Microbiological and Clinical Short-Term Evaluation of the Efficacy of an Herbal Tincture as an Adjunctive Treatment in the Management of Stage II, Grade A Periodontitis
Abstract
1. Introduction
2. Materials and Methods
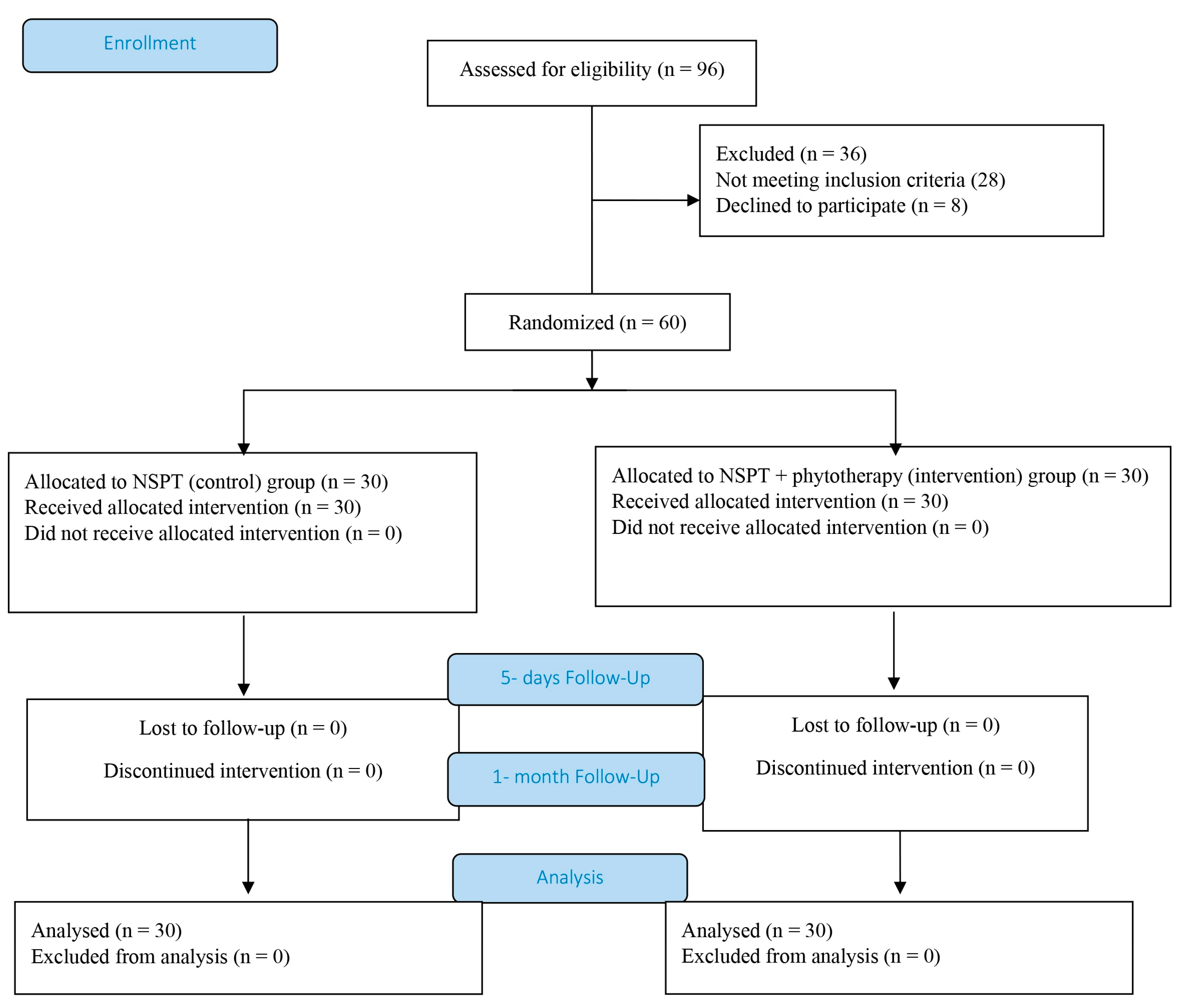
2.1. Study Design
2.2. High-Performance Liquid Chromatography (HPLC) of Tinctura Paradentoica®
2.3. Ethical Approval
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Sample Size Calculation
2.7. Clinical Examinations
2.8. Sampling and Polymerase Chain Reaction (PCR) Analysis
2.9. Statistical Analysis
3. Results
3.1. High-Performance Liquid Chromatography (HPLC) of Tinctura Paradentoica® Evaluation
3.2. Data Analysis
3.3. Clinical Evaluation
3.4. Microbiological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSPT | Non-surgical periodontal therapy |
| PCR | Polymerase chain reaction |
| CAL | Clinical attachment level |
| BOP | Bleeding on probing |
| PI | Green–Vermilion plaque index |
| Pg | Porphyromonas gingivalis |
| Tf | Tannerella forsythensis |
| Td | Treponema denticola |
| ICMJE | International Committee of Medical Journal Editors |
| CONSORT | Consolidated Standards of Reporting Trials |
References
- Hashim, N.T.; Babiker, R.; Chaitanya, N.C.S.K.; Mohammed, R.; Priya, S.P.; Padmanabhan, V.; Ahmed, A.; Dasnadi, S.P.; Islam, M.S.; Gismalla, B.G.; et al. New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential. Molecules 2025, 30, 807. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol 2000 2023, 1–17. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral. Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red Complex: Polymicrobial Conglomerate in Oral Flora. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Meštrović, T.; Matijević, T.; Mesarić, D.; Katalinić, D.; Erić, S.; Milostić-Srb, A.; Flam, J.; Škrlec, I. Aggregatibacter actinomycetemcomitans: From the Oral Cavity to the Heart Valves. Microorganisms 2024, 12, 1451. [Google Scholar] [CrossRef]
