Evaluation of the PreTect HPV-Proofer E6/E7 mRNA Assay for the Detection of Precancerous Cervical Lesions in the Greek Female Population
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. HPV DNA Genotyping
- High-risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59
- Probably high-risk types: 26, 34, 53, 66, 67, 68a, 68b, 69, 70, 73, 82IS39, 82MM4
- Low-risk types: 6, 11, 40, 42, 43, 44, 54, 55, 57, 61, 62, 72, 81CP8304, 83MM7, 84MM8, 90, 91
2.3. HPV mRNA Test
2.4. Cytological Analysis
2.5. Colposcopy and Biopsy
2.6. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Comparison of Biopsy Results with HPV DNA Test Outcomes
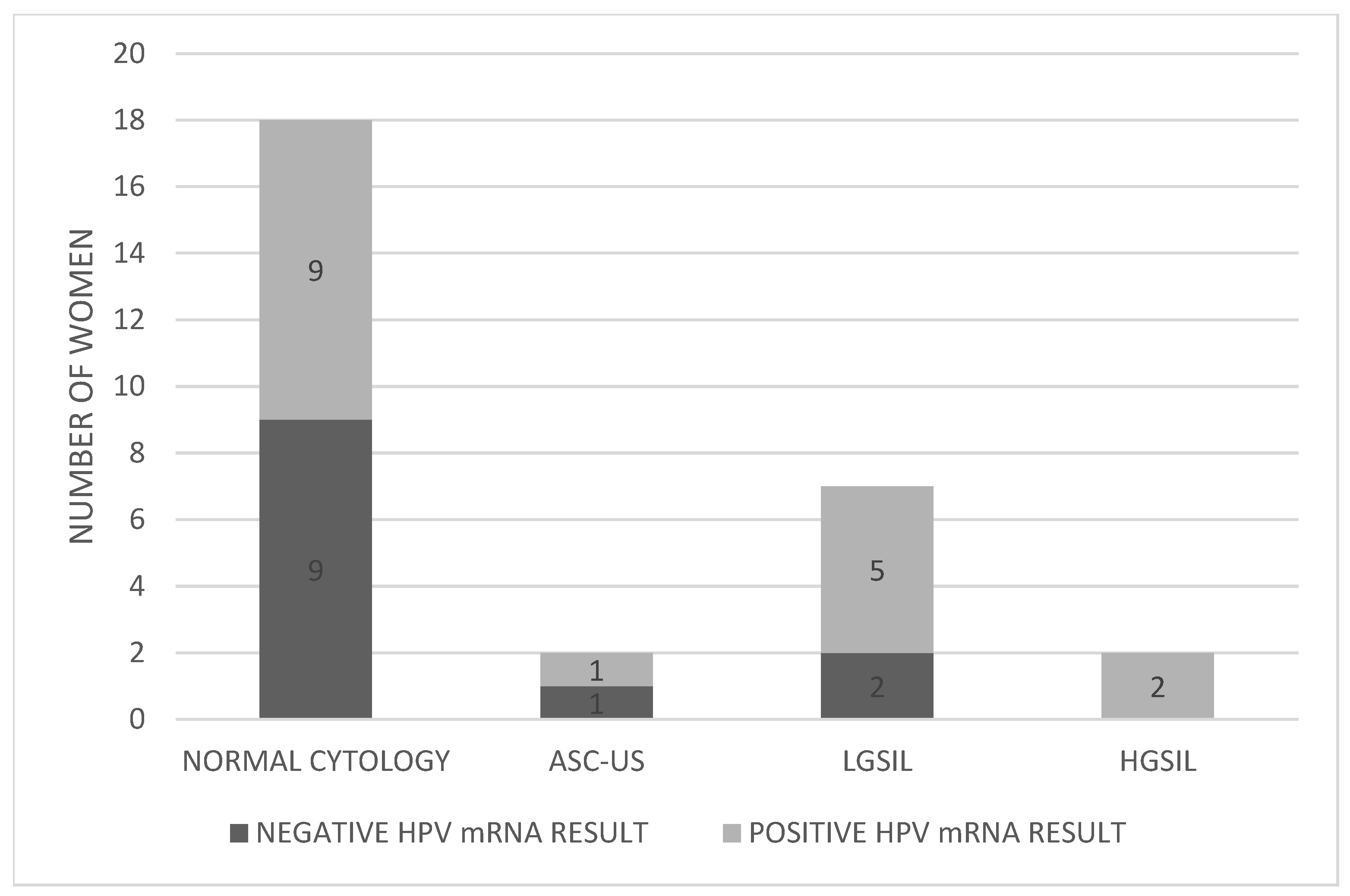
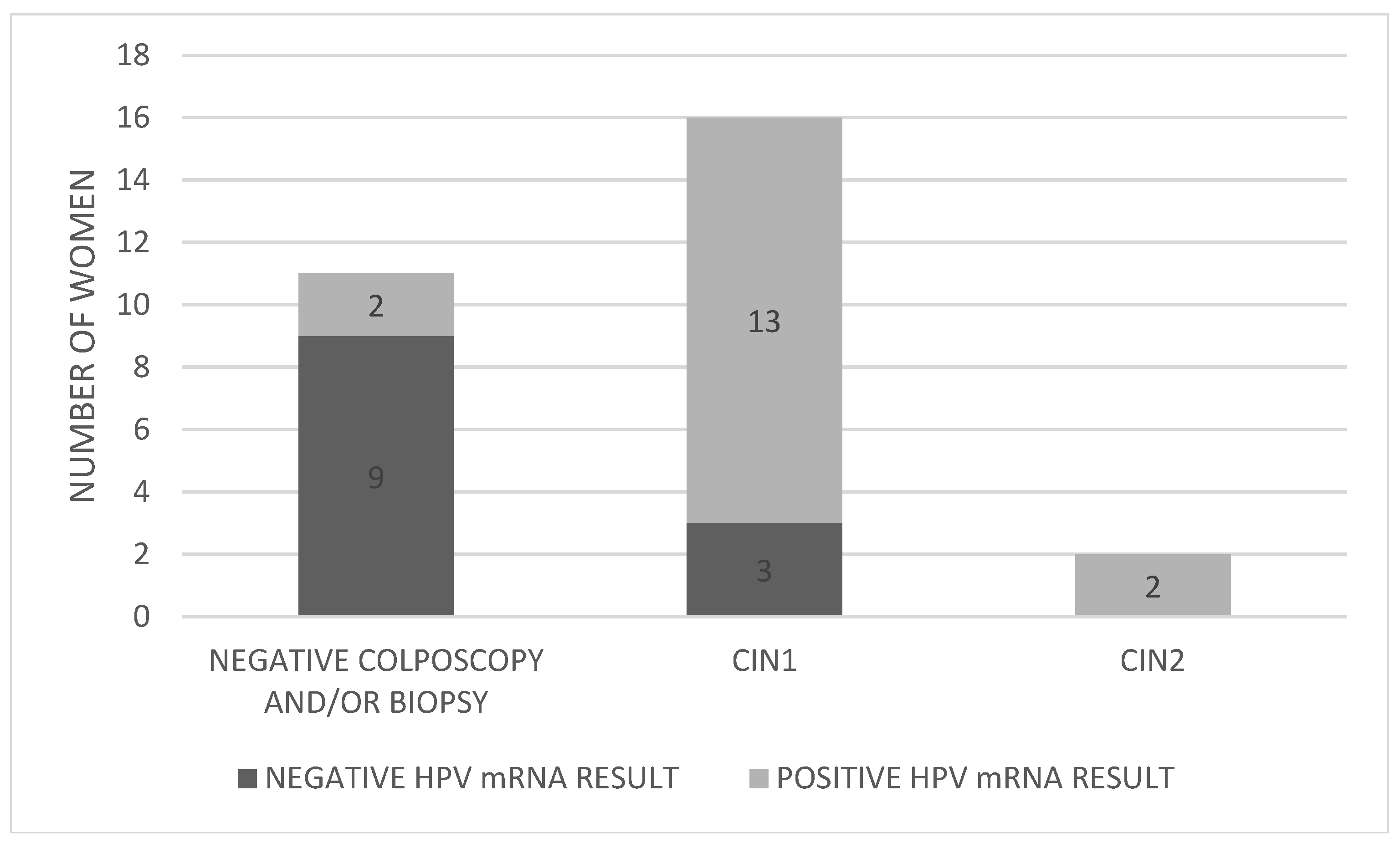
3.3. Comparison of Biopsy Results with HPV mRNA Test Outcomes
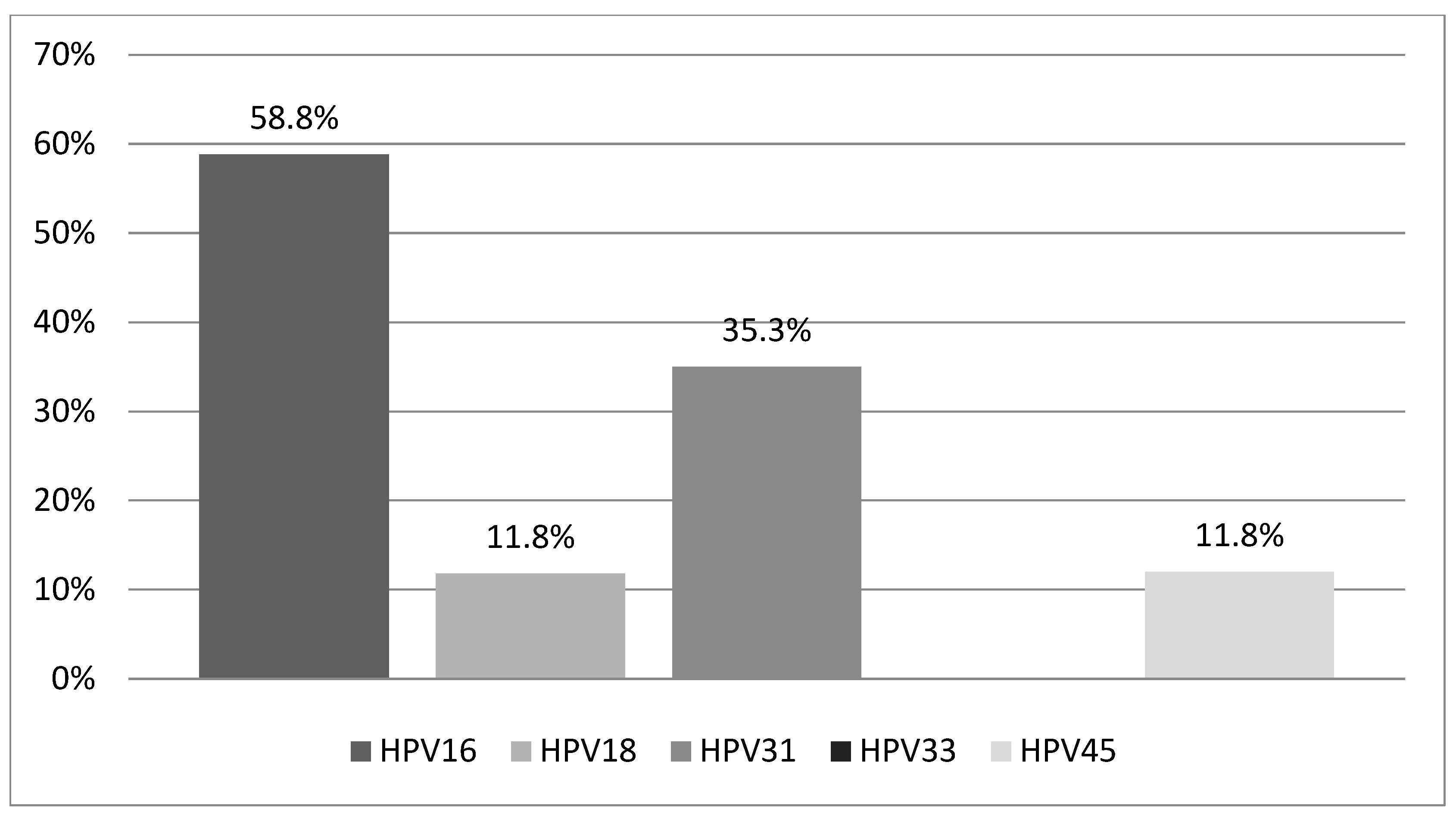
3.4. Distribution of HPV Genotypes Among Women with Histological Lesions
3.5. Comparison Between HPV DNA and HPV mRNA Test Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World: Summary Report 10 March 2023. 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 6 December 2024).
- Unim, B.; Meggiolaro, A.; Semyonov, L.; Maffongelli, E.; La Torre, G. Role of Pap-test in cervical cancer prevention: A systematic review and meta-analysis: Brigid Unim. Eur. J. Public Health 2014, 24 (Suppl. S2). cku165-115. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021.
