Pathogen Safety Issues Around the “Blood Scandals” 1995–2024—A Perspective Built on Experience
Abstract
1. Introduction
2. The Guilty Pathogens
3. The Third Pillar
4. The “Blood Scandal” Inquiries—Some Examples
5. Some Common Features in “Blood Scandal” Inquiries
- The timeline of the plasma industry’s progression to products safe from viral transmission, both in the commercial and the publicly funded agencies;
- The issue of self-sufficiency in plasma products;
- The exposure of patients to clinical studies;
- The development of regulatory oversight of the industry;
- Informed consent and patient reactions.
5.1. The Progression Towards Viral Inactivation
5.2. The Issue of Self-Sufficiency in Plasma Products
5.3. The Exposure of Patients to Clinical Studies
5.4. The Development of Regulatory Oversight of the Industry
“Where uncertainties or countervailing public health concerns preclude completely eliminating potential risks, the FDA should encourage, and where necessary require, the blood industry to implement partial solutions that have little risk of causing harm.”
“When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically. In this context the proponent of an activity, rather than the public, should bear the burden of proof.”[66]
“Preventive action should be taken when there is evidence that a potentially disease-causing agent is or may be blood borne, even when there is no evidence that recipients have been affected. If harm can occur, it should be assumed that it will occur. If there are no measures that will entirely prevent the harm, measures that may only partially prevent transmission should be taken.”[67]
5.5. Informed Consent and Patient Reactions
- It disallowed patients, or in many instances, their parents, from opting out of the treatment offered, because of the possibility of pathogen transmission;
- It obviated the possibility of alternatives to the treatments offered, again leading the patients to an option which led to infection.
6. Final Reflections
Funding
Acknowledgments
Conflicts of Interest
References
- Farrugia, A. The Evolution of the Safety of Plasma Products from Pathogen Transmission—A Continuing Narrative. Pathogens 2023, 12, 318. [Google Scholar] [CrossRef]
- Larsson, S.A. Life expectancy of Swedish haemophiliacs, 1831–1980. Br. J. Haematol. 1985, 59, 593–602. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Varekamp, I.; Smit, C.; Bröcker-Vriends, A.H.J.T.; Van Dijck, H.; Vandenbroucke, J.P.; Hermans, J.; Suurmeijer, T.P.B.M.; Briët, E. Mortality and causes of death in Dutch haemophiliacs, 1973–1986. Br. J. Haematol. 1989, 71, 71–76. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Gdovin, S.; Gringeri, A.; Colombo, M.; Mele, A.; Schinaia, N.; Ciavarella, N.; Emerson, S.U.; Purcell, R.H.; The Italian Collaborative Group. Transmission of hepatitis A to patients with hemophilia by factor VIII concentrates treated with organic solvent and detergent to inactivate viruses. Ann. Intern. Med. 1994, 120, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huzly, D.; Umhau, M.; Bettinger, D.; Cathomen, T.; Emmerich, F.; Hasselblatt, P.; Hengel, H.; Herzog, R.; Kap-pert, O.; Maassen, S.; et al. Transfusion-transmitted hepatitis E in Germany, 2013. Eurosurveillance 2014, 19, 20812. [Google Scholar] [CrossRef]
- Laurian, Y.; Dussaix, E.; Parquet, A.; Chalvon-Demersay, A.; d’Oiron, R.; Tchernia, G. Transmission of human parvovirus B19 by plasma derived factor VIII concentrates. Nouv. Rev. Fr. Hematol. 1994, 36, 449–453. [Google Scholar] [PubMed]
