Human Tuberculosis in Migrant and Autocthonous Patients: A Ten-Year Single-Centre Experience
Abstract
1. Introduction
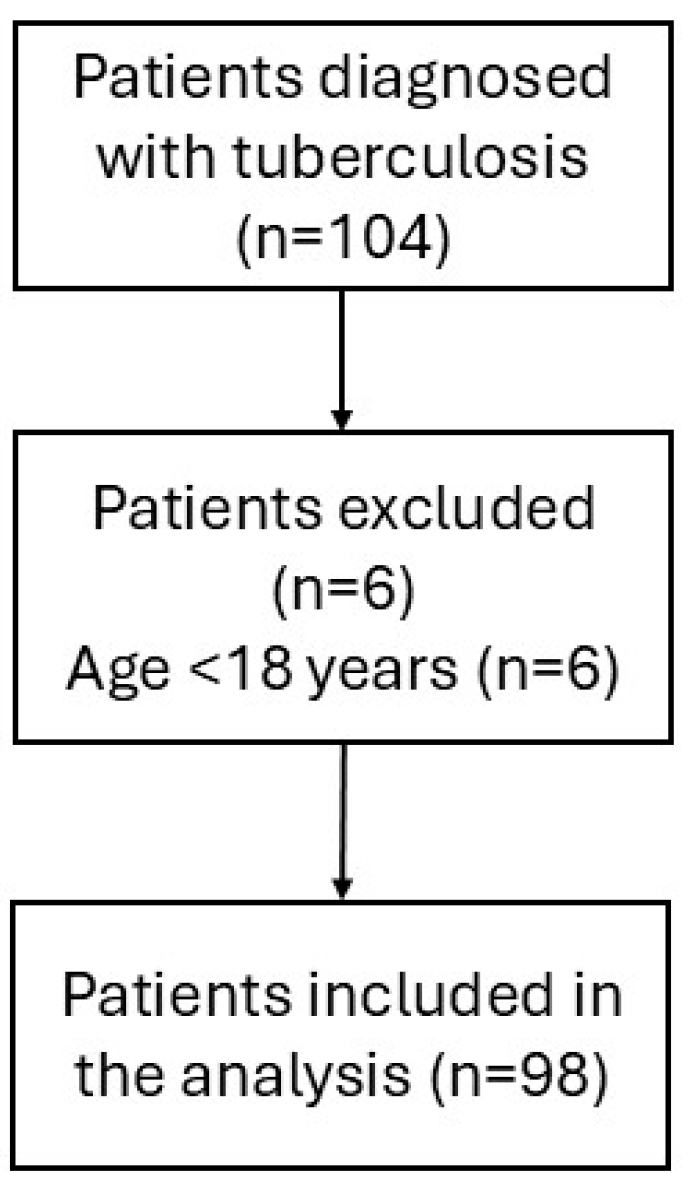
2. Material and Methods
3. Results
3.1. General Cohort Description
3.2. Migrant vs. Autochthonous Comparison
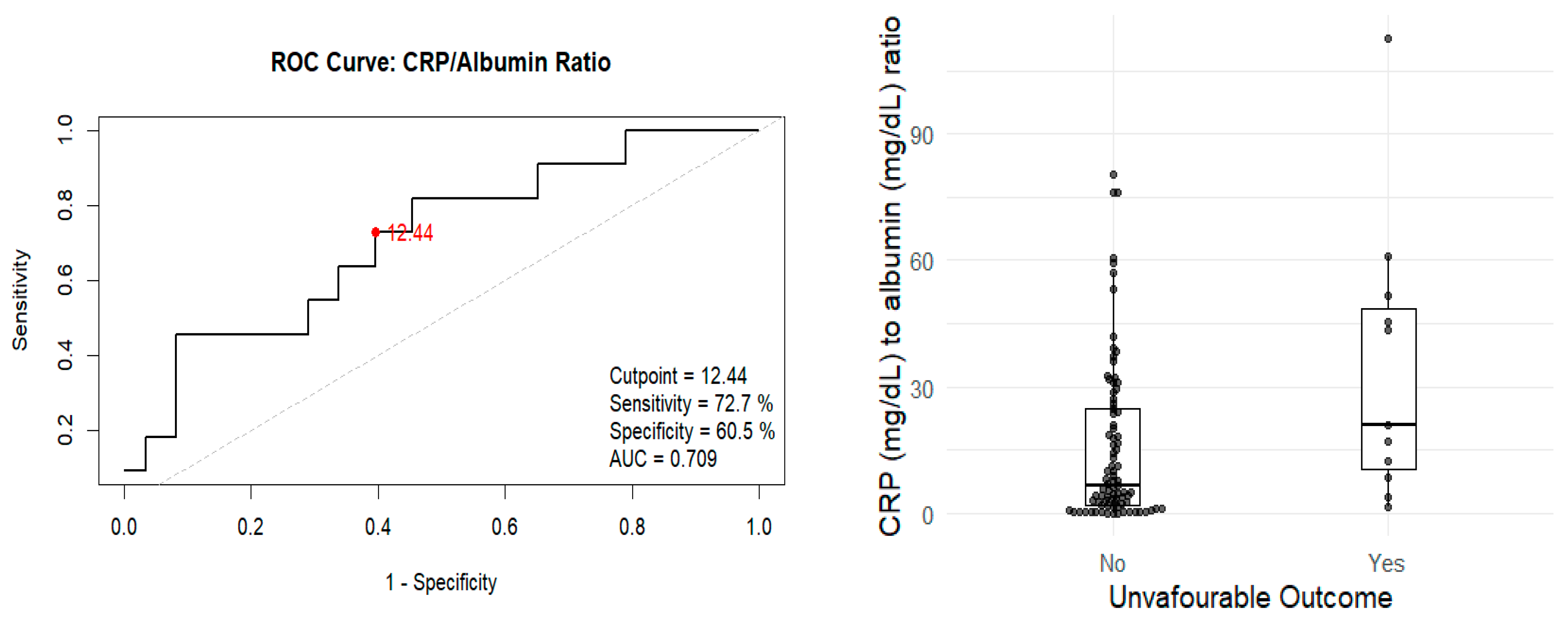
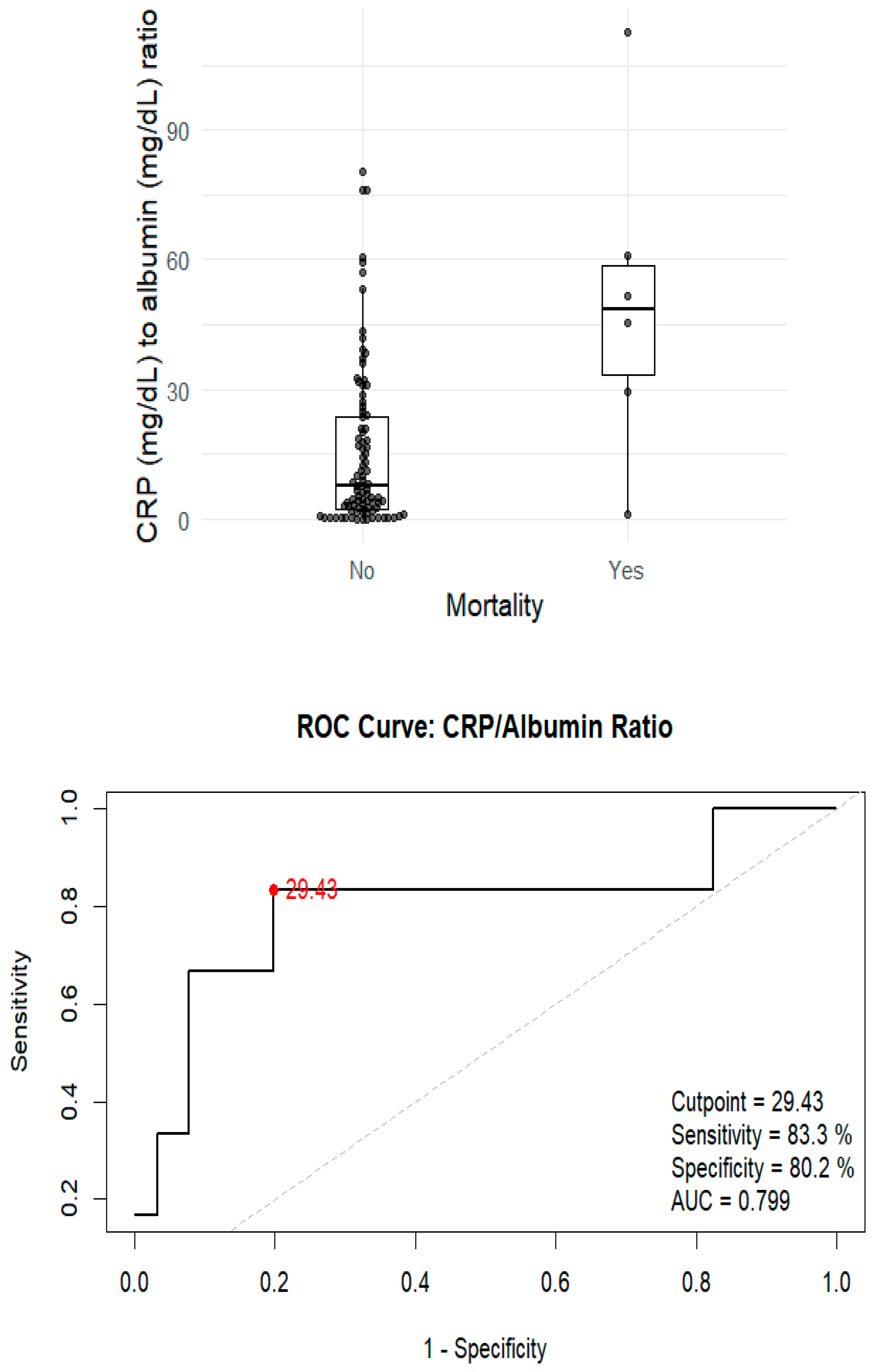
3.3. Analysis of Unfavourable Outcomes and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2023; WHO: Geneva, Switzerland, 2023. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 7 July 2025).
- Sánchez-Montalvá, A.; Salvador, F.; Molina-Morant, D.; Molina, I. Tuberculosis e inmigración, Tuberculosis and immigration. Enferm. Infecc. Microbiol. Clin. 2018, 36, 446–455. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; European Centre for Disease Prevention and Control. Tuberculosis Surveillance and Monitoring in Europe; WHO/ECDC: Geneva, Switzerland, 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2023-2021-data (accessed on 7 July 2025).
