Hansen’s Disease in Ecuador: Current Status, Knowledge Gaps, and Research Priorities: A Literature Review
Abstract
1. Introduction
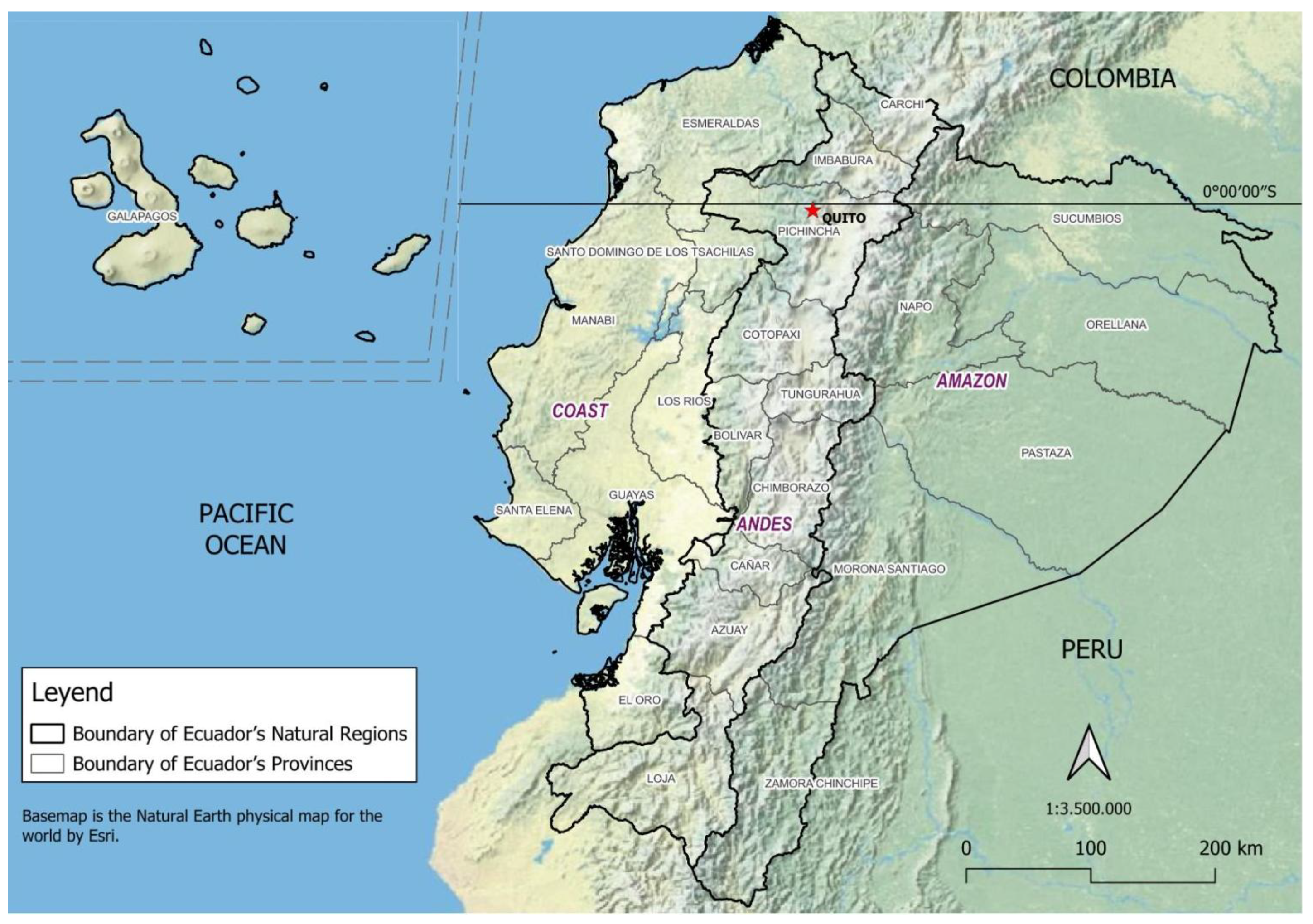
2. Geographical Location and Populations of Ecuador
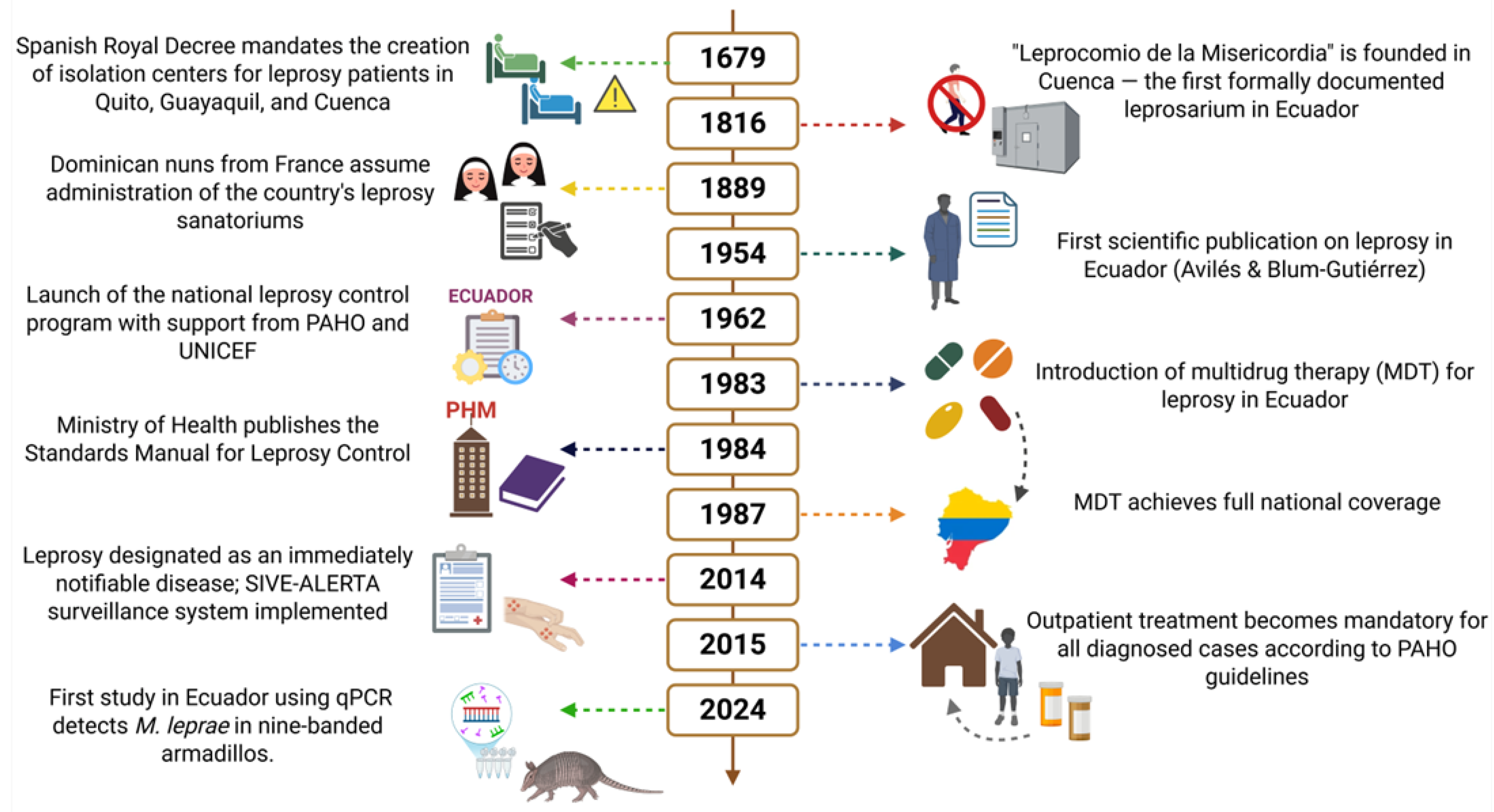
3. Historical Background
4. Study Design, Methods, and Ethics
5. Results
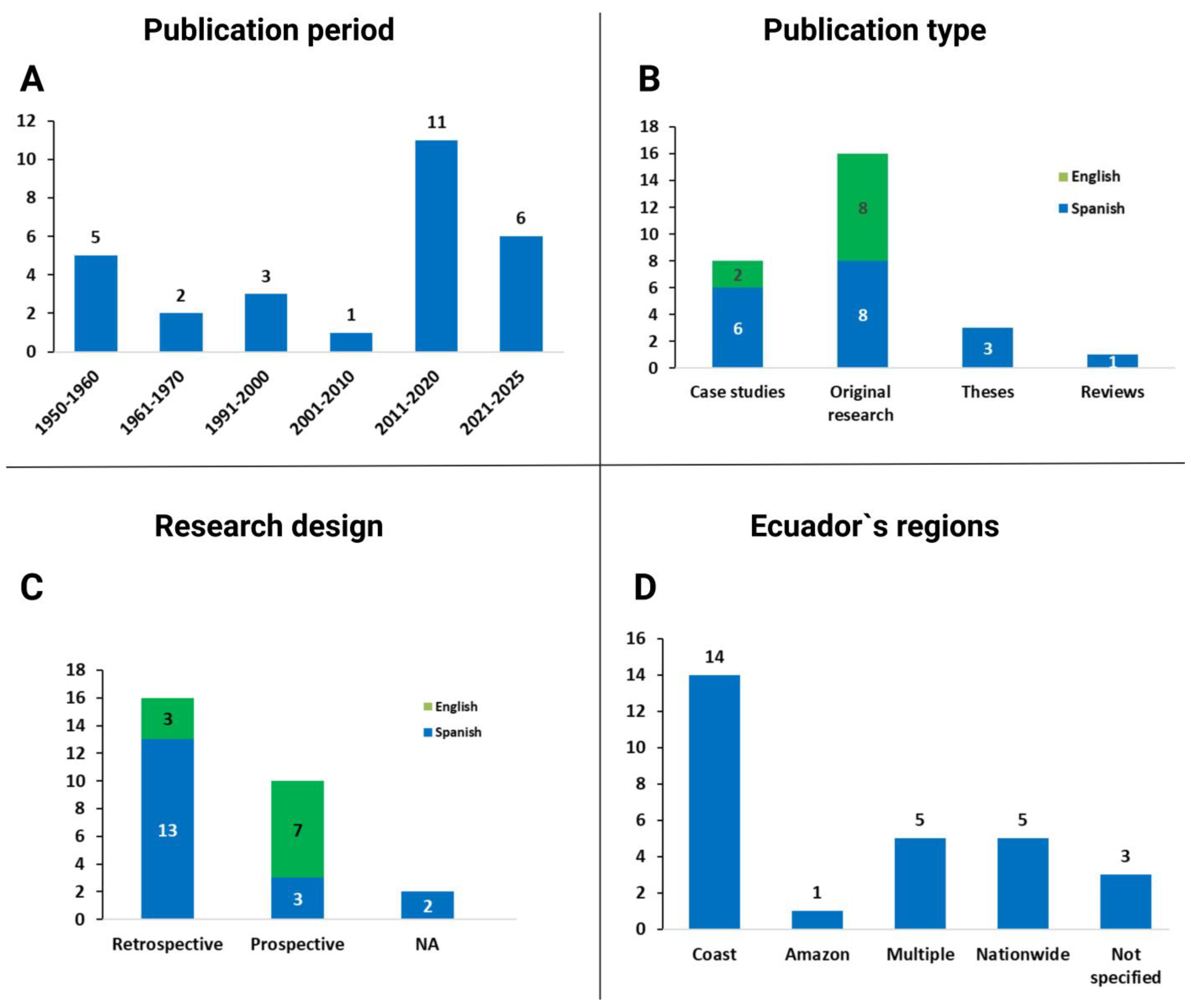
5.1. The Published Literature in Ecuador
5.2. Geographic Distribution and Trends of Human Infections
5.3. Demographic Distribution of Leprosy Cases
5.4. Diagnosis, Laboratory Tests, and Differential Diagnosis
5.5. Clinical Features and Classification of Leprosy in Ecuador
5.6. Etiology, Animal, and Environmental Studies
5.7. Treatment and Chemoprophylaxis of Leprosy
5.8. Control Strategies, Bacillus Calmette–Guérin (BCG) Vaccination
5.9. Disability Outcomes and Social Stigma of Leprosy
6. Discussion
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Leprosy/Hansen Disease Elimination Dossier: Tool Accompanying the Technical Guidance on Interruption of Transmission and Elimination of Leprosy. World Health Organization. Regional Office for South-East Asia. 2025. Available online: https://iris.who.int/handle/10665/380448 (accessed on 26 July 2025).