- Schmid, R. History of viral hepatitis: A tale of dogmas and misinterpretations. J. Gastroenterol. Hepatol. 2001, 16, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.R.; Havens, W.P., Jr.; Sabin, A.B.; Philip, C.B. Transmission experiments in serum jaundice and infectious hepatitis. JAMA 1945, 128, 911–915. [Google Scholar] [CrossRef]
- Krugman, S.; Giles, J.P.; Hammond, J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiologi-cal, and immunological types of infection. JAMA 1967, 200, 365–373. [Google Scholar] [CrossRef]
- Alter, H.J.; Klein, H.G. The hazards of blood transfusion in historical perspective. Blood 2008, 112, 2617–2626. [Google Scholar] [CrossRef]
- Alter, H. Hepatitis C virus-from Hippocrates to cure. Hamdan Med. J. 2016, 9, 309. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, C.; Shi, J.-J.; Zhang, J.-Y.; Wang, F.-S. Hepatitis C: Milestones from discovery to clinical cure. Mil. Med. Res. 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Perkins, H.A.; Busch, M.P. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion 2010, 50, 2080–2099. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Nasir, J.A.; Hinde, A. The prevalence of hepatitis C virus infection in β-thalassemia patients in Pa-kistan: A systematic review and meta-analysis. BMC Public Health 2020, 20, 587. [Google Scholar] [CrossRef]
- Nishiya, A.S.; Ferreira, S.C.; Salles, N.A.; Rocha, V.; Mendrone-Júnior, A. Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil. Viruses 2022, 14, 2214. [Google Scholar] [CrossRef]
- Busch, M.P.; Bloch, E.M.; Kleinman, S. Prevention of transfusion-transmitted infections. Blood 2019, 133, 1854–1864. [Google Scholar] [CrossRef]
- Lynch, T.J.; Weinstein, M.J.; Tankersley, D.L.; Fratantoni, J.C.; Finlayson, J.S. Considerations of pool size in the manufacture of plasma derivatives. Transfusion 1996, 36, 770–775. [Google Scholar] [CrossRef]
- Soucie, J.M.; Richardson, L.C.; Evatt, B.L.; Linden, J.V.; Ewenstein, B.M.; Stein, S.F.; Leissinger, C.; Manco-Johnson, M.; Sexauer, C.L. Risk factors for infection with HBV and HCV in a largecohort of hemophiliac males. Transfusion 2001, 41, 338–343. [Google Scholar] [CrossRef]
- Horowitz, B.; Prince, A.M.; Hamman, J.; Watklevicz, C. Viral safety of solvent/detergent-treated blood products. Blood Coagul. Fibrinolysis 1994, 5, S21. [Google Scholar] [CrossRef]
- Burnouf-Radosevich, M.; Appourchaux, P.; Huart, J.J.; Burnouf, T. Nanofiltration, a new specific virus elimination method applied to high-purity factor IX and factor XI concentrates. Vox Sang. 1994, 67, 132–138. [Google Scholar] [PubMed]
- Picard, A. Krever Inquiry. In The Canadian Encyclopedia. 2006. Available online: https://thecanadianencyclopedia.ca/en/article/krever-inquiry (accessed on 8 August 2025).
- Institute of Medicine (US) Committee to Study HIV Transmission Through Blood and Blood Products. HIV and the Blood Supply: An Analysis of Crisis Decisionmaking; Leveton, L.B., Sox, H.C., Stoto, M.A., Eds.; National Academies Press (US): Washington, DC, USA, 1995. Available online: http://www.ncbi.nlm.nih.gov/books/NBK232417/ (accessed on 21 March 2024).
- Lindsay, A. Report of the Tribunal of Inquiry into the Infection with HIV and Hepatitis C of Persons with Haemophilia and Related Matters|Department of Health. 2002. Available online: http://health.gov.ie/blog/publications/report-of-the-tribunal-of-inquiry-into-the-infection-with-hiv-and-hepatitis-c-of-persons-with-haemophilia-and-related-matters/ (accessed on 28 September 2015).