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Delpero, E.; Selk, A. Shifting from cytology to HPV testing for cervical cancer screening in Canada. Can. Med. Assoc. J. 2022, 194, E613–E615. [Google Scholar] [CrossRef]
- Liang, L.A.; Einzmann, T.; Franzen, A.; Schwarzer, K.; Schauberger, G.; Schriefer, D.; Radde, K.; Zeissig, S.R.; Ikenberg, H.; Meijer, C.J.; et al. Cervical cancer screening: Comparison of conventional Pap smear test, liquid-based cytology, and human papillomavirus testing as stand-alone or co-testing strategies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 474–484. [Google Scholar] [CrossRef]
- Origoni, M.; Cristoforoni, P.; Carminati, G.; Stefani, C.; Costa, S.; Sandri, M.T.; Mariani, L.; Preti, M. E6/E7 mRNA testing for human papillomavirus-induced high-grade cervical intraepithelial disease (CIN2/CIN3): A promising perspective. Ecancermedicalscience 2015, 9, 533. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Coquillard, G.; Palao, B.; Patterson, B.K. Quantification of intracellular HPV E6/E7 mRNA expression increases the specificity and positive predictive value of cervical cancer screening compared to HPV DNA. Gynecol. Oncol. 2011, 120, 89–93. [Google Scholar] [CrossRef]
- Benevolo, M.; Vocaturo, A.; Caraceni, D.; French, D.; Rosini, S.; Zappacosta, R.; Terrenato, I.; Ciccocioppo, L.; Frega, A.; Rossi, P.G. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J. Clin. Microbiol. 2011, 49, 2643–2650. [Google Scholar] [CrossRef]
- Sørbye, S.W.; Fismen, S.; Gutteberg, T.J.; Mortensen, E.S.; Skjeldestad, F.E. HPV mRNA is more specific than HPV DNA in triage of women with minor cervical lesions. PLoS ONE 2014, 9, e112934. [Google Scholar] [CrossRef]
- Westre, B.; Giske, A.; Guttormsen, H.; Sørbye, S.W.; Skjeldestad, F.E. 5-type HPV mRNA versus 14-type HPV DNA test: Test performance, over-diagnosis and overtreatment in triage of women with minor cervical lesions. BMC Clin. Pathol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Ronco, G.; Mancuso, P.; Carozzi, F.; Allia, E.; Bisanzi, S.; Gillio-Tos, A.; De Marco, L.; Rizzolo, R.; Gustinucci, D.; et al. NTCC2 Working Group. Performance of HPV E6/E7 mRNA assay as primary screening test: Results from the NTCC2 trial. Int. J. Cancer 2022, 151, 1047–1058. [Google Scholar] [CrossRef]
- Frega, A.; Pavone, M.; Sesti, F.; Leone, C.; Bianchi, P.; Cozza, G.; Colombrino, C.; Lukic, A.; Marziani, R.; De Sanctis, L.; et al. Sensitivity and specificity values of high-risk HPV DNA, p16/ki-67 and HPV mRNA in young women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL). Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10672–10677. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 2012; Volume 100.
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.S.; Pouryasin, A.; Yarandi, F.; Pirzadeh, L.; Alipour, A.; Khodadad, S.; Pouryasin, M. Assessment of cervical cancer molecular-based screening tools: HPV-DNA detection versus E6/E7 mRNA testing. Iran. J. Public Health 2020, 49, 1734–1742. [Google Scholar] [CrossRef]
- Dabeski, D.; Duvlis, S.; Basheska, N.; Antovska, V.; Stojovski, M.; Trajanova, M.; Dimitrov, G.; Dabeski, A.; Gureva-Gjorgievska, N. Comparison between HPV DNA testing and HPV E6/E7 mRNA testing in women with squamous cell abnormalities of the uterine cervix. Prilozi 2019, 40, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Simon, M.; de Sanjosé, S.; Clarke, M.A.; Poljak, M.; Rezhake, R.; Berkhof, J.; Nyaga, V.; Gultekin, M.; Canfell, K.; et al. Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 950–960. [Google Scholar] [CrossRef]
- Andersson, E.; Kärrberg, C.; Rådberg, T.; Blomqvist, L.; Zetterqvist, B.-M.; Ryd, W.; Lindh, M.; Horal, P. Type-specific human papillomavirus E6/E7 mRNA detection by real-time PCR improves identification of cervical neoplasia. J. Clin. Microbiol. 2011, 49, 3794–3799. [Google Scholar] [CrossRef]
- Sharma, B.; Lakhanpal, V.; Singh, K.; Oberoi, L.; Bedi, P.K.; Devi, P. Evaluation of HPV E6/E7 mRNA detection in clinically suspected cases of cervical cancer with abnormal cytology: Time to upgrade the screening protocols. J. Lab. Physicians 2022, 14, 336–342. [Google Scholar] [CrossRef]
- Mello, V.; Sundstrom, R.K. Cervical intraepithelial neoplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544371/ (accessed on 4 March 2025).
