Optimization of (Dithioperoxo)thiolate-Based Antifungal Agents for Triazole-Resistant Aspergillus Fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
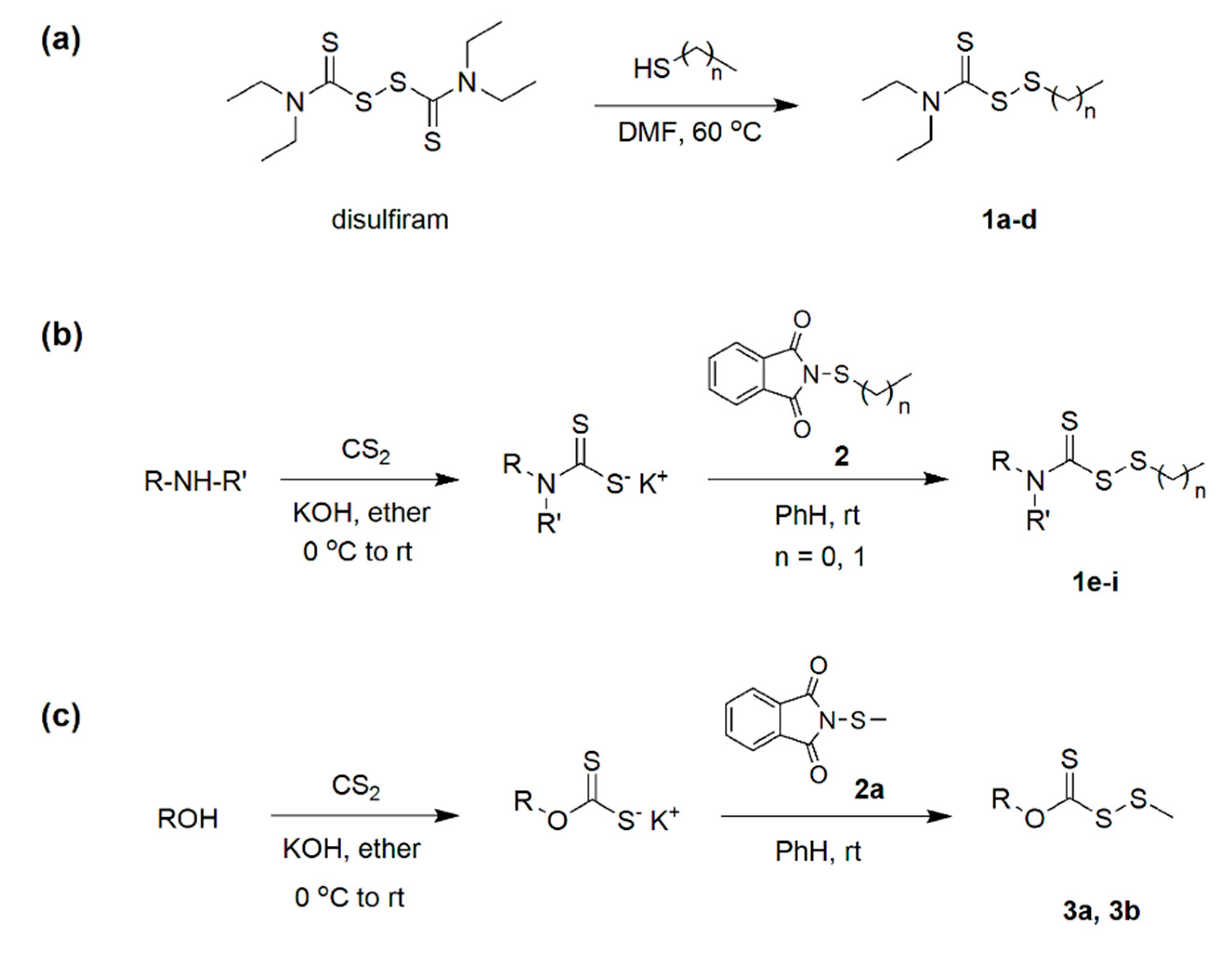
2.1.1. Preparation of Carbamo(dithioperoxo)thiolates (1a–d); General Procedure
2.1.2. Preparation of N-Alkylthiophthalimide (2); General Procedure
2.1.3. Preparation of Carbamo(dithioperoxo)thiolates (1e–i); General Procedure
2.1.4. Preparation of Carbano(dithioperoxo)thioates (3); General Procedure
2.2. Antimicrobial Studies
2.2.1. MIC Determination
2.2.2. Growth Studies
2.2.3. Checkerboard Studies
2.3. Cytotoxicity Studies
2.3.1. IC50 Determination
2.3.2. Hematological Analysis
3. Results
3.1. (Dithioperoxo)thiolate Synthesis
3.2. Susceptibility Testing
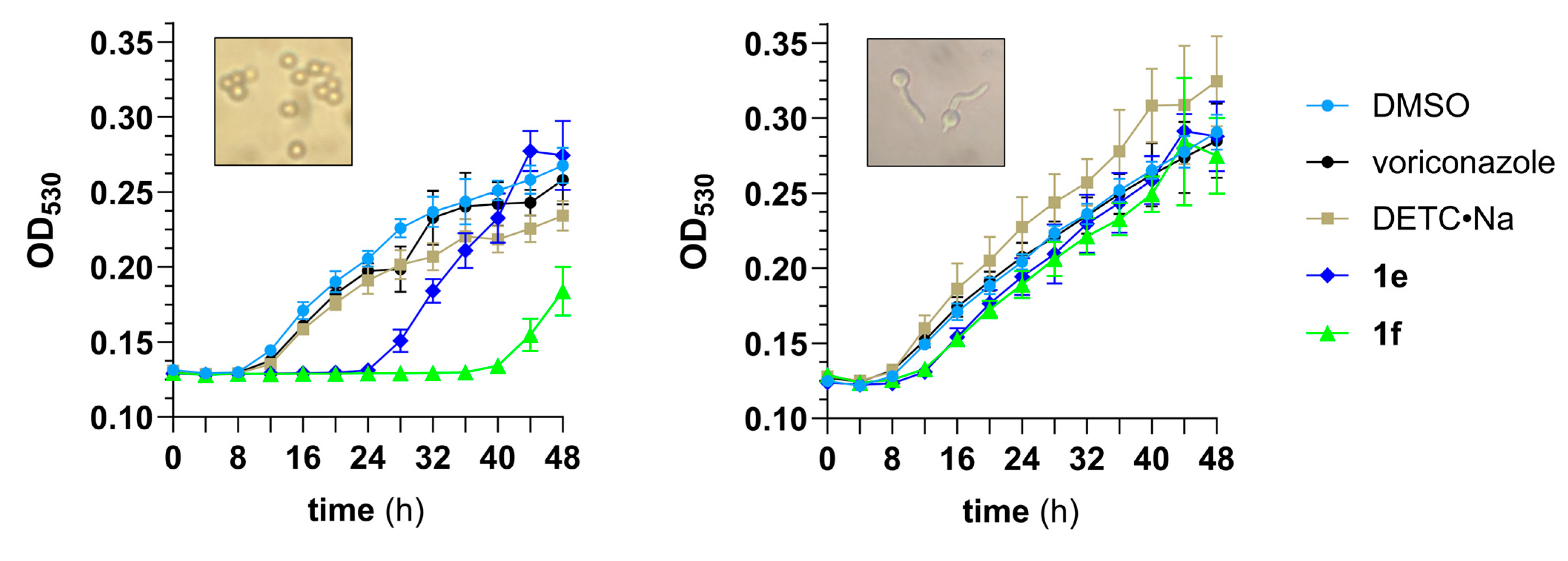
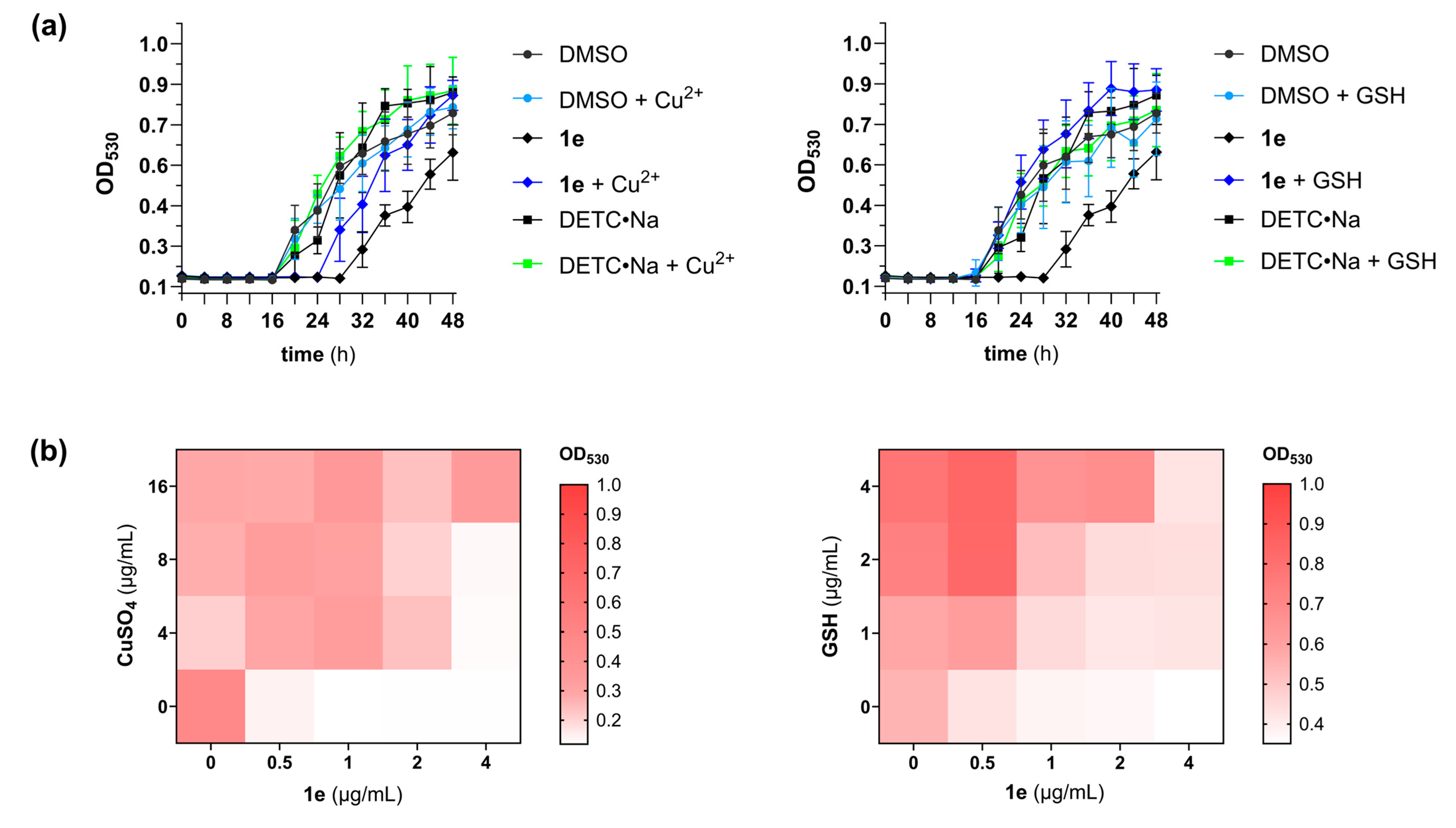
3.3. Pharmacodynamic Studies
3.4. Cytotoxicity Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segal, B.H.; Walsh, T.J. Current approaches to diagnosis and treatment of invasive aspergillosis. Am. J. Respir. Crit. Care Med. 2006, 173, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Lewis, J.S., 2nd; Wiederhold, N.P.; Hakki, M.; Thompson, G.R., 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob. Agents Chemother. 2022, 66, e0017722. [Google Scholar] [CrossRef]
- CLSI. Voriconazole Breakpoints for Aspergillus fumigatus, 1st ed; CLSI rationale document FR01; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2024; ISBN 978-1-68440-230-4. [Google Scholar]
- Slavin, M.A.; Chen, Y.-C.; Cordonnier, C.; Cornely, O.A.; Cuenca-Estrella, M.; Donnelly, J.P.; Groll, A.H.; Lortholary, O.; Marty, F.M.; Nucci, M.; et al. When to Change Treatment of Acute Invasive Aspergillosis: An Expert Viewpoint. J. Antimicrob. Chemother. 2021, 77, 16–23. [Google Scholar] [CrossRef]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Hagiwara, D.; Watanabe, A.; Kamei, K.; Goldman, G.H. Epidemiological and Genomic Landscape of Azole Resistance Mechanisms in Aspergillus Fungi. Front. Microbiol. 2016, 7, 1382. [Google Scholar] [CrossRef]