- Ramos, J.M.; Masiá, M.; Rodríguez, J.C.; Padilla, I.; Soler, M.J.; Gutiérrez, F. Tuberculosis en inmigrantes: Diferencias clinicoepidemiológicas con la población autóctona (1999–2002). Enferm. Infecc. Microbiol. Clin. 2004, 22, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ministerio del Interior. Inmigración Irregular 2024; Gobierno de España: Madrid, Spain, 2024. Available online: https://www.interior.gob.es/opencms/es/prensa/balances-e-informes/ (accessed on 7 July 2025).
- Wikman-Jorgensen, P.; López-Velez, R.; Llenas-García, J.; Treviño, B.; Pascual, R.; Molina, I.; Domínguez, Á.; Torrús, D.; Giardín, J.M.R.; Monge-Maillo, B.; et al. Latent and active tuberculosis infections in migrants and travellers: A retrospective analysis from the Spanish +REDIVI collaborative network. Travel Med. Infect. Dis. 2020, 36, 101460. [Google Scholar] [CrossRef] [PubMed]
- Godoy, S.; Alsedà, M.; Parrón, I.; Millet, J.-P.; Caylà, J.A.; Follia, N.; Carol, M.; Orcau, A.; Toledo, D.; Ferrús, G.; et al. Exposure Time to a Tuberculosis Index Case as a Marker of Infection in Immigrant Populations. Pathogens 2025, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Zenner, D.; White, P.J.; Muzyamba, M.C.; Loutet, M.; Dhavan, P.; Mosca, D.; Hayward, A.C.; Abubakar, I. Prevalence of and risk factors for active tuberculosis in migrants screened before entry to the UK: A population-based cross-sectional study. Lancet Infect. Dis. 2016, 16, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Guillén, A.; Carbonell, X.; Alfranca, R.; Beranuy, M.; Parés-Bayerri, A.; Font-Mayolas, S. “Healthy immigrant effect” among individuals experiencing homelessness in Spain?: Foreign-born individuals had higher average age at death in 15-year retrospective cohort study. BMC Public Health 2023, 23, 1212. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tuberculosis: Extensively Drug-Resistant Tuberculosis (XDR-TB). Available online: https://www.who.int/news-room/questions-and-answers/item/tuberculosis-extensively-drug-resistant-tuberculosis-(XDR-TB) (accessed on 7 July 2025).