- Steffen, M. The Nation’s Blood Medicine, Justice, and the State in France. In Blood Feuds: Aids, Blood, and the Politics of Medical Disaster; Feldman, E., Bayer, R., Eds.; Oxford University Press: Mumbai, India, 1999. [Google Scholar] [CrossRef]
- Barraclough, B. Report of the Expert Advisory Group on Hepatitis C and Plasma in 1990. 1993. Available online: https://online.fliphtml5.com/isbjv/rjau/ (accessed on 8 August 2025).
- McLucas, J. Hepatitis C and the Blood Supply in Australia. 2004. Available online: https://www.aph.gov.au/parliamentary_business/committees/senate/community_affairs/completed_inquiries/2002-04/hepc/report/index (accessed on 8 August 2025).
- Penrose, G. The Penrose Inquiry. 2015. Available online: https://webarchive.nrscotland.gov.uk/20170401015224/http://www.penroseinquiry.org.uk/finalreport/text/354876_chapter_24.html (accessed on 27 June 2025).
- About the Inquiry|Infected Blood Inquiry. 2024. Available online: https://www.infectedbloodinquiry.org.uk/about (accessed on 8 July 2025).
- Makris, M.; O’Mahony, B. Learnings from the United Kingdom infected blood inquiry. Res Pract Thromb Haemost. 2024, 8, 102458. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A. The UK Infected Blood Inquiry: A Personal Reflection. Haemophilia. 2025. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/hae.70043 (accessed on 27 June 2025).
- Foster, P.R. The UK Infected Blood Inquiry: A Personal Reflection. Haemophilia. 2025. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/hae.70103 (accessed on 8 August 2025).
- Feldman, P.A. Scientific Review of the UK Infected Blood Inquiry Report: A Personal Reflection. Haemophilia. 2025. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/hae.70100 (accessed on 8 August 2025).
- Feldman, E.; Bayer, R. Blood Feuds: AIDS, Blood, and the Politics of Medical Disaster; New Edition; Oxford University Press: New York, NY, USA, 1999; 400p. [Google Scholar]
- Human Rights Watch. Life Doesn’t Wait. 2006. Available online: https://www.hrw.org/report/2006/08/01/life-doesnt-wait/romanias-failure-protect-and-support-children-and-youth-living (accessed on 26 August 2025).
- Guan, Y. China’s Blood-Borne HIV Catastrophe Revisited. Mod. China 2020, 46, 372–399. [Google Scholar] [CrossRef]
- Isfordink, C.J.; van Erpecum, K.J.; van der Valk, M.; Mauser-Bunschoten, E.P.; Makris, M. Viral hepatitis in haemophilia: Historical perspective and current management. Br. J. Haematol. 2021, 195, 174–185. [Google Scholar] [CrossRef]
- Mannucci, P.M. AIDS, hepatitis and hemophilia in the 1980s: Memoirs from an insider. J. Thromb. Haemost. 2003, 1, 2065–2069. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Colombo, M.; Rizzetto, M. Nonprogressive course of non-A, non-B chronic hepatitis in multitransfused hemophiliacs. Blood 1982, 60, 655–658. [Google Scholar] [CrossRef][Green Version]
- Heimburger, N.; Schwinn, H.; Gratz, P.; Lüben, G.; Kumpe, G.; Herchenhan, B. Factor VIII concentrate, highly purified and heated in solution (author’s transl). Arzneimittelforschung 1981, 31, 619–622. [Google Scholar][Green Version]
- Schimpf, K.; Mannucci, P.M.; Kreutz, W.; Brackmann, H.H.; Auerswald, G.; Ciavarella, N.; Mösseler, J.; DeRosa, V.; Kraus, B.; Bruegkmann, C.; et al. Absence of hepatitis after treatment with a pasteurized factor VIII concentrate in patients with hemophilia and no previous transfusions. N. Engl. J. Med. 1987, 316, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef]
- Petricciani, J.C.; McDougal, J.S.; Evatt, B.L. Case for concluding that heat-treated, licensed anti-haemophilic factor is free from HTLV-III. Lancet 1985, 19, 890–891. [Google Scholar] [CrossRef] [PubMed]
- White, G.C.; Matthews, T.J.; Weinhold, K.J.; Haynes, B.F.; Cromartie, H.L.; McMillan, C.W.; Bolognesi, D.P. HTLV-III seroconversion associated with heat-treated factor VIII concentrate. Lancet 1986, 1, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.; Shaw, D. Blood on Their Hands: How Greedy Companies, Inept Bureaucracy, and Bad Science Killed Thousands of Hemophiliacs; Rutgers University Press: Toronto, ON, Canada, 2017; 304p, Available online: https://dokumen.pub/blood-on-their-hands-how-greedy-companies-inept-bureaucracy-and-bad-science-killed-thousands-of-hemophiliacs-9780813576244.html (accessed on 8 July 2025).