- Shanholtzer, C.N.; Rice, C.; Watson, K.; Carreon, H.; Long, T.E. Effect of copper on the antifungal activity of disulfiram (Antabuse®) in fluconazole-resistant Candida strains. Med. Mycol. 2022, 60, myac016. [Google Scholar] [CrossRef]
- Custodio, M.M.; Sparks, J.; Long, T.E. Disulfiram: A Repurposed Drug in Preclinical and Clinical Development for the Treatment of Infectious Diseases. Antiinfect. Agents 2022, 20, e040122199856. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.R.; Moore, J.A.; Long, T.E. Antibacterial activity of disulfiram and its metabolites. J. Appl. Microbiol. 2019, 126, 79–86. [Google Scholar] [CrossRef]
- Long, T.E.; Naidu, S.T.; Hissom, E.G.; Meka, Y.; Chavva, H.; Brown, K.C.; Valentine, M.E.; Fan, J.; Denvir, J.; Primerano, D.A.; et al. Disulfiram induces redox imbalance and perturbations in central glucose catabolism and metal homeostasis to inhibit the growth of Staphylococcus aureus. Sci. Rep. 2025, 15, 15658. [Google Scholar] [CrossRef]
- Lewis, A.D.; Riedel, T.M.; Kesler, M.B.A.; Varney, M.E.; Long, T.E. Pharmacological evaluation of disulfiram analogs as antimicrobial agents and their application as inhibitors of fosB-mediated fosfomycin resistance. J. Antibiot. 2022, 75, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.G.; Frazier, K.R.; Saralkar, P.; Hossain, M.F.; Geldenhuys, W.J.; Long, T.E. Disulfiram-based disulfides as narrow-spectrum antibacterial agents. Bioorg. Med. Chem. Lett. 2018, 28, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.G.; Long, T.E. Allicin-inspired thiolated fluoroquinolones as antibacterials against ESKAPE pathogens. Bioorg. Med. Chem. Lett. 2016, 26, 5545–5549. [Google Scholar] [CrossRef]
