New Insights into the Pathogenesis of Experimental Cytomegalovirus Retinal Necrosis with an Emphasis on Inflammasomes and Pyroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viruses
2.3. Induction of MAIDS
2.4. Intraocular Injection of MCMV
2.5. Quantification of Infectious MCMV
2.6. Quantitative Real-Time Reverse Transcriptase PCR (RT-PCR) Assay
2.7. Western Blot Assay
2.8. Histopathologic Analysis
2.9. Statistical Analysis
3. Results
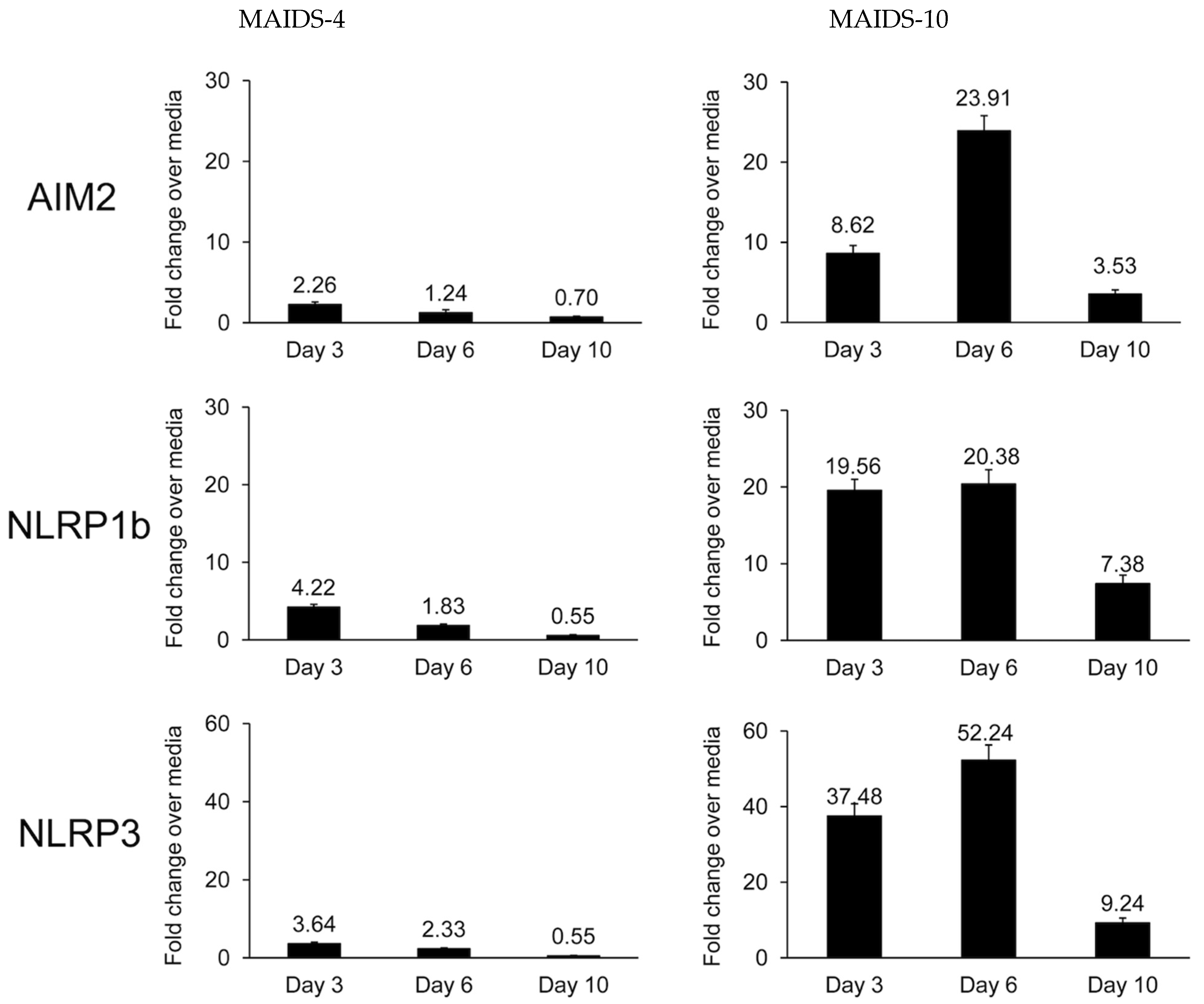
3.1. MCMV-Infected Eyes of Mice with MAIDS-10 Show Significant Stimulation of NLRP3, NLRP1b, and AIM2 Transcripts When Compared with MCMV-Infected Eyes of MAIDS-4 Mice
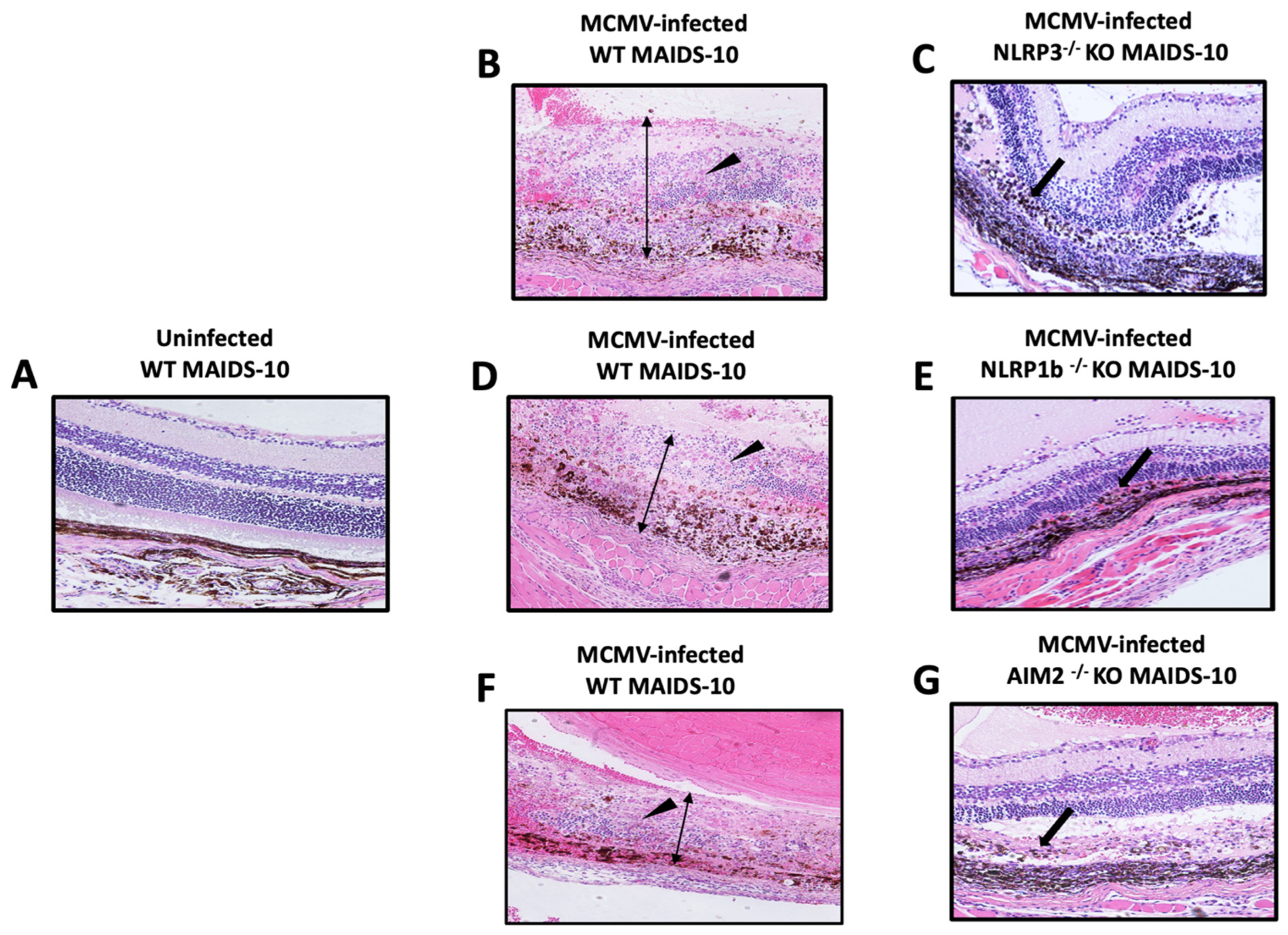
3.2. MCMV-Infected Eyes of MAIDS-10 Mice Deficient in NLRP3, NLRPb, or AIM2 Fail to Develop Full-Thickness Retinal Necrosis as Occurs Within MCMV-Infected Eyes of MAIDS-10 Wildtype Mice
3.3. MCMV-Infected Eyes of MAIDS-10 Mice Deficient in NLRP3, NLRPb, or AIM2 All Show an Atypical Pattern of Retinal Disease
3.4. MCMV-Infected Eyes of MAIDS-10 Mice Deficient in Either NLRP3, NLRP1b, or AIM Harbor Significant Amounts of Infectious Virus but Significantly Less than That Observed for MCMV-Infected Eyes of Wildtype MAIDS-10 Mice
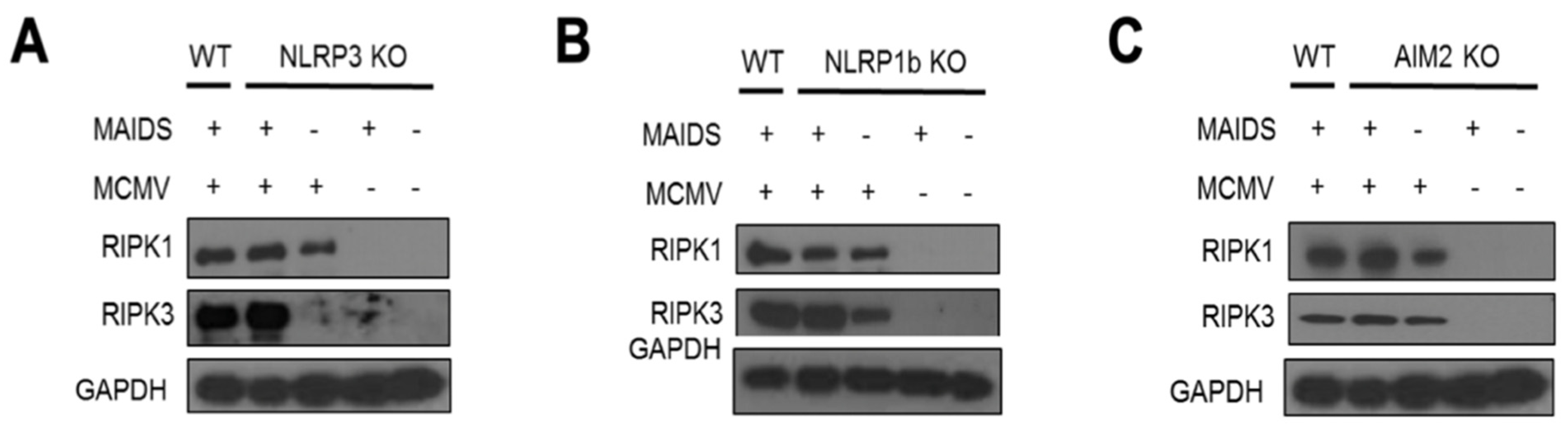
3.5. RIPK1 and RIPK3 of the Necroptosis Pathway Are Stimulated Within MCMV-Infected Eyes of MAIDS-10 Mice Deficient in Either NLRP3, NLRP1b, or AIM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus retinitis: A review. J. Ocul. Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome—Bench to bedside: LXVII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2011, 151, 198–216. [Google Scholar] [CrossRef]
- Heiden, D.; Ford, N.; Wilson, D.; Rodriguez, W.R.; Margolis, T.; Janssens, B.; Bedelu, M.; Tun, N.; Goemaere, E.; Saranchuk, P.; et al. Cytomegalovirus retinitis: The neglected disease of the AIDS pandemic. PLoS Med. 2007, 4, e344. [Google Scholar] [CrossRef] [PubMed]
- Hofman, F.; Hinton, D.R. Tumor necrosis factor-a in the retina of acquired immune deficiency syndrome. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1829–1835. [Google Scholar]
- Bammidi, S.; Koontz, V.; Gautam, P.; Hose, S.; Singa, D.; Ghosh, S. Neutrophils in ocular diseases. Int. J. Mol. Sci. 2024, 25, 7736. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; VandeWalle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermine D for non-canonical inflammasome signaling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Chien, H.; Dix, R.D. Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: Apoptosis, necroptosis, and pyroptosis. J. Virol. 2012, 86, 10961. [Google Scholar] [CrossRef] [PubMed]