- Evatt, B.L. The AIDS epidemic in haemophilia patients II: Pursuing absolute viral safety of clotting factor concentrates 1985–1988. Haemophilia 2012, 18, 649–654. [Google Scholar] [CrossRef]
- Horowitz, B.; Wiebe, M.E.; Lippin, A.; Stryker, M.H. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion 1985, 25, 516–522. [Google Scholar] [CrossRef]
- Horowitz, M.S.; Rooks, C.; Horowitz, B.; Hilgartner, M.W. Virus safety of solvent/detergent-treated antihaemophilic factor concentrate. Lancet. 1988, 2, 186–189. [Google Scholar] [CrossRef]
- Foster, P. Statement of Dr Peter Foster to the Infected Blood Inquiry. 2022. Available online: https://www.infectedbloodinquiry.org.uk/sites/default/files/documents/WITN6914001%20-%20Written%20Statement%20of%20Dr%20Peter%20Foster%207%20March%202022.pdf (accessed on 11 August 2025).
- Smith, J. Statement of Dr James Smith to the Infected Blood Inquiry. 2020. Available online: https://www.infectedbloodinquiry.org.uk/search?keywords=Statement+No+WITN3433001 (accessed on 11 August 2025).
- McDougal, J.S.; Martin, L.S.; Cort, S.P.; Mozen, M.; Heldebrant, C.M.; Evatt, B.L. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J. Clin. Investig. 1985, 76, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, L.; Feldman, P.A. Interim Results O1 Surveillance for Nandii in Patients Receiving Heated Concentrates Produced in England. Dev. Biol. Stand. 1986, 67, 323–325. [Google Scholar]
- McGrath, K.M.; Herrington, R.W.; Turner, P.J.; Schiff, P.; Thomas, K.B.; Taylor, L.; Ekert, H.; Gust, I.D. Use of heat-treated clotting-factor concentrates in patients with haemophilia and a high exposure to HTLV-III. Med. J. Aust. 1985, 143, 11–13. [Google Scholar] [CrossRef]
- Macleod, A.; Cuthbertson, B.; Foster, P. Pasteurisation of Factor VIII and Factor IX concentrates. In Proceedings of the 18th Congress of the International Society of Blood Transfusion, Munich, Germany, 22–27 July 1984; p. 34. [Google Scholar]
- Farrugia, A. Studies on the Procurement of Blood Coagulation Factor VIII; University of Edinburgh: Edinburgh, UK, 1984; Available online: https://era.ed.ac.uk/handle/1842/20432 (accessed on 8 October 2020).