- Heldreth, B.; Long, T.E.; Jang, S.; Reddy, G.S.K.; Turos, E.; Dickey, S.; Lim, D.V. N-Thiolated β-lactam antibacterials: Effects of the N-organothio substituent on anti-MRSA activity. Bioorg. Med. Chem. 2006, 14, 3775–3784. [Google Scholar] [CrossRef]
- Satyanarayana, A.N.V.; Pattanayak, P.; Nayar, P.P.; Chatterjee, T. Iodine-mediated, chalcogen–chalcogen bond formation in water: Green synthesis of carbamo(dithioperoxo)thioates, carbamo(selenothioperoxo)thioates, carbono(dithioperoxo)thioates, and carbono(selenothioperoxo)thioates. Green Chem. 2025, 27, 1054–1061. [Google Scholar] [CrossRef]
- Schroll, A.L.; Eastep, S.J.; Barany, G. Synthesis and characterization of methoxy(thiocarbonyl)sulfenyl chloride. J. Org. Chem. 1990, 55, 1475–1479. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI standard M27; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI standard M38; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Moody, J.A. Synergism testing: Broth microdilution checkboard and broth macrodilution methods. In Clinical Procedures Handbook; Isenberg, H.D., Ed.; ASM Press: Washington, DC, USA, 1992; pp. 5.18.1–5.18.28. [Google Scholar]
- Adeluola, A.A.; Bosomtwe, N.; Long, T.E.; Amin, A.R.M.R. Context-dependent activation of p53 target genes and induction of apoptosis by actinomycin D in aerodigestive tract cancers. Apoptosis 2022, 27, 342–353. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Chavva, H.; Meka, Y.; Long, T.E. Antimicrobial pharmacodynamics of vancomycin and disulfiram (Antabuse®) in Staphylococcus aureus. Front. Microbiol. 2023, 13, 1092257. [Google Scholar] [CrossRef]
- Sato, I.; Shimizu, M.; Hoshino, T.; Takaya, N. The glutathione system of Aspergillus nidulans involves a fungus-specific glutathione S-transferase. J. Biol. Chem. 2009, 284, 8042–8053. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, P.; Gao, M.; Lan, W.; Sun, S.; Ma, Z.; Sultani, R.; Cui, Y.; Umar, M.N.; Khan, S.W.; et al. Amphotericin B Tamed by Salicylic Acid. ACS Omega 2022, 7, 14690–14696. [Google Scholar] [CrossRef]