- WHO. Towards Zero Leprosy: Global Leprosy (Hansen’s Disease) Strategy 2021–2030. World Health Organization. 2021. Available online: https://iris.who.int/handle/10665/340774 (accessed on 26 July 2025).
- WHO. World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. 2021. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 26 July 2025).
- Hernandez-Bojorge, S.; Gardellini, T.; Parikh, J.; Rupani, N.; Jacob, B.; Hoare, I.; Calvopiña, M.; Izurieta, R. Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador. Trop. Med. Infect. Dis. 2024, 9, 246. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Interruption of Transmission and Elimination of Leprosy Disease—Technical Guidance. 2023. Available online: https://www.who.int/publications/i/item/9789290210467 (accessed on 26 July 2025).
- MSP. Subsecretaria Nacional de Vigilancia, Prevención y Control de la Salud Pública. Dirección Nacional de Vigilancia Epidemiológica Enfermedades de la Piel. 2024. Available online: https://www.salud.gob.ec/wp-content/uploads/2025/02/Lepra-SE-08.pdf (accessed on 23 February 2025).
- Baquero Suárez, J.M.; Sánchez, D.G.; Moreira, O.D. Diagnóstico de lepra en una comunidad ecuatoriana. Rev. Cuba Med. Gen. Integral 2019, 35. [Google Scholar]
- Dávila-Rodríguez, J.J.; Rosero, C.; Tello, S.; Yanchapaxi, S. Lepra histioide, una variante rara: Primer reporte en Ecuador. Actas Dermo-Sifiliográficas 2019, 110, 867–869. [Google Scholar] [CrossRef]
- Roldan-Vasquez, A.; Roldan-Vasquez, E.; Vasquez, A.M. Uveitic Glaucoma and Hansen’s disease, A case report. Am. J. Ophthalmol. Case Rep. 2021, 22, 101096. [Google Scholar] [CrossRef] [PubMed]
- Martínez Borrero, P.; Coello, M.; Ortega, P.; Merchán, M.; Palacios, P.B.D. Lepra: Revisión bibliográfica a propósito de un caso clínico. ATENEO 2021, 23, 71–77. [Google Scholar]
- Polanco, C.R.A.; Lucas, M.A.R.; Vinces, L.W.L.; Contino, C.G.D. Multibacillary lepromatous leprosy detected in Olmedo, Ecuador. Case report. Salud Cienc. Tecnol. 2024, 4, 899. [Google Scholar] [CrossRef]
- Ramírez Honores, J.F.; Abendaño, Y.I.C.; Ceron, Y.M.P.; Romero, M.D.G.; Saa, B.A.R. Lepra Virchowiana o Lepromatosa en Paciente Masculino. Reporte de Caso. Cienc. Lat. Rev. Científica Multidiscip. 2024, 8, 9119–9126. [Google Scholar] [CrossRef]
- World Bank Group. World Bank Open Data. Ecuador Data 2023. 2023. Available online: https://data.worldbank.org (accessed on 26 July 2025).
- INEC. INEC Buenas Cifras Mejores Vidas. Censo de Población y Vivienda 2022. 2022. Available online: https://www.ecuadorencifras.gob.ec/estadisticas/ (accessed on 26 July 2025).