- Thomas, D.P.; Hampton, K.K.; Dasani, H.; Lee, C.A.; Giangrande, P.L.; Harman, C.; Lee, M.L.; Preston, F.E. A cross-over pharmacokinetic and thrombogenicity study of a prothrombin complex concentrate and a purified factor IX concentrate. Br. J. Haematol. 1994, 87, 782–788. [Google Scholar] [CrossRef]
- Littlewood, J.D.; Dawes, J.; Smith, J.K.; Feldman, P.A.; Haddon, M.E.; McQuillan, T.A.; Foster, P.R.; Ferguson, J.; Prowse, C.V. Studies on the effect of heat treatment on the thrombogenicity of factor IX concentrates in dogs. Br. J. Haematol. 1987, 65, 463–468. [Google Scholar] [CrossRef]
- Garsia, R.J.; Gatenby, P.A.; Basten, A.; Kenny, D.F.; Gallagher, K.J.; Rickard, K.A.; Gust, I.D.; Maskill, W. Australian hemophiliac recipients of voluntary donor blood products longitudinally evaluated for AIDS. A clinical and laboratory study, 1983–1986. Aust. N. Z. J. Med. 1987, 17, 371–378. [Google Scholar] [CrossRef]
- France’s blood scandal draws blood. Nature 1992, 359, 759. [CrossRef] [PubMed]
- Aronstam, A.; Arblaster, P.G.; Rainsford, S.G.; Turk, P.; Slattery, M.; Alderson, M.R.; Hall, D.E.; Kirk, P.J. Prophylaxis in haemophilia: A double-blind controlled trial. Br. J. Haematol. 1976, 33, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bassingham, A.; Smith, J. Treloar’s College Was ‘Using the Boys as Guinea Pigs’ to Treat Haemophilia. 2024. Available online: https://www.bbc.com/news/uk-england-gloucestershire-68884338 (accessed on 11 August 2025).
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- March, A. Witness Statement No: WITN1369014 to the Infected Blood Inquiry. 2020. Available online: https://www.infectedbloodinquiry.org.uk/sites/default/files/25-50/25-50/WITN1369060%20-%20Tainted%20Blood%27s%20Accusations%20Document.pdf (accessed on 10 August 2025).
- Foster, P.R.; Bienek, C. Fractionated Products; Regan, F.A.M., Barbara, J.A.J., Contreras, M., Eds.; Transfusion Microbiology; Cambridge University Press: Cambridge, UK, 2008; pp. 259–304. Available online: https://www.cambridge.org/core/books/transfusion-microbiology/fractionated-products/A3F5C27C822FADCA41D4FA78184744D4 (accessed on 16 January 2023).
- Mannucci, P.M.; Colombo, M. Revision of the Protocol Recommended for Studies of Safety from Hepatitis of Clotting Factor Concentrates. Thromb. Haemost. 1989, 61, 532–534. [Google Scholar] [CrossRef]
- Harden, V.A. Koch’s postulates and the etiology of AIDS: An historical perspective. Hist. Philos. Life Sci. 1992, 14, 249–269. [Google Scholar]
- Precautionary Principle. Wikipedia. 2025. Available online: https://en.wikipedia.org/w/index.php?title=Precautionary_principle&oldid=1305080569 (accessed on 12 August 2025).
- Mathias, R.G. The Krever Commission and public health. Can. J. Public Health. 1998, 89, 6–8. [Google Scholar] [CrossRef]
- Adler, J. The Problems with Precaution: A Principle Without Principle. American Enterprise Institute—AEI. 2011. Available online: https://www.aei.org/articles/the-problems-with-precaution-a-principle-without-principle/ (accessed on 12 August 2025).
- Leach Bennett, J.; Devine, D.V. Risk-based decision making in transfusion medicine. Vox Sang. 2018, 113, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. Disease transmission by blood products: Past, present and future. Pathophysiol Haemost Thromb. 2002, 32 (Suppl. 1), 1–4. [Google Scholar] [CrossRef]
- Russo, R.G. (Ed.) Informed Consent. In Medical Ethics; Springer Nature: Cham, Switzerland, 2023; pp. 233–250. [Google Scholar] [CrossRef]
- Devereux, E. Nurses Failed Patients During Infected Blood Scandal, Inquiry Finds. Nursing Times. 2024. Available online: https://www.nursingtimes.net/policies-and-guidance/nurses-failed-patients-during-infected-blood-scandal-inquiry-finds-21-05-2024/ (accessed on 12 August 2025).
