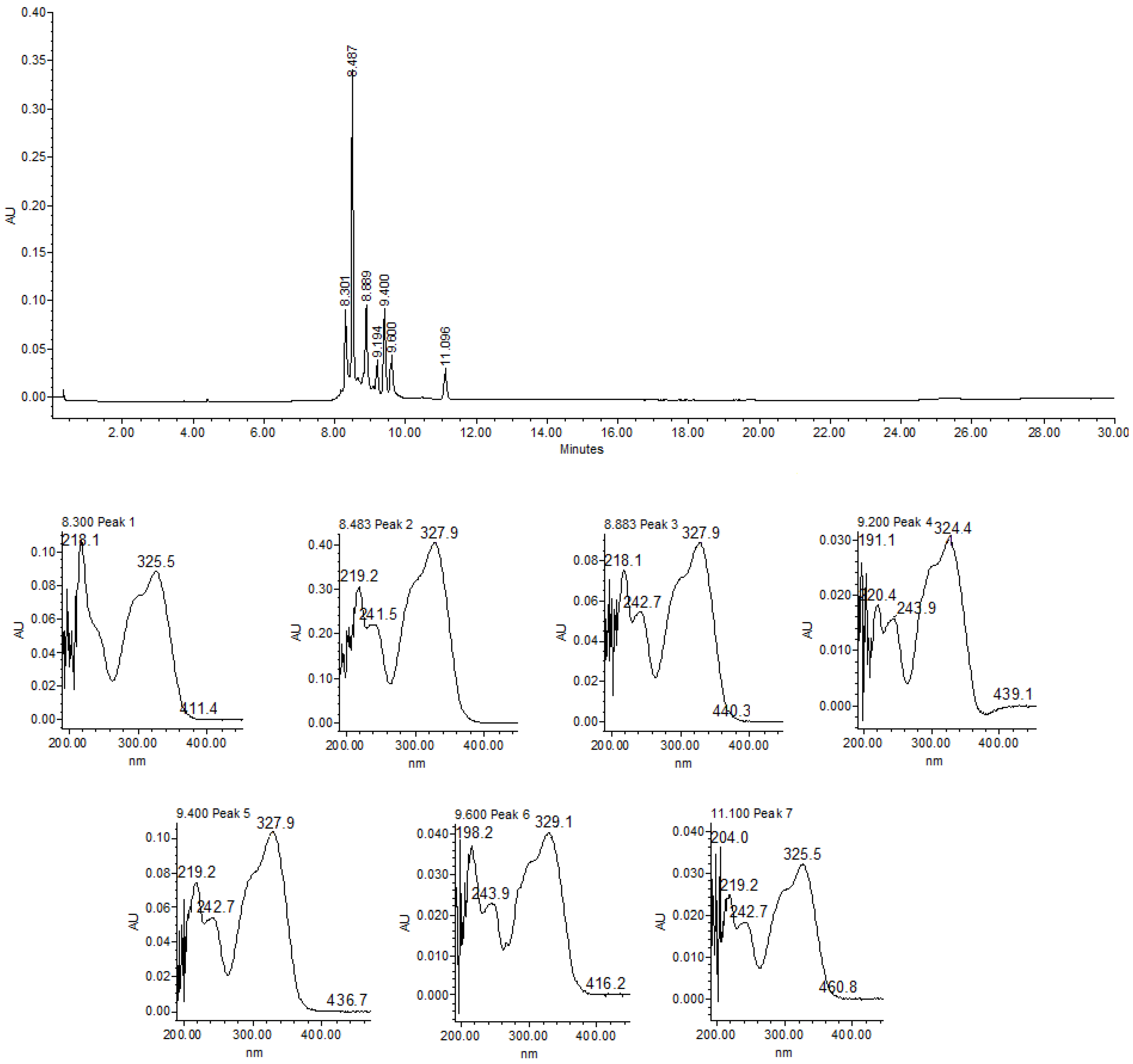
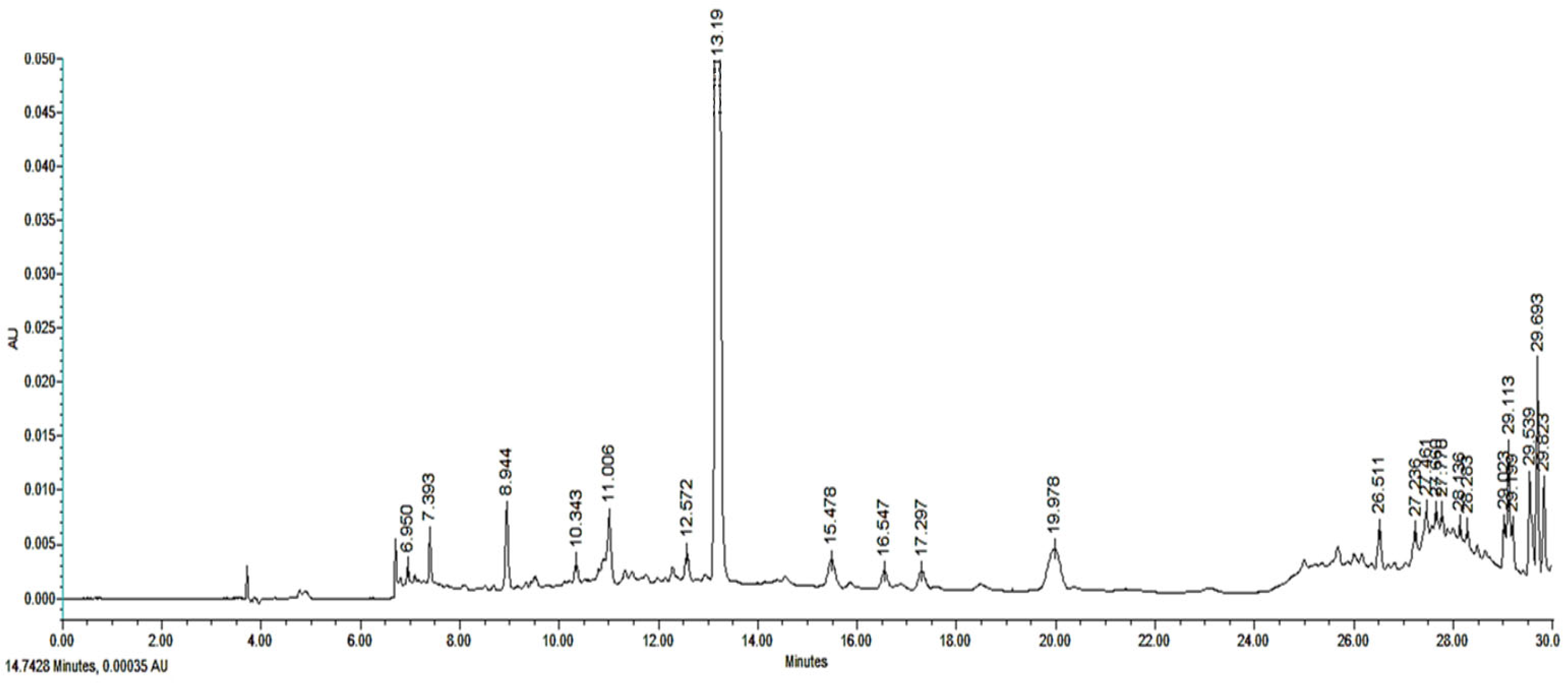
| Treatments | Mean of Eggs and Larvae Recovered | %EHI ± s.d | |
|---|---|---|---|
| Eggs | Larvae | ||
| Distilled water | 3.08 | 126.16 | 2.43 ± 0.59 d |
| Methanol (3%) | 4.25 | 127.25 | 3.19 ± 2.13 d |
| Thiabendazole (0.1 mg/mL) | 129.91 | 0 | 100.00 a |
| HA-E (mg/mL) | |||
| 40.0 | 111.91 | 0 | 100.00 a |
| 20.0 | 99.50 | 15.50 | 86.69 ± 23.04 ab |
| 10.0 | 100.75 | 24.50 | 81.12 ± 26.70 ab |
| 5.0 | 41.75 | 80.25 | 34.39 ± 5.81 cd |
| Aq-F (mg/mL) | |||
| 10.0 | 99.25 | 15.00 | 86.57 ± 7.19 ab |
| 5.0 | 33.87 | 76.62 | 31.56 ± 8.30 cd |
| 2.5 | 3.83 | 115.25 | 3.16 ± 1.14 d |
| EtOAc-F (mg/mL) | |||
| 4.0 | 128.83 | 0.00 | 100.00 a |
| 2.0 | 124.08 | 0.50 | 99.54 ± 0.78 ab |
| 1.0 | 74.67 | 41.00 | 62.49 ± 28.00 cd |
| 0.5 | 16.50 | 97.50 | 14.46 ± 12.02 d |
| Variation coefficient | 21.12 | ||
| R2 | 0.94 | ||
| Treatments | Mean of Eggs and Larvae Recovered | %EHI ± s.d | |
|---|---|---|---|
| Eggs | Larvae | ||
| Distilled water | 3 | 126.75 | 2.40 ± 1.28 mn |
| Methanol (3%) | 4.75 | 127 | 3.67 ± 1.59 mn |
| Thiabendazole (0.1 mg/mL) | 132.25 | 0 | 100.00 a |
| TdR2 (mg/mL) | |||
| 2.0 | 123 | 0 | 100.00 a |
| 1.0 | 123.75 | 4.5 | 96.55 ± 1.18 abc |
| 0.5 | 122.25 | 19.75 | 86. 14 ± 1.00 de |
| 0.25 | 96.75 | 32.75 | 74.80 ± 4.28 fg |
| 0.125 | 48 | 55.5 | 46.67 ± 7.03 h |
| TdR3 (mg/mL) | |||