- Aviles, F.; Blum-Gutierrez, E. Acerca de 60 casos de lepra estudiados en el Instituto nacional de higiene. Rev. Ecuat. Hig. Med. Trop. 1954, 11, 99–107. [Google Scholar]
- Blum-Gutierrez, E. Programa de control de lepra en el Ecuador consideraciones sobe los dos primeros años de actividades. Bol. Oficina Sanit. Panam. 1967, 120–131. [Google Scholar]
- Aviles, F.; Blum-Gutierrez, E. La Lepra en el Ecuador Algunas observaciones sobre el problema. Rev. Ecuat. Hig. Med. Trop. 1956, 13, 95–103. [Google Scholar]
- Blum-Gutierrez, E. El programa de control de la lepra en Ecuador. Boletín Oficina Sanit. Panam. 1967, 62–72. [Google Scholar]
- Zárate, N.O.V. La lepra en el Ecuador. Hansen. Int. 1992, 17, 33–41. [Google Scholar] [CrossRef]
- Blum-Gutierrez, E. La Lepra en el Ecuador Sugerencias para su control. Rev. Ecuat. Hig. Med. Trop. 1957, 14, 39–42. [Google Scholar]
- MSP. Manual de Procedimientos del Subsistema de Vigilancia Epidemiológica Alerta Acción SIVE–ALERTA. 2014. Available online: https://mail.google.com/mail/u/0/#inbox/FMfcgzQbdrSjMsvPmGnnfnsdzzHpdPDv?projector=1&messagePartId=0.1 (accessed on 18 June 2025).
- Hosokawa, A.; Nonaka, S.; Jurado, M.; Furuya, M.; Eshita, Y.; Mimori, T.; Katakura, K.; Eduardo, A.; Gomez, L.; Izumi, S.; et al. Seroepidemiological surveys for leprosy in Ecuador. Jpn. J. Trop. Med. Hyg. 1994, 22, 179–184. [Google Scholar] [CrossRef][Green Version]
- Hosokawa, A.; Shigeo, N.; Alava, P.J.J.; Gomez, L.E.A.; Jurado, S.H.M.; Yoshihisa, H. Case report of leprosy and a trial of screenings for the family members in Ecuador. Jpn. J. Trop. Med. Hyg. 1994, 22, 219–223. [Google Scholar] [CrossRef]
- PAHO. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy—PAHO/WHO|Pan American Health Organization. 2018. Available online: https://www.paho.org/en/documents/guidelines-diagnosis-treatment-and-prevention-leprosy (accessed on 26 July 2025).
- Romero-Alvarez, D.; Calvopiña, M.; Cisneros-Vásquez, E.; Garzon-Chavez, D.; Warren, A.K.; Bennett, L.S.; Janapati, R.R.; Bastidas-Caldes, C.; Cabezas-Moreno, M.; de Waard, J.H.; et al. Mycobacterium leprae in Nine-Banded Armadillos (Dasypus novemcinctus), Ecuador. Emerg. Infect. Dis. 2024, 30, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Leprosy. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 26 July 2025).
- Blum-Gutierrez, E. Estudio del estado inmunoalergico de la lepra en nuestro medio. Rev. Ecuat. Hig. Med. Trop. 1956, 13, 39–42. [Google Scholar]
- MSP. Informe de Cierre Eventos Dirección Nacional de Vigilancia Epidemiológica. 2018. Available online: https://www.salud.gob.ec/wp-content/uploads/2019/12/INFORME-CIERRE-DE-EVENTOS-2018.pdf (accessed on 21 February 2025).
- Blum-Gutierrez, E. Lepromina preparada a partir de ganglio linfatico formalizado. Rev. Ecuat. Hig. Med. Trop. 1954, 11, 1–2. [Google Scholar]
- WHO. Global Leprosy (Hansen Disease) Update. 2023: Elimination of Leprosy Disease Is Possible—Time to Act! 2024. Available online: https://www.who.int/publications/i/item/who-wer9937-501-521 (accessed on 26 July 2025).