- Farrugia, A.; Smit, C.; Buzzi, A. The legacy of haemophilia: Memories and reflections from three survivors. Haemophilia 2022, 28, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M. Hemophilia treatment innovation: 50 years of progress and more to come. J. Thromb. Haemost. 2023, 21, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Makris, M.; Calizzani, G.; Fischer, K.; Gilman, E.A.; Hay, C.R.M.; Lassila, R.; Lambert, T.; Ludlam, C.A.; Mannucci, P.M. EUHASS: The European Haemophilia Safety Surveillance system. Thromb. Res. 2011, 127 (Suppl. 2), S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P. Nonlinear systems in medicine. Yale J. Biol. Med. 2002, 75, 247–260. [Google Scholar] [PubMed]
- Helou, M.A.; DiazGranados, D.; Ryan, M.S.; Cyrus, J.W. Uncertainty in Decision-Making in Medicine: A Scoping Review and Thematic Analysis of Conceptual Models. Acad. Med. 2020, 95, 157–165. [Google Scholar] [CrossRef] [PubMed]
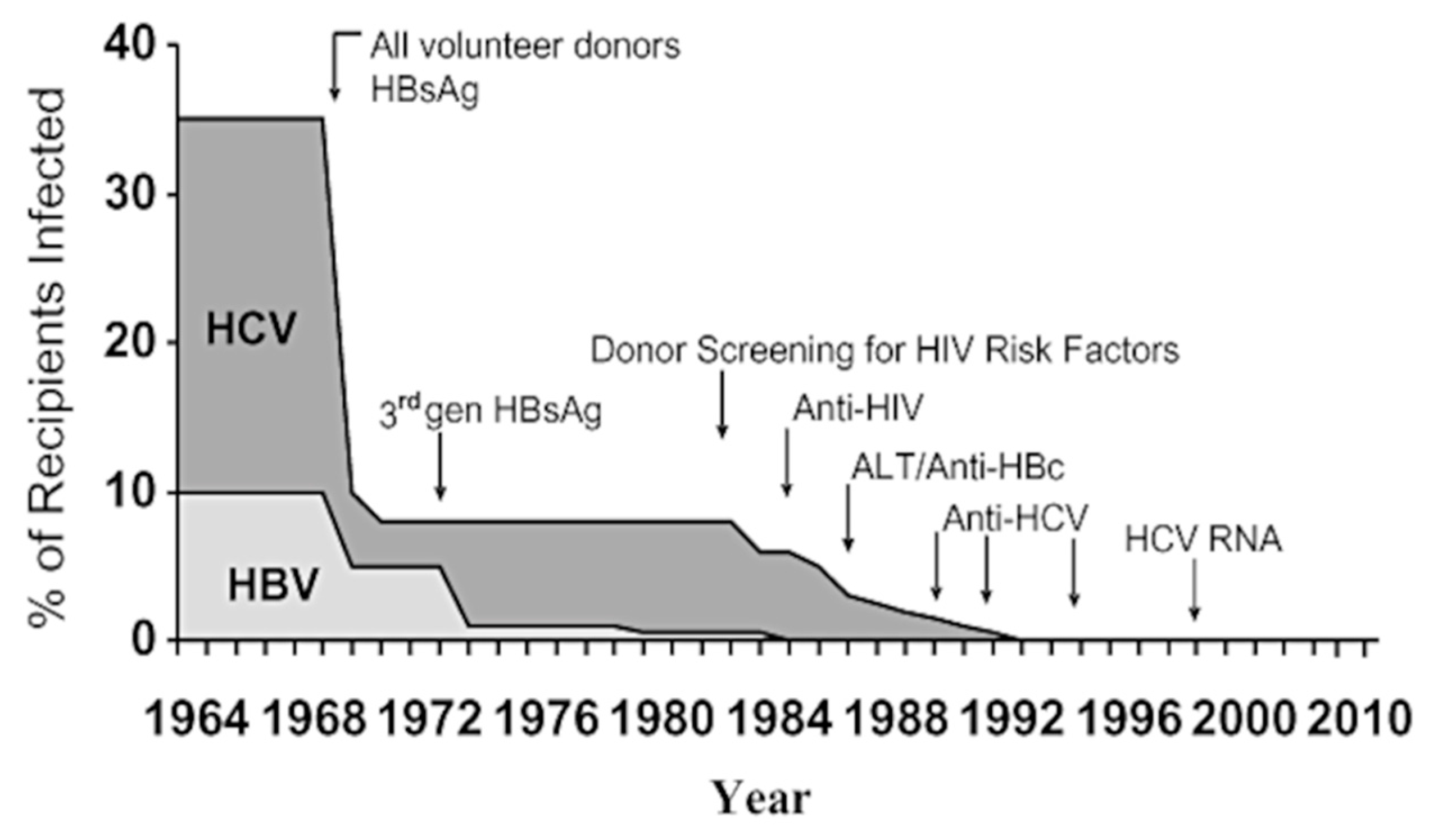
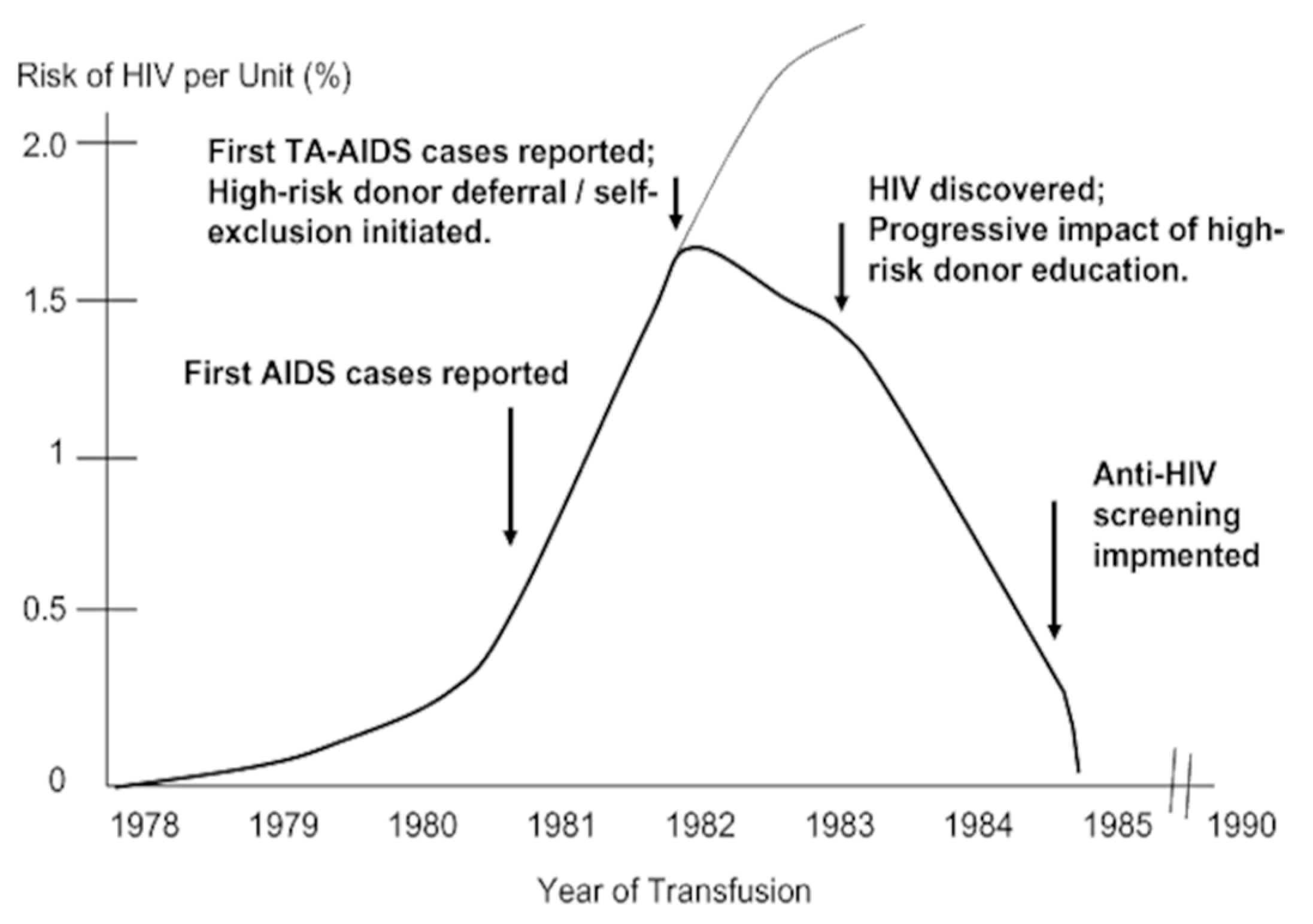