| 2.0 | 147.75 | 0 | 100.00 a |
| 1.0 | 146.5 | 0 | 100.00 a |
| 0.5 | 138 | 1 | 99.28 ± 0.59 ab |
| 0.25 | 134.75 | 3.25 | 97.62 ± 1.13 abc |
| 0.125 | 130 | 15.75 | 89. 33 ± 1.94 bcd |
| 0.062 | 98.75 | 29.5 | 76.56 ± 4.77 efg |
| TdR4 (mg/mL) | |||
| 2.0 | 136.75 | 0 | 100.00 a |
| 1.0 | 96.75 | 1 | 98.97 ± 1.19 ab |
| 0.5 | 81 | 2.5 | 97.12 ± 1.74 ab |
| 0.25 | 97.5 | 13 | 88.61 ± 3.50 cd |
| 0.125 | 66.5 | 33.25 | 66.79 ± 10.40 g |
| 0.062 | 21.5 | 99 | 17.96 ± 1.41 jkl |
| TdR5 (mg/mL) | |||
| 2.0 | 135.25 | 28.25 | 82.88 ± 5.18 def |
| 1.0 | 75.5 | 86.5 | 46.90 ± 5.74 h |
| 0.5 | 30 | 156.75 | 16.11 ± 4.10 jkl |
| 0.25 | 18.75 | 159.75 | 10.49 ± 2.31 klmn |
| TdR6 (mg/mL) | |||
| 2.0 | 115.25 | 2.25 | 98.13 ± 1.84 abc |
| 1.0 | 101.25 | 19.5 | 84.10 ± 3.39 def |
| 0.5 | 43.25 | 84.4 | 34.06 ± 4.35 i |
| 0.25 | 30.75 | 118.75 | 20.57 ± 2.52 jk |
| TdR7 (mg/mL) | |||
| 2.0 | 136 | 16.75 | 89.03 ± 6.46 bcd |
| 1.0 | 119 | 39.75 | 75.02 ± 5.43 fg |
| 0.5 | 32.5 | 109.25 | 22.55 ± 7.10 j |
| 0.25 | 20.25 | 129.75 | 13.57 ± 2.59 jklm |
| Variation coefficient | 0.99 | ||
| R2 | 6.36 | ||
| Treatments | EC50 (mg/mL) | Confidence Interval | EC90 (mg/mL) | Confidence Interval | Prediction Equations | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | ||||
| HA-E | 6.80 | 6.41 | 7.49 | 18.51 | 16.83 | 20.69 | y = 0.3439ln(x) − 0.1596 |
| Aq-F | 6.36 | 6.02 | 6.69 | 10.69 | 10.00 | 11.57 | y = 0.6626ln(x) − 0.7263 |