- Augusco, M.A.C.; Sarri, D.A.; Panontin, J.F.; Rodrigues, M.A.M.; Fernandes, R.d.M.N.; Silva, J.F.M.d.; Cardoso, C.A.L.; Rambo, M.K.D.; Scapin, E. Extracts from the Leaf of Couroupita guianensis (Aubl.): Phytochemical, Toxicological Analysis and Evaluation of Antioxidant and Antimicrobial Activities against Oral Microorganisms. Plants 2023, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Lauten, J.D.; Boyd, L.; Hanson, M.B.; Lillie, D.; Gullion, C.; Madden, T.E. A clinical study: Melaleuca, Manuka, Calendula and green tea mouth rinse. Phytother. Res. 2005, 19, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.A. Management of two cases of desquamative gingivitis with clobetasol and Calendula officinalis gel. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech. Repub. 2010, 154, 335–338. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Siles-Sánchez, M.D.L.N.; Rodríguez García-Risco, M.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable extraction techniques for obtaining antioxidant and anti-inflammatory compounds from the Lamiaceae and Asteraceae species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell. Long. 2021, 2021, 6643827. [Google Scholar] [CrossRef] [PubMed]
- Idir, F.; Van Ginneken, S.; Coppola, G.A.; Grenier, D.; Steenackers, H.P.; Bendali, F. Origanum vulgare ethanolic extracts as a promising source of compounds with antimicrobial, anti-biofilm, and anti-virulence activity against dental plaque bacteria. Front. Microbiol. 2021, 2, 999839. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Blumentha, L.M.; Busse, N.J.R.; Goldberg, A. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines; American Botanical Council and Boston, Integrative Medicine Communications: Austin, TX, USA, 1998; pp. 198–201. [Google Scholar]
- Tomczyk, M.; Latté, K.P. Potentilla—A phytochemical and pharmacological profile review. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef]
- Melzig, M.F.; Böttger, S. Tormentillae Rhizoma—Review for an Underestimated European Herbal Drug. Planta. Med. 2020, 86, 1050–1057. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Mentha x Piperita, L., Folium. Available online: https://www.ema.europa.eu/en/medicines/herbal/menthae-piperitae-aetheroleum (accessed on 6 April 2025).