- Calvopina, M.; Gomez, E.; Sindermann, H.; Cooper, P.; Hashiguchi, Y. Relapse of new world diffuse cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana after miltefosine treatment. Am. J. Trop. Med. Hyg. 2006, 75, 1074–1077. [Google Scholar] [CrossRef]
- Franco, J. Prevalencia de Lepra y Contagio de Familiares o Personas Que Estan en Contacto con Pacientes Que Tienen la Enfermedad de Hansen en el Cantón Atahualpa de la Provincia de El Oro, Periodo Febrero-Agosto 2011. [Loja-Ecuador]: Universidad Nacional de Loja. 2012. Available online: https://dspace.unl.edu.ec/jspui/bitstream/123456789/5682/1/Franco%20Tinoco%20%20Joffre%20Bismark.pdf (accessed on 14 February 2025).
- Terán, M.; Sarmiento, G.T.; Plaza, E.P. Prevalencia de lepra en el cantón Salitre, provincia del Guayas, hospital “Oswaldo Jervis Alarcón”. Medicina 2010, 16, 36–42. [Google Scholar]
- Goldman, L.; Schafer, A. Goldman-Cecil. Tratado de Medicina Interna, 26th ed.; Elsevier: Philadelphia, PA, USA, 2020; Available online: https://www.clinicalkey.es/#!/browse/book/3-s2.0-C2019104870X (accessed on 26 July 2025).
- MSP. Lineamientos de la Campaña de Vacunación y Recuperación del Esquema Regular. 2021. Available online: https://www.salud.gob.ec/wp-content/uploads/2022/04/Lineamiento_plan_recuperacion_de_vacunacion_version_30_09_2021-signed-signed-signed.pdf (accessed on 12 March 2025).
- Polo-Checa, A.M.; Sanmartín-Plaza, M.M.; Toro-Manzanares, X.M.; Roldan-Fernández, J.V. Características de la enfermedad de Hansen y contagio a familiares en El Oro. Estudio descriptivo. Rev. Fac. Cienc. Médicas Univ. Cuenca 2017, 35, 23–30. [Google Scholar]
- Baltodano, P.A.; Wan, E.L.; Noboa, J.; Rosson, G.D.; Dellon, A.L. Selecting a Test for Leprous Neuropathy Screening. J. Reconstr. Microsurg. 2015, 31, 607–613. [Google Scholar] [CrossRef]
- Baltodano, P.A.; Rochlin, D.H.; Noboa, J.; Sarhane, K.A.; Rosson, G.D.; Dellon, A.L. Prevalence of leprous neuropathy determined by neurosensory testing in an endemic zone in Ecuador: Development of an algorithm to identify patients benefiting from early neurolysis. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 966–971. [Google Scholar] [CrossRef]
- Dellon, A.L. Multiple Crush Concept Applied to Multiple Nerves in Leprous Neuropathy. Clin. Podiatr. Med. Surg. 2016, 33, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.L.; Rivadeneira, A.F.; Jouvin, R.M.; Dellon, A.L. Treatment of Peripheral Neuropathy in Leprosy: The Case for Nerve Decompression. Plast. Reconstr. Surg. Glob. Open 2016, 4, e637. [Google Scholar] [CrossRef]
- Wan, E.L.; Noboa, J.; Baltodano, P.A.; Jousin, R.M.; Ericson, W.B.; Wilton, J.P.; Rosson, G.D.; Dellon, A.L. Nerve decompression for leprous neuropathy: A prospective study from Ecuador. Lepr. Rev. 2017, 88, 95–108. [Google Scholar] [CrossRef]
- INEC. Home—Instituto Nacional de Estadística y Censos. 2022. Available online: https://www.ecuadorencifras.gob.ec/institucional/home/ (accessed on 27 July 2025).