| EtOAc-F | 0.84 | 0.80 | 0.88 | 1.39 | 1.39 | 1.50 | y = 0.6807ln(x) + 0.616 |
| TdR2 | 0.139 | 0.125 | 0.154 | 0.553 | 0.505 | 0.612 | y = 0.2502ln(x) + 0.992 |
| TdR3 | 0.028 | 0.022 | 0.034 | 0.123 | 0.112 | 0.136 | y = 0.2348ln(x) + 1.3346 |
| TdR4 | 0.111 | 0.104 | 0.117 | 0.282 | 0.260 | 0.308 | y = 0.3689ln(x) + 1.3109 |
| TdR5 | 1.074 | 1.016 | 1.132 | 2.771 | 2.515 | 3.121 | y = 0.3629ln(x) + 0.474 |
| TdR6 | 0.560 | 0.531 | 0.589 | 1.349 | 1.251 | 1.469 | y = 0.3912ln(x) + 0.7267 |
| TdR7 | 0.783 | 0.700 | 0.777 | 1.915 | 1.775 | 2.086 | y = 0.361ln(x) + 0.6093 |
| Treatments | Mean of Larvae Recovered | %Mortality ± s.d | |
|---|---|---|---|
| Dead | Alive | ||
| Distilled water | 0.7 | 60.7 | 1.27 ± 1.60 e |
| Methanol (3%) | 0.3 | 70.7 | 0.53 ± 1.03 e |
| PVP (60 mg/mL) | 2.2 | 72.0 | 2.83 ± 2.44 e |
| Ivermectin (5 mg/mL) | 73.3 | 12.7 | 100 a |
| EtOAc-F (mg/mL) | |||
| 60 | 76.8 | 14.0 | 84.61 ± 4.13 a |
| 30 | 73.3 | 19.7 | 78.35 ± 6.87 ab |
| 15 | 65.5 | 27.8 | 69.72 ± 9.97 bc |
| 7.50 | 55.1 | 37.7 | 58.64 ± 10.47 c |
| 3.75 | 41.1 | 49.8 | 44.11 ± 9.62 d |
| Variation coefficient | 12.25 | ||
| R2 | 0.96 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortes-Morales, J.A.; Olmedo-Juárez, A.; Tapia-Molina, V.M.; González-Cortazar, M.; Zamilpa, A.; Gives, P.M.-d.; Villa-Mancera, A.; Sachman-Ruiz, B.; Olvera, F.A. Bioactive Compounds from Tithonia diversifolia Aerial Parts Against Eggs and Infective Larvae of the Parasitic Nematode Haemonchus contortus. Pathogens 2025, 14, 884. https://doi.org/10.3390/pathogens14090884