- Mocarski, E.S. Cytomegalovirus biology viewed through a cell death suppression lens. Viruses 2024, 16, 1820. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wnag, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmootic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef]
- Xue, Y.; Tuipulotu, D.E.; Tan, W.H.; Kay, C.; Man, M. Emerging activators and retulators of inflammasomes and pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-b. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.D.; Dietrich, W.F. NLRP1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006, 38, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Alston, C.I.; Oh, J.J.; Duncan, L.-A.M.; Nemeno, J.G.E.; Byfield, S.N.; Dix, R.D. Mechanisms of AIDS-related cytomegalovirus retinitis. Future Virol. 2019, 14, 545–560. [Google Scholar] [CrossRef]
- Oh, J.J.; Carter, J.J.; Dix, R.D. A mouse model that mimics AIDS-related cytomegalovirus retinitis: Insights into pathogenesis. Pathogens 2021, 10, 850. [Google Scholar] [CrossRef]
- Sweet, C. The pathogenicity of cytomegalovirius. FEMS Microbiol. Rev. 1999, 23, 457–482. [Google Scholar] [CrossRef]
- Atherton, S.S.; Newell, C.K.; Kanter, M.Y.; Cousins, S.W. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supracilliary route. Curr. Eye Res. 1991, 10, 667–677. [Google Scholar] [CrossRef]
- Duan, Y.; Ji, Z.; Atherton, S.S. Dissemination and replication of MCMV after supraciliary inoculation in immunosuppressed BALB/c mice. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1124–1131. [Google Scholar]
- Morse, H.C., III; Yetter, R.A.; Via, C.S.; Hardy, R.R.; Cerny, A.; Hayakawa, K.; Hugin, A.W.; Miller, M.W.; Holmes, K.L.; Shearer, G.M.; et al. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J. Immunol. 1989, 143, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Morse, H.C., III; Chattopadhyat, S.K.; Makino, M.; Fredrickson, T.N.; Hügin, A.W.; Hartley, J.W. Retrovirus-induced immunodeficiency in a mouse: MAIDS as a model of AIDS. AIDS 1992, 6, 607–621. [Google Scholar] [CrossRef]