| % Recipients Infected | Transfused Population | Haemophilia Population | |||
|---|---|---|---|---|---|
| 1982 | Hepatitis (All Types) | AIDS/HIV | HBV | HCV | HIV |
| 1982 | 10 | 1.5 | 30 | 60 | 18 |
| 1985 | 8 | 0.25 | 15 | 38 | 0 |
| Plasma Fractionator and Method for Heat-Treated Factor VIII Concentrate | Date Applied for FDA Licensing | Date License Granted by FDA |
|---|---|---|
| Baxter Healthcare (dry heat, 60 °C for 72–74 h) | June 1982 | March 1983 |
| Miles, Inc. (formerly Cutter Biological) | ||
| August 1983 November 1983 | January 1984 February 1984 |
| Alpha Therapeutics (wet heat, 60 °C for 20 h) | December 1982 | February 1984 |
| Armour Pharmaceutical (dry heat, 60 °C for 30 h) | December 1982 | January 1984 |
| Country | Year in Which Virally Inactivated FVIII Was Issued | Year in Which Virally Inactivated FIX Was Issued | ||
|---|---|---|---|---|
| Process Which Could Destroy HIV [50] | Process Which Could Destroy HIV, HBV, and HCV [51] | Process Which Could Destroy HIV, HBV and HCV | Source | |
| Scotland | Q4 1984 | Q4 1986 | Q3 1985 | Ref. [48] |
| England | Q3 1983 (only 3 patients treated) | Q3 1985 | Q4 1985 | Ref. [49] |
| Australia | Q4 1984 [50] | ca Q1 1989 | Q4 1984 (HIV only) Q2 1993 (special access) Q2 1998 (market approval) | Ref. [52] Personal information from author |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrugia, A. Pathogen Safety Issues Around the “Blood Scandals” 1995–2024—A Perspective Built on Experience. Pathogens 2025, 14, 868. https://doi.org/10.3390/pathogens14090868
Farrugia A. Pathogen Safety Issues Around the “Blood Scandals” 1995–2024—A Perspective Built on Experience. Pathogens. 2025; 14(9):868. https://doi.org/10.3390/pathogens14090868
Chicago/Turabian StyleFarrugia, Albert. 2025. "Pathogen Safety Issues Around the “Blood Scandals” 1995–2024—A Perspective Built on Experience" Pathogens 14, no. 9: 868. https://doi.org/10.3390/pathogens14090868
APA StyleFarrugia, A. (2025). Pathogen Safety Issues Around the “Blood Scandals” 1995–2024—A Perspective Built on Experience. Pathogens, 14(9), 868. https://doi.org/10.3390/pathogens14090868