Cortes-Morales JA, Olmedo-Juárez A, Tapia-Molina VM, González-Cortazar M, Zamilpa A, Gives PM-d, Villa-Mancera A, Sachman-Ruiz B, Olvera FA. Bioactive Compounds from Tithonia diversifolia Aerial Parts Against Eggs and Infective Larvae of the Parasitic Nematode Haemonchus contortus. Pathogens. 2025; 14(9):884. https://doi.org/10.3390/pathogens14090884
Chicago/Turabian StyleCortes-Morales, Jorge Alberto, Agustín Olmedo-Juárez, Victoria Michelle Tapia-Molina, Manases González-Cortazar, Alejandro Zamilpa, Pedro Mendoza-de Gives, Abel Villa-Mancera, Bernardo Sachman-Ruiz, and Filiberto Anzures Olvera. 2025. "Bioactive Compounds from Tithonia diversifolia Aerial Parts Against Eggs and Infective Larvae of the Parasitic Nematode Haemonchus contortus" Pathogens 14, no. 9: 884. https://doi.org/10.3390/pathogens14090884
APA StyleCortes-Morales, J. A., Olmedo-Juárez, A., Tapia-Molina, V. M., González-Cortazar, M., Zamilpa, A., Gives, P. M.-d., Villa-Mancera, A., Sachman-Ruiz, B., & Olvera, F. A. (2025). Bioactive Compounds from Tithonia diversifolia Aerial Parts Against Eggs and Infective Larvae of the Parasitic Nematode Haemonchus contortus. Pathogens, 14(9), 884. https://doi.org/10.3390/pathogens14090884