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Egemen, D.; Cheung, L.C.; Chen, X.M.; Demarco, M.; Perkins, R.B.M.; Kinney, W.; Poitras, N.B.; Befano, B.B.; Locke, A.; Guido, R.S.; et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J. Low. Genit. Tract Dis. 2020, 24, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kafasi, A.; Kaparos, G.; Pitiriga, V.C.; Spanakis, N.; Vlachos, N.; Thomakos, N.; Stournaras, S.; Tsakris, A. Prevalence of HPV genotypes among Greek women in association with their potential to cause precancerous lesions. Microorganisms 2024, 12, 1404. [Google Scholar] [CrossRef]
- Na, J.; Li, Y.; Wang, J.; Wang, X.; Lu, J.; Han, S. The correlation between multiple HPV infections and the occurrence, development, and prognosis of cervical cancer. Front. Microbiol. 2023, 14, 1220522. [Google Scholar] [CrossRef]
- Cattani, P.; Siddu, A.; D’Onghia, S.; Marchetti, S.; Santangelo, R.; Vellone, V.G.; Zannoni, G.F.; Fadda, G. RNA (E6/E7) assays versus DNA (E6/E7) assays for risk evaluation for women infected with human papillomavirus. J. Clin. Microbiol. 2009, 47, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, K.S.; Whitley, M.J.; Cubie, H.A. Human papillomavirus type specific DNA and RNA persistence—Implications for cervical disease progression and monitoring. J. Med. Virol. 2004, 73, 65–70. [Google Scholar] [CrossRef]
- Rossi, N.M.; Dai, J.; Xie, Y.; Wangsa, D.; Heselmeyer-Haddad, K.; Lou, H.; Boland, J.F.; Yeager, M.; Orozco, R.; Freites, E.A.; et al. Extrachromosomal amplification of human papillomavirus episomes is a mechanism of cervical carcinogenesis. Cancer Res. 2023, 83, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Pett, M.R.; Ward, D.; Stanley, M.A.; Roberts, I.; Scarpini, C.G.; Coleman, N. In vitro progression of human papillomavirus 16 episome-associated cervical neoplasia displays fundamental similarities to integrant-associated carcinogenesis. Cancer Res. 2010, 70, 4081–4091. [Google Scholar] [CrossRef] [PubMed]
| Case Number | HPV DNA Test | HPV mRNA Test | Pap Test | Colposcopy/ Biopsy |
|---|---|---|---|---|
| 1 | 16 (High Risk) | Negative | Normal | Negative |
| 2 | 16 (High Risk), 45 (High Risk) | HPV 16, 45 | HGSIL | CIN2 |
| 3 | 16 (High Risk) | HPV 16 | LGSIL | CIN1 |
| 4 | 45 (High Risk), 56 (High Risk) | Negative | LGSIL | CIN1 |
| 5 | 16 (High Risk) | HPV 16 | Normal | CIN1 |
| 6 | 18 (High Risk) | Negative | Normal | Negative |
| 7 | 6 (Low Risk), 16 (High Risk), 18 (High Risk), 53 (Probably High Risk), 61 (Low Risk), 62 (Low Risk), 67 (Probably High Risk), 68a (Probably High Risk), 90 (Low Risk) | HPV 16, 18 | LGSIL | CIN1 |
| 8 | 31 (High Risk) | HPV 31 | Normal | CIN1 |
| 9 | 18 (High Risk), 44 (LowRisk) | Negative | Normal | Negative |
| 10 | 16 (High Risk), 42 (Low Risk), 73 (Probably High Risk) | HPV 16 | Normal | CIN1 |
| 11 | 18 (High Risk), 61 (LowRisk) | Negative | Normal | Negative |
| 12 | 16 (High Risk) | Negative | ASC-US | Negative |
| 13 | 18 (High Risk) | Negative | Normal | Negative |
| 14 | 33 (High Risk) | Negative | Normal | Negative |
| 15 | 51 (High Risk), 56 (High Risk), 45 (High Risk), 35 (High Risk), 73 (Probably High Risk), 82MM4 (Probably High Risk), 54 (Low Risk), 90 (Low Risk) | HPV 45 | Normal | CIN1 |