- Kumar, P.; Ansari, S.H.; Ali, J. Herbal remedies for the treatment of periodontal disease—A patent review. Recent. Pat. Drug. Deliv. Formul. 2009, 3, 221–228. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Pollard, A.; Claydon, N.; Franceschetti, G.; Khan, I.; West, N. Efficacy of alternative or additional methods to professional mechanical plaque removal during supportive periodontal therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Leira, Y.; Moreno, F.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 7, 155–175. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Hrishi, T.S.; Kundapur, P.P.; Naha, A.; Thomas, B.S.; Kamath, S.; Bhat, G.S. Effect of adjunctive use of green tea dentifrice in periodontitis patients—A Randomized Controlled Pilot Study. Int. J. Dent. Hygiene. 2016, 14, 178–183. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Evidence-Based Clinical Practice Guideline on the Nonsurgical Treatment of Chronic Periodontitis by Means of Scaling and Root Planing with or without Adjuncts. J. Am. Dent. Assoc. 2015, 146, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Skotnicka, A.; Ruchała, M.A.; Cielecka-Piontek, J. Can Plant Materials Be Valuable in the Treatment of Periodontal Diseases? Practical Review. Pharmaceutics 2021, 13, 2185. [Google Scholar] [CrossRef]
- Gościniak, A.; Konarska, A.; Weryszko-Chmielewska, E.; Materska, M.; Sulborska-Różycka, A.; Dmitruk, M.; Chilczuk, B. Phenolic Compounds in Flowers and Herb of Achillea millefolium L.: Histochemical and Phytochemical Studies. Molecules 2025, 30, 2084. [Google Scholar] [CrossRef]
- Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of Extraction of Phenolic Compounds with Antimicrobial Properties from Origanum vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Braswell, L.; Offenbacher, S. Inhibition of gingivitis by topical application of ebselen and rosmarinic acid. Agents Actions. 1986, 19, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Kitić, D.; Petrović, M.B.; Jevtović-Stoimenov, T.; Jović, M.; Petrović, A.; Živanović, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59. [Google Scholar] [CrossRef]
- Zdarilova, A.; Svobodova, A.; Simanek, V.; Ulrichova, J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicol. Vitr. 2009, 23, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The effect of luteolin in the prevention of periodontal disease in Wistar rats. J. Periodontol. 2019, 90, 1481–1489. [Google Scholar] [CrossRef]
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral. Health 2019, 19, 229. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 6, 9966750. [Google Scholar] [CrossRef]
- Kariu, T.; Hamada, N.; Lakshmyya, K. Luteolin inhibits Porphyromonas gingivalis growth and alleviates alveolar bone destruction in experimental murine periodontitis. Biosci. Biotechnol. Biochem. 2023, 19, 37–43. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, R. Computation screening of narcissoside a glycosyloxyflavone for potential novel coronavirus 2019 (COVID-19) inhibitor. Biomed. J. 2020, 43, 363–367. [Google Scholar] [CrossRef]
- Johnston, W.; Rosier, B.T.; Artacho, A.; Paterson, M.; Piela, K.; Delaney, C.; Brown, J.L.; Ramage, G.; Mira, A. Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci. Rep. 2021, 11, 9796. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Heck, K.; Walter, E.; Ern, C.; Bumm, C.V.; Folwaczny, M. Probing pocket depth reduction after non-surgical periodontal therapy: Tooth-related factors. J. Periodontol. 2024, 95, 29–39. [Google Scholar] [CrossRef]