- Truman, R.W.; Singh, P.; Sharma, R.; Busso, P.; Rougemont, J.; Paniz-Mondolfi, A.; Kapopoulou, A.; Brisse, S.; Scollard, D.M.; Gillis, T.P.; et al. Probable Zoonotic Leprosy in the Southern United States. N. Engl. J. Med. 2011, 364, 1626–1633. [Google Scholar] [CrossRef]
- Vargas Roman, V.C.; Bezemer, J.; Calvopiña, M.; Ortega, F.; Salazar, N.B.; Schallig, H.D.; de Vries, H.J. Multi-sensorial perceptions of risk: The aesthetics behind (muco)cutaneous leishmaniasis-related stigma in Ecuador. Anthropol. Med. 2023, 30, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Lucio, R.; Villacrés, N.; Henríquez, R. Sistema de salud de Ecuador. Salud Pública México 2011, 53, s177–s187. [Google Scholar]
- Middleton, D.R. Migration and Urbanization in Ecuador: A View from the Coast. Urban Anthropol. 1979, 8, 313–332. [Google Scholar]
- Singh, P.; Busso, P.; Paniz-Mondolfi, A.; Aranzazu, N.; Monot, M.; Honore, N.; de Faria Fernandes Belone, A.; Virmond, M.; Villarreal-Olaya, M.E.; Rivas, C.; et al. Molecular drug susceptibility testing and genotyping of Mycobacterium leprae strains from South America. Antimicrob. Agents Chemother. 2011, 55, 2971–2973. [Google Scholar] [CrossRef]
- UNICEF. Desnutrición Crónica Infantil Uno de los Mayores Problemas de Salud Pública en Ecuador. 2021. Available online: https://www.unicef.org/ecuador/sites/unicef.org.ecuador/files/2021-03/Desnutricion-Cronica-Infantil.pdf (accessed on 20 July 2025).
- Ploemacher, T.; Faber, W.R.; Menke, H.; Rutten, V.; Pieters, T. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Negl. Trop. Dis. 2020, 14, e0008276. [Google Scholar] [CrossRef]
- Tió-Coma, M.; Wijnands, T.; Pierneef, L.; Schilling, A.K.; Alam, K.; Roy, J.C.; Faber, W.R.; Menke, H.; Pieters, T.; Stevenson, K.; et al. Detection of Mycobacterium leprae DNA in soil: Multiple needles in the haystack. Sci. Rep. 2019, 9, 3165. [Google Scholar] [CrossRef] [PubMed]
- Feijó, A.; Vilela, J.F.; Cheng, J.; Schetino, M.A.A.; Coimbra, R.T.F.; Bonvicino, C.R.; Santos, F.R.; Patterson, B.D.; Cordeiro-Estrela, P. Phylogeny and molecular species delimitation of long-nosed armadillos (Dasypus: Cingulata) supports morphology-based taxonomy. Zool. J. Linn. Soc. 2019, 186, 813–825. [Google Scholar] [CrossRef]
- Wheat, W.H.; Casali, A.L.; Thomas, V.; Spencer, J.S.; Lahiri, R.; Williams, D.L.; McDonnell, G.E.; Gonzalez-Juarrero, M.; Brennan, P.J.; Jackson, M. Long-term Survival and Virulence of Mycobacterium leprae in Amoebal Cysts. PLoS Negl. Trop. Dis. 2014, 8, e3405. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.M.; Lima, A.; Heringer, S.; Sanders, V.; Pessotti, H.A.; Deps, P. Systematic Review of Hansen Disease Attributed to Mycobacterium lepromatosis. Emerg. Infect. Dis. 2023, 29, 1376–1385. [Google Scholar] [CrossRef]
- Avanzi, C.; Singh, P.; Truman, R.W.; Suffys, P.N. Molecular epidemiology of leprosy: An update. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 86, 104581. [Google Scholar] [CrossRef]
- Calvopina, M.; Armijos, R.X.; Hashiguchi, Y. Epidemiology of leishmaniasis in Ecuador: Current status of knowledge—A review. Mem. Inst. Oswaldo Cruz 2004, 99, 663–672. [Google Scholar] [CrossRef]
- Lastória, J.C.; Abreu, M.A.M.M.D. Leprosy: A review of laboratory and therapeutic aspects—Part 2. An. Bras. Dermatol. 2014, 89, 389–401. [Google Scholar] [CrossRef]
- Bruno, A.; Alfaro-Núñez, A.; de Mora, D.; Armas, R.; Olmedo, M.; Garcés, J.; Garcia-Bereguiain, M.A. First case of human infection with highly pathogenic H5 avian Influenza A virus in South America: A new zoonotic pandemic threat for 2023? J. Travel Med. 2023, 30, taad032. [Google Scholar] [CrossRef]
- Parreiras de Jesus, A.C.; Fraga, V.G.; Pimenta-Carvalho, S.A.; Guimarães, T.M.P.D.; Araújo, M.S.S.; de Carvalho, J.C.; Santos, M.B.; Araújo, M.G.; Pascoal-Xavier, M.A.; Lyon, S.; et al. Identifying promising peptide targets for leprosy serological tests: From prediction to ELISA. J. Genet. Eng. Biotechnol. 2025, 23, 100475. [Google Scholar] [CrossRef] [PubMed]
- MSP. Prevención, Diagnóstico, Tratamiento y Control de la Tuberculosis Guía de Práctica Clínica. 2018. Available online: https://www.salud.gob.ec/wp-content/uploads/2018/03/GP_Tuberculosis-1.pdf (accessed on 25 January 2025).