| 16 | 16 (High Risk), 66 (Probably High Risk), 91 (Low Risk) | HPV 16 | Normal | CIN1 |
| 17 | 16 (High Risk), 51 (High Risk) | Negative | Normal | CIN1 |
| 18 | 18 (High Risk), 73 (Probably High Risk) | Negative | Normal | Negative |
| 19 | 31 (High Risk), 58 (High Risk) | HPV 31 | Normal | CIN1 |
| 20 | 31 (High Risk), 73 (Probably High Risk), 44 (Low Risk) | HPV 31 | Normal | CIN1 |
| 21 | 31 (High Risk), 59 (High Risk) | HPV 31 | LGSIL | CIN1 |
| 22 | 16 (High Risk), 59 (High Risk), 34 (Probably High Risk), 70 (Probably High Risk), 62 (Low Risk), 44 (Low Risk) | HPV 16 | LGSIL | CIN1 |
| 23 | 31 (High Risk) | HPV 31 | Normal | CIN1 |
| 24 | 31 (High Risk) | HPV 31 | ASC-US | Negative |
| 25 | 18 (High Risk), 66 (Probably High Risk), 62 (Low Risk) | Negative | Normal | Negative |
| 26 | 16 (High Risk), 66 (Probably High Risk) | HPV 16 | LGSIL | CIN1 |
| 27 | 16 (High Risk), 52 (High Risk) | Negative | LGSIL | CIN1 |
| 28 | 16 (High Risk) | HPV 16 | HGSIL | CIN2 |
| 29 | 16 (High Risk), 18 (High Risk), 66 (Probably High Risk), 54 (Low Risk), 61 (Low Risk) | HPV 16, 18 | Normal | Negative |
| Comparison | Fisher’s p-Value | Interpretation |
|---|---|---|
| CIN1 vs. Negative | p ≈ 0.0057 | Statistically significant |
| CIN2 vs. Negative | p ≈ 0.099 | Not significant (small numbers) |
| CIN2 vs. CIN1 | p ≈ 1.0 | Not significant |
| Metric | CIN1 | CIN2 | ||
|---|---|---|---|---|
| HPV DNA Test | HPV mRNA Test | HPV DNA Test | HPV mRNA Test | |
| True Positives | 16 | 13 | 2 | 2 |
| False Positives | 13 | 4 | 27 | 15 |
| False Negatives | 0 | 3 | 0 | 0 |
| True Negatives | 0 | 9 | 0 | 12 |
| PPV | 55.2% | 76.5% | 6.9% | 11.8% |
| NPV | — | 75.0% | — | 100% |
| Test Sensitivity | 100% | 81.3% | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafasi, A.; Pitiriga, V.C.; Spanakis, N.; Vlachos, N.; Thomakos, N.; Stournaras, S.; Tsakris, A.; Kaparos, G. Evaluation of the PreTect HPV-Proofer E6/E7 mRNA Assay for the Detection of Precancerous Cervical Lesions in the Greek Female Population. Pathogens 2025, 14, 853. https://doi.org/10.3390/pathogens14090853
Kafasi A, Pitiriga VC, Spanakis N, Vlachos N, Thomakos N, Stournaras S, Tsakris A, Kaparos G. Evaluation of the PreTect HPV-Proofer E6/E7 mRNA Assay for the Detection of Precancerous Cervical Lesions in the Greek Female Population. Pathogens. 2025; 14(9):853. https://doi.org/10.3390/pathogens14090853
Chicago/Turabian StyleKafasi, Athanasia, Vassiliki C. Pitiriga, Nikolaos Spanakis, Nikolaos Vlachos, Nikolaos Thomakos, Stamatios Stournaras, Athanasios Tsakris, and Georgios Kaparos. 2025. "Evaluation of the PreTect HPV-Proofer E6/E7 mRNA Assay for the Detection of Precancerous Cervical Lesions in the Greek Female Population" Pathogens 14, no. 9: 853. https://doi.org/10.3390/pathogens14090853
APA StyleKafasi, A., Pitiriga, V. C., Spanakis, N., Vlachos, N., Thomakos, N., Stournaras, S., Tsakris, A., & Kaparos, G. (2025). Evaluation of the PreTect HPV-Proofer E6/E7 mRNA Assay for the Detection of Precancerous Cervical Lesions in the Greek Female Population. Pathogens, 14(9), 853. https://doi.org/10.3390/pathogens14090853