- Handelman, M.; Meir, Z.; Shadkchan, Y.; Kandil, A.A.; Amano, O.; Mariscal, M.; López-Berges, M.S.; Osherov, N.; Mitchell, A.P. Evolution of the pathogenic mold Aspergillus fumigatus on high copper levels identifies novel resistance genes. mSphere 2024, 9, e0025324. [Google Scholar] [CrossRef] [PubMed]
- Baselt, R.C.; Sunderman, F.W., Jr.; Mitchell, J.; Horak, E. Comparisons of antidotal efficacy of sodium diethyldithiocarbamate, D-penicillamine and triethylenetetramine upon acute toxicity of nickel carbonyl in rats. Res. Commun. Chem. Pathol. Pharmacol. 1977, 18, 677–688. [Google Scholar] [PubMed]
- Blume, L.; Long, T.E.; Turos, E. Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials. Int. J. Mol. Sci. 2023, 24, 8659. [Google Scholar] [CrossRef] [PubMed]
- Loi, V.V.; Huyen, N.T.T.; Busche, T.; Tung, Q.N.; Gruhlke, M.C.H.; Kalinowski, J.; Bernhardt, J.; Slusarenko, A.J.; Antelmann, H. Staphylococcus aureus responds to allicin by global S-thioallylation—Role of the Brx/BSH/YpdA pathway and the disulfide reductase MerA to overcome allicin stress. Free Radic. Biol. Med. 2019, 139, 55–69. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I. Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Baltussen, T.J.H.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Molecular Mechanisms of Conidial Germination in Aspergillus spp. Microbiol. Mol. Biol. Rev. 2019, 84, e00049-19. [Google Scholar]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Johansson, B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. Suppl. 1992, 369, 15–26. [Google Scholar] [CrossRef] [PubMed]