- Jolicoeur, P. Murine acquired immunodeficiency syndrome (MAIDS): An animal model to study AIDS pathogenesis. FASEB J. 1991, 5, 238–2405. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Nemeno, J.G.E.; Oh, J.; Houghton, J.E.; Dix, R.D. Atypical cytomegalovirus retinal disease in pyroptosis-deficient mice with murine acquired immunodeficiency syndrome. Exp. Eye Res. 2021, 209, 108651. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Cray, C.; Cousins, S.W. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr. Eye Res. 1994, 13, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Cousins, S.W. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: Correlation with intraocular levels of tumor necrosis factor-a and interferon-g. Curr. Eye Res. 2004, 29, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.J.; Carter, J.J.; Nemeno, J.G.E.; Dix, R.D. Parthanatos-associated proteins are stimulated intraocularly during development of experimental murine cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression. J. Med. Virol. 2019, 92, 394–398. [Google Scholar] [CrossRef]
- Scarpette, G.A.M.; Carter, J.J.; Nemeno, J.G.E.; Dix, R.D. Evidence for the involvement of interleukin-1a during development of experimental cytomegalovirus retinitis in immunosuppressed mice. Cytokine 2021, 144, 155596. [Google Scholar] [CrossRef]
- Mocarski, E.S.; Upton, J.W.; Kaiser, W.J. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat. Rev. Immuno. 2011, 12, 79–88. [Google Scholar] [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.-M. Phorphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interaction protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.-Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Mocarski, E.S.; Guo, H.; Kaiser, W.J. Necroptosis: The Trojan Horse in cell autonomous antiviral host defense. Virology 2015, 160, 479–489. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Patho. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Guo, H.; Koehler, H.S.; Dix, R.D.; Mocarski, E.S. Programmed cell death-dependent host defense in ocular herpes simplex virus infection. Front Microbiol. 2022, 13, 869064. [Google Scholar] [CrossRef]
- Guo, H.; Kaiser, W.J.; Mocarski, E.S. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med. Microbiol. Immunol. 2015, 204, 439–448. [Google Scholar] [CrossRef]
- Skaletskaya, A.; Bartle, L.M.; Chittenden, T.; McCormick, A.L.; Mocarski, E.S.; Goldmacher, V.S. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 2001, 98, 7829–7834. [Google Scholar] [CrossRef]