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, A.; Willershausen, B.; Steinmeier, E.M.; Kreisler, M. Efficacy of subgingival irrigation using herbal extracts on gingival inflammation. J. Periodontol. 2003, 74, 616–622. [Google Scholar] [CrossRef]
- Soukoulis, S.; Hirsch, R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Aust. Dent. J. 2004, 49, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori, A.I.; Mahmood, M.S. The Effects of Salvia officinalis Gel as an Adjunct to Scaling and Root Planning in Patients with Periodontitis (Clinical and Immunological Study). Int. J. Drug Deliv. Technol. 2020, 10, 232–237. [Google Scholar] [CrossRef]
- Milićević, R.; Brajović, G.; Nikolić-Jakoba, N.; Popović, B.; Pavlica, D.; Leković, V.; Milašin, J. Identification of periodontopathogen microorganisms by PCR technique. Srp. Arh. Celok. Lek. 2008, 136, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ozmeric, N.; Preus, N.R.; Olsen, I. Genetic diversity of Porphyromonas gingivalis and its possible importance to pathogenicity. Acta. Odontol. Scand. 2000, 58, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Predin, T.; Djuric, M.; Nikolic, N.; Mirnic, J.; Gusic, I.; Petrovic, D.; Milasin, J. Clinical and microbiological effects of quadrant versus full-mouth root planning-a randomized study. J. Dent. Sci. 2014, 9, 400–406. [Google Scholar] [CrossRef]
- Günther, M.; Karygianni, L.; Argyropoulou, A.; Anderson, A.C.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. The Antimicrobial Effect of Rosmarinus officinalis Extracts on Oral Initial Adhesion Ex Vivo. Clin. Oral Investig. 2022, 26, 4369–4380. [Google Scholar] [CrossRef]
- Tambur, Z.Z.; Aleksić, E.D.; Čabrilo Lazić, M.P.; Opačić, D.N.; Kalevski, K.D.; Puletić, M.Z.; Avramov, S.N.; Biočanin, V.M. The investigation of antibacterial activity of hyperlight fluid fusion subcellular essential complex. J. Infect. Dev. Ctries. 2023, 17, 643–648. [Google Scholar] [CrossRef]
- He, J.; Huang, W.; Pan, Z.; Cui, H.; Qi, G.; Zhou, X.; Chen, H. Quantitative analysis of microbiota in saliva, supragingival, and subgingival plaque of Chinese adults with chronic periodontitis. Clin. Oral. Investig. 2012, 16, 1579–1588. [Google Scholar] [CrossRef]
- Reddahi, S.; Bouziane, A.; Dib, K.; Tligui, H.; Ennibi, O.K. qPCR detection and quantification of Aggregatibacter actinomycetemcomitans and other periodontal pathogens in saliva and gingival crevicular fluid among periodontitis patients. Pathogens 2023, 12, 76. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 388, e081123. [Google Scholar] [CrossRef] [PubMed]
- Milovanova-Palmer, J.; Pendry, B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J. Herb. Med. 2018, 12, 33–48. [Google Scholar] [CrossRef]
- Abullais Saquib, S.; Abdullah AlQahtani, N.; Ahmad, I.; Arora, S.; Mohammed Asif, S.; Ahmed Javali, M.; Nisar, N. Synergistic antibacterial activity of herbal extracts with antibiotics on bacteria responsible for periodontitis. J. Infect. Dev. Ctries. 2021, 15, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.S.; Kesic, L.G.; Kitic, D.V.; Milašin, J.M.; Obradovic, R.R.; Bojovic, M.D.; Simonovic, A.A. Periodontal Disease and Phytotherapy. J. Org. Hyg. Health 2015, 3, 172. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.M.; Casamassima, L.; Trilli, I.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Effectiveness of Herbal Medicines with Anti-Inflammatory, Antimicrobial, and Antioxidant Properties in Improving Oral Health and Treating Gingivitis and Periodontitis: A Systematic Review. Nutrients 2025, 17, 762. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.G.; Silveira Souto, M.L.; Franco, G.C.N.; Holzhausen, M.; Pannuti, C.M. Efficacy of local phytotherapy in the nonsurgical treatment of periodontal disease: A systematic review. J. Periodontal. Res. 2018, 53, 288–297. [Google Scholar] [CrossRef]