| Compd | n | Species a|Modal MIC: µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans AR-0761 b | C. glabrata AR-1104 b | C. krusei AR-0397 b | C. tropicalis AR-1098 b | C. auris AR-1104 b | C. neoformans NIH-306 c | A. fumigatus AR-0731 c | ||
| 1a | 1 | 0.5 (2) | 1 (5) | 0.5 (2) | 1 (5) | 2 (10) | 1 (5) | 2 (10) |
| 1b | 3 | 1 (2) | 2 (8) | 2 (8) | 2 (8) | 4 (17) | 1 (4) | 4 (17) |
| 1c | 5 | 1 (4) | 2 (8) | 2 (8) | 2 (8) | 4 (15) | 1 (4) | 8 (30) |
| 1d | 7 | 2 (7) | 2 (7) | 4 (17) | 16 (55) | 16 (55) | 2 (7) | >32 (>109) |
| disulfiram | 1 (3) | 8 (27) | 1 (3) | 8 (27) | 2 (7) | 8 (27) | >16 (>54) | |
| fluconazole | >16 (>52) | >16 (>52) | >16 (>52) | >16 (>52) | >16 (>52) | 8 (23) | >16 (>52) | |
| voriconazole | >16 (>45) | 4 (12) | 2 (6) | 8 (23) | 1 (3) | ≤0.5 (≤1) | 4 (12) | |
| amphotericin B | 1 (1) | 0.5 (0.5) | 1 (1) | 1 (1) | 1 (1) | 0.5 (0.5) | 1 (1) | |
| Compd | R | R’ | n | Species|Modal MIC: µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans AR-0761 a | C. glabrata AR-1104 a | C. krusei AR-0397 a | C. tropicalis AR-1098 a | C. auris AR-1104 a | C. neoformans NIH-306 b | A. fumigatus AR-0731 b | ||||
| 1e | Et | Et | 0 | 1 (5) | 0.5 (3) | 1 (5) | 0.5 (3) | 1 (5) | 2 (10) | 2 (10) |
| 1f | Me | Me | 0 | 1 (6) | 2 (12) | 2 (12) | 2 (12) | 2 (12) | 2 (12) | 1 (6) |
| 1g | Me | Me | 1 | 0.5 (3) | 1 (6) | 1 (6) | 1 (6) | 1 (6) | 1 (6) | 1 (6) |
| 1h | Me | Bn | 1 | 1 (4) | 0.5 (2) | 0.5 (2) | 2 (8) | 2 (8) | 0.5 (2) | 4 (16) |
| 1i | Me | MeO | 0 | 1 (6) | 1 (5.5) | 1 (6) | 2 (11) | 1 (6) | 4 (22) | 4 (22) |
| 3a | Me | 0 | 16 (104) | 8 (52) | 16 (104) | 16 (104) | 8 (52) | 8 (52) | >16 (>104) | |
| 3b | Et | 0 | 16 (95) | 8 (48) | 16 (95) | 16 (95) | 8 (48) | 8 (48) | >16 (>95) | |
| Strain | Cyp51A Mutation | Compd|Modal MIC: µg/mL (µM) | |||||
|---|---|---|---|---|---|---|---|
| 1a | 1e | 1f | 1g | DETC•Na a | Voriconazole | ||
| AR-0732 | F495I, L98H, S297T, TR34 | 2 (10) | 2 (10) | 1 (6) | 2 (11) | >16 (>71) | 2 (6) |