- Zivarifar, H.; Ahrari, F.; Karbalaei, M. Computational investigation of the global prevalence of multidrug resistant Mycobacterium leprae: A systematic review and meta-analysis. J. Clin. Tuberc. Mycobact. Dis. 2024, 37, 100495. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Yang, J.; Jin, G.; Shao, Y.; Li, Y.; Wei, P.; Zhang, L. Drug Resistance (Dapsone, Rifampicin, Ofloxacin) and Resistance-Related Gene Mutation Features in Leprosy Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12443. [Google Scholar] [CrossRef]
- Barreto, J.; Rosa, P.S.; Adams, L.; Aguilar, Z.; Bakare, N.; Chaplan, S.R.; Akli, R.D.; Ernault, E.; Kulke, S.; Lounis, N.; et al. Bedaquiline Monotherapy for Multibacillary Leprosy. N. Engl. J. Med. 2024, 391, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- MSP. Información de Enfermedades de la Piel o Reemergentes 2025—Ministerio de Salud Pública. 2025. Available online: https://www.salud.gob.ec/informacion-de-enfermedades-de-la-piel-o-reemergentes-2025/ (accessed on 27 July 2025).
- Brandsma, J.W.; Van Brakel, W.H. WHO disability grading: Operational definitions. Lepr. Rev. 2003, 74, 366–373. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leprosy/Hansen Disease: Contact Tracing and Post-Exposure Prophylaxis. Technical Guidance. 2020. Available online: https://iris.who.int/bitstream/handle/10665/336679/9789290228073-eng.pdf (accessed on 18 March 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvopiña, M.; Izquierdo-Condoy, J.S.; Ortiz-Prado, E.; Vasconez-Gonzalez, J.; Vaca, L.; Guamán, E. Hansen’s Disease in Ecuador: Current Status, Knowledge Gaps, and Research Priorities: A Literature Review. Pathogens 2025, 14, 832. https://doi.org/10.3390/pathogens14080832
Calvopiña M, Izquierdo-Condoy JS, Ortiz-Prado E, Vasconez-Gonzalez J, Vaca L, Guamán E. Hansen’s Disease in Ecuador: Current Status, Knowledge Gaps, and Research Priorities: A Literature Review. Pathogens. 2025; 14(8):832. https://doi.org/10.3390/pathogens14080832
Chicago/Turabian StyleCalvopiña, Manuel, Juan S. Izquierdo-Condoy, Esteban Ortiz-Prado, Jorge Vasconez-Gonzalez, Lorena Vaca, and Elías Guamán. 2025. "Hansen’s Disease in Ecuador: Current Status, Knowledge Gaps, and Research Priorities: A Literature Review" Pathogens 14, no. 8: 832. https://doi.org/10.3390/pathogens14080832
APA StyleCalvopiña, M., Izquierdo-Condoy, J. S., Ortiz-Prado, E., Vasconez-Gonzalez, J., Vaca, L., & Guamán, E. (2025). Hansen’s Disease in Ecuador: Current Status, Knowledge Gaps, and Research Priorities: A Literature Review. Pathogens, 14(8), 832. https://doi.org/10.3390/pathogens14080832