- Rao, Z.; Shu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.Y.; Moriyama, M.; Iwasaki, A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020, 205, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; So, K.-F.; Lam, W.C.; Lo, A. Novel programmed cell death as therapeutic targets in age-related macular degeneration. Int. J. Mol. Sci. 2020, 21, 7279. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of photoreceptor death in retinitis pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-J.; Fan, C.-L.; Hu, X.-M.; Ban, X.-X.; Wan, H.; He, Y.; Zhang, Q.; Xiong, K. Regulated cell death of retinal ganglion cells in glaucoma: Molecular insights and therapeutic potentials. Cell Mol. Neurobiol. 2023, 43, 3161–3178. [Google Scholar] [CrossRef]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Den, C.; Li, J.; Jiang, N.; Huang, S.; Long, E.; Zhuo, Y. LncRNA H19 initiates microglial pyroptosis and neuronal cell death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020, 27, 176–191. [Google Scholar] [CrossRef]
- Deng, Y.; Ostermann, E.; Brune, W.A. Cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis. Nat. Commun. 2024, 15, 786. [Google Scholar] [CrossRef]
- Deng, Y.; Agueda-Pinto, A.; Burne, W. No time to die: How cytomegaloviruses suppress apoptosis, necroptosis, and pyroptosis. Viruses 2024, 16, 1272. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, D.; Huang, H.; Lu, Y.; Liao, Y.; Liu, L.; Liu, X.; Fang, F. Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome. Virol. J. 2017, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Imre, G. Pyroptosis in health and disease. Am. J. Physiol. Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Schneider, D.H.; Hisamuddin, A.M.; Dix, R.D. Murine cytomegalovirus and human cytomegalovirus differ in pyroptosis induction in different cell types during productive replication. Viruses 2025, 17, 1106. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Y.; Meng, X.; Li, J.; Liu, X.; Liu, H. PANoptosis: Mechanisms, biology, and role in disease. Immunol. Rev. 2024, 321, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cao, P.; Wang, Y.; Zhang, Q.; Zhang, D.; Wang, Y.; Wang, L.; Gong, Z. PANoptosis: A cell death characterized by pyroptosis, apoptosis, and necroptosis. J. Inflamm. Res. 2023, 12, 1523–1532. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Pandeya, A.; Li, L.; Li, Z.; Wei, Y. Gasdermin D (GSDMD) as a new target for the treatment of infection. Medchemcomm 2019, 10, 660–667. [Google Scholar] [CrossRef]