| AR-0733 | L98H, TR34 | 2 (10) | 2 (10) | 1 (6) | 1 (6) | >16 (>71) | 4 (12) |
| AR-0734 | L98H, TR34 | 4 (19) | 2 (10) | 1 (6) | 4 (22) | >16 (>71) | 4 (12) |
| AR-0735 | F495I, L98H, S297T, TR34 | 2 (10) | 2 (10) | 1 (6) | 2 (11) | >16 (>71) | 2 (6) |
| AR-1283 | M220V | 2 (10) | 2 (10) | 2 (12) | 1 (6) | >16 (>71) | 1 (3) |
| AR-1292 | M220K | 2 (10) | 2 (10) | 2 (12) | 1 (6) | >16 (>71) | 2 (6) |
| AR-1293 | G54R | 1 (5) | 1 (5) | 2 (12) | 1 (6) | >16 (>71) | 2 (6) |
| AR-1294 | T289A, TR46, Y121 | 1 (5) | 1 (5) | 2 (12) | 2 (11) | ≥16 (≥71) | >16 (>45) |
| AR-1295 | T289A, TR46, Y121 | 4 (19) | 2 (10) | 2 (12) | 4 (22) | ≥16 (≥71) | >16 (>45) |
| AR-1296 | T289A, TR46, Y121 | 1 (5) | 1 (5) | 2 (12) | 4 (22) | >16 (>71) | >16 (>45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuturi, S.; Jobe, K.L.; Varney, M.E.; Hambuchen, M.D.; Amin, A.R.M.R.; Long, T.E. Optimization of (Dithioperoxo)thiolate-Based Antifungal Agents for Triazole-Resistant Aspergillus Fumigatus. Pathogens 2025, 14, 878. https://doi.org/10.3390/pathogens14090878
Karuturi S, Jobe KL, Varney ME, Hambuchen MD, Amin ARMR, Long TE. Optimization of (Dithioperoxo)thiolate-Based Antifungal Agents for Triazole-Resistant Aspergillus Fumigatus. Pathogens. 2025; 14(9):878. https://doi.org/10.3390/pathogens14090878
Chicago/Turabian StyleKaruturi, Surya, Kaitlyn L. Jobe, Melinda E. Varney, Michael D. Hambuchen, A. R. M. Ruhul Amin, and Timothy E. Long. 2025. "Optimization of (Dithioperoxo)thiolate-Based Antifungal Agents for Triazole-Resistant Aspergillus Fumigatus" Pathogens 14, no. 9: 878. https://doi.org/10.3390/pathogens14090878
APA StyleKaruturi, S., Jobe, K. L., Varney, M. E., Hambuchen, M. D., Amin, A. R. M. R., & Long, T. E. (2025). Optimization of (Dithioperoxo)thiolate-Based Antifungal Agents for Triazole-Resistant Aspergillus Fumigatus. Pathogens, 14(9), 878. https://doi.org/10.3390/pathogens14090878