| Bacteria | Primer | Base Pairs in DNA | Annealing Temperature |
|---|---|---|---|
| Porphyromonas gingivalis (Pg) | PGF PGR | 5′-AGGCAGCTTGCCATACTGCG -3′ 5′-ACTGTTAGCAACTACCGATGT-3′ | 55 °C |
| Tannerella forsythensis (Tf) | TanF TanR | 5′-GCGTATGTAACCTGCCCGCA-3′ 5′-TGCTTCAGTGTCAGTTATACCT-3′ | 55 °C |
| Treponema denticola (Td) | TDF TDR | 5′-TAATACCGAATGTGCTCATTTACAT-3′ 5′-TCAAAGAAGCATTCCCTCTTCTTCTTA-3′ | 60 °C |
| Compound | Content (µg/mL) |
|---|---|
| Chlorogenic acid | 162.85 ± 4.12 |
| Rutin | 52.59 ± 1.39 |
| Gallic acid | 11.12 ± 0.23 |
| Rosmarinic acid | 1102.79 ± 21.56 |
| Isorhamnetin | 24.17 ± 0.49 |
| Narcissoside | 227.34 ± 3.78 |
| Vitexin | 73.17 ± 2.15 |
| Luteolin-7-O-glucoside | 358.06 ± 5.64 |
| Hyperoside | 19.89 ± 0.33 |
| NSPT (Control) Group | Intervention Group | |
|---|---|---|
| Gender (man/woman) | 11/19 | 11/19 |
| Mean age | 42.03 ± 15.39 (38.50) | 45.03 ± 16.13 (39.00) |
| Index | NSPT Group | Intervention Group |
|---|---|---|
| PI Before | 1.92 ± 0.56 #†***(2.00) | 1.93 ± 0.68 #†***(2.00) |
| After 5th | 0.00 ± 0.00 (0.00) | 0.00 ± 0.00 (0.00) |
| After a month | 1.01 ± 0.49 $* (1.00) | 0.66 ± 0.33 (0.70) |
| BOP Before | 1.33 ± 0.48 #†*** (1.00) | 1.33 ± 0.48 #†*** (1.00) |
| After 5th | 0.52 ± 0.50 $** (0.75) | 0.23 ± 0.50 (0.00) |
| After a month | 0.58 ± 0.66 $* (0.20) | 0.37 ± 0.50 (0.00) |
| CAL Before | 2.89 ± 0.35 †*** (3.00) | 2.80 ± 0.31 †*** (3.00) |
| After a month | 2.37 ± 0.35 $*** (2.42) | 2.22 ± 0.26 (2.17) |
| Bacteria | NSPT Group | Intervention Group | |
|---|---|---|---|
| Tf | Before | 50.00% (15/30) | 80.00% (24/30) a**C*** |
| After | 53.33% (16/30) | 33.33% (10/30) | |
| Td | Before | 40.00% (12/30) | 60.00% (18/30) C*** |
| After | 40.00% (12/30) | 16.67% (5/30) | |
| Pg | Before | 50.00% (15/30) | 90.00% (27/30) a*** |
| After | 43.33% (13/30) | 86.67% (26/30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, M.; Kesić, L.; Miladinović, B.; Obradović, R.; Pejčić, A.; Bojović, M.; Šavikin, K.; Živković, J.; Stanković, I.; Kitić, D. Microbiological and Clinical Short-Term Evaluation of the Efficacy of an Herbal Tincture as an Adjunctive Treatment in the Management of Stage II, Grade A Periodontitis. Pathogens 2025, 14, 861. https://doi.org/10.3390/pathogens14090861
Petrović M, Kesić L, Miladinović B, Obradović R, Pejčić A, Bojović M, Šavikin K, Živković J, Stanković I, Kitić D. Microbiological and Clinical Short-Term Evaluation of the Efficacy of an Herbal Tincture as an Adjunctive Treatment in the Management of Stage II, Grade A Periodontitis. Pathogens. 2025; 14(9):861. https://doi.org/10.3390/pathogens14090861
Chicago/Turabian StylePetrović, Milica, Ljiljana Kesić, Bojana Miladinović, Radmila Obradović, Ana Pejčić, Marija Bojović, Katarina Šavikin, Jelena Živković, Ivana Stanković, and Dušanka Kitić. 2025. "Microbiological and Clinical Short-Term Evaluation of the Efficacy of an Herbal Tincture as an Adjunctive Treatment in the Management of Stage II, Grade A Periodontitis" Pathogens 14, no. 9: 861. https://doi.org/10.3390/pathogens14090861
APA StylePetrović, M., Kesić, L., Miladinović, B., Obradović, R., Pejčić, A., Bojović, M., Šavikin, K., Živković, J., Stanković, I., & Kitić, D. (2025). Microbiological and Clinical Short-Term Evaluation of the Efficacy of an Herbal Tincture as an Adjunctive Treatment in the Management of Stage II, Grade A Periodontitis. Pathogens, 14(9), 861. https://doi.org/10.3390/pathogens14090861