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6676. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.D.; Borase, H.; Gagan, S.; Sharma, P.; Kapoor, D.; Yadavalli, T.; Jain, S.; Joseph, J.; Bagga, B.; Shukla, D. Rapid NETosis is an effector mechanism to combat ocular herpes infection. Investig. Ophthalmol Vis. Sci. 2024, 65, 36. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.; Alston, C.I.; Dix, R.D. Suppressor of cytokine signaling (SOCS) 1 and SOCS3 are stimulated within the eye during experimental murine cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression. J. Virol. 2018, 92, e00526-18. [Google Scholar] [CrossRef] [PubMed]

| Signaling Pathway | Consequences | Proinflammatory? | Pathway Suppression via Virus-Encoded Gene b | |
|---|---|---|---|---|
| Extrinsic Apoptosis | TNFR1 | Cell lysis | M36 (MCMV) | |
| TNF | Phagocytosis | No | UL36 (HCMV) | |
| Caspase-8 | ||||
| Caspase-3 | ||||
| Canonical Pyroptosis | Inflammasomes | Cell lysis | M84 (MCMV) | |
| Caspase-1 | Release of | Yes | UL83 (HCMV) | |
| Gasdermin D | ||||
| Necroptosis | RIPK1 | Cell lysis | M45 (MCMV) | |
| RIPK3 | Release of | Yes | ||
| MLKL | cellular contents |
| Wildtype MAIDS-10 | 89% (8/9) |
|---|---|
| NLRP3−/− MAIDS-10 | 0% (0/4) |
| NLRP1b−/− MAIDS-10 | 0% (0/4) |
| AIM2−/− MAIDS-10 | 0% (0/4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dix, R.D.; Carter, J.J.; Koehler, H.; Guo, H. New Insights into the Pathogenesis of Experimental Cytomegalovirus Retinal Necrosis with an Emphasis on Inflammasomes and Pyroptosis. Pathogens 2025, 14, 879. https://doi.org/10.3390/pathogens14090879
Dix RD, Carter JJ, Koehler H, Guo H. New Insights into the Pathogenesis of Experimental Cytomegalovirus Retinal Necrosis with an Emphasis on Inflammasomes and Pyroptosis. Pathogens. 2025; 14(9):879. https://doi.org/10.3390/pathogens14090879
Chicago/Turabian StyleDix, Richard D., Jessica J. Carter, Heather Koehler, and Hongyan Guo. 2025. "New Insights into the Pathogenesis of Experimental Cytomegalovirus Retinal Necrosis with an Emphasis on Inflammasomes and Pyroptosis" Pathogens 14, no. 9: 879. https://doi.org/10.3390/pathogens14090879
APA StyleDix, R. D., Carter, J. J., Koehler, H., & Guo, H. (2025). New Insights into the Pathogenesis of Experimental Cytomegalovirus Retinal Necrosis with an Emphasis on Inflammasomes and Pyroptosis. Pathogens, 14(9), 879. https://doi.org/10.3390/pathogens14090879