- World Health Organization. Tuberculosis: Multidrug-Resistant (MDR-TB) or Rifampicin-Resistant TB (RR-TB). Available online: https://www.who.int/news-room/questions-and-answers/item/tuberculosis-multidrug-resistant-tuberculosis-(mdr-tb) (accessed on 7 July 2025).
- Palomo López, P.; Redondo Mena, C. Legislación vigente y ética en investigación clínica. Rev. Int. Cienc. Podol. 2012, 6, 81–93. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado. Ley Orgánica 3/2018, de 5 de Diciembre, de PROTECCIÓN de Datos Personales y Garantía de los Derechos Digitales; Gobierno de España: Madrid, Spain, 2018. Available online: https://www.boe.es/eli/es/lo/2018/12/05/3/con (accessed on 7 July 2025).
- Centro Nacional de Epidemiología. Protocolos de la Red Nacional de Vigilancia Epidemiológica; Instituto de Salud Carlos III (ISCIII): Madrid, Spain, 2022.
- Pichiule-Castañeda, M.; Rodero-Garduño, I.; Febrel-Bordeje, C.; Ribeiro-Alexandre-d, M.C.; Rodríguez-Baena, E.; Córdoba-Deorador, E.; Sánchez-Díaz, J.; Gil-Montalbán, E.; Barbas-Del-Buey, J.; Jiménez-Bueno, S.; et al. Tendencia de la tuberculosis en la Comunidad de Madrid en la población autóctona y extranjera (2009–2018). Rev. Esp. Salud Pública 2020, 94, e202009113. [Google Scholar] [PubMed]
- Jinyi, W.; Zhang, Y.; Wang, K.; Peng, P. Global, regional, and national mortality of tuberculosis attributable to alcohol and tobacco from 1990 to 2019: A modelling study based on the Global Burden of Disease study 2019. J. Glob. Health 2024, 14, 04023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.X.; Yan, L.J.; Qie, D.L.; Shao, L.J.; Li, N.; Chen, G. Risk factors for mortality in patients with tuberculosis admitted to intensive care units. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Jarsberg, L.G.; Kedia, K.; Wendler, J.; Wright, A.T.; Piehowski, P.D.; Gritsenko, M.A.; Shi, T.; Lewinsohn, D.M.; Sigal, G.B.; Weiner, M.H.; et al. Nutritional markers and proteome in patients undergoing treatment for pulmonary tuberculosis differ by geographic region. PLoS ONE 2021, 16, e0250586. [Google Scholar] [CrossRef] [PubMed]
- Chaulk, C.P.; Moonan, P.K. Over the limit: Tuberculosis and excessive alcohol use. Int. J. Tuberc. Lung Dis. 2020, 24, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Dohál, M.; Dvořáková, V.; Šperková, M.; Pinková, M.; Ghodousi, A.; Omrani, M.; Porvazník, I.; Rasmussen, E.M.; Škereňová, M.; Krivošová, M.; et al. Tuberculosis in Ukrainian War Refugees and Migrants in the Czech Republic and Slovakia: A Molecular Epidemiological Study. J. Epidemiol. Glob. Health 2024, 14, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Min, J.; Myong, J.P.; Lee, Y.H.; Park, Y.J.; Kim, Y.; Kim, G.; Park, G.; Lee, S.S.; Park, J.S.; et al. Incidence of Tuberculosis Among Immigrants in Korea Who Participated in a Latent Tuberculosis Infection Screening Program. J. Korean Med. Sci. 2024, 39, e207. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA; ECDC: Stockholm, Sweden, 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Public%20health%20guidance%20on%20screening%20and%20vaccination%20of%20migrants%20in%20the%20EU%20EEA.pdf?utm_source=chatgpt.com (accessed on 15 January 2025).
| Variables | Total (n = 98) | Native Population (n = 69) | Migrant Population (n = 29) | p-Value |
|---|---|---|---|---|
| Time in Spain < 5 years | 16 (16%) | 0 | 16 (55.2%) | 0.001 |
| Sex | ||||
| Male | 66 (67%) | 47 (68%) | 19 (65%) | 0.988 |
| Female | 32 (32.6%) | 22 (31%) | 10 (34.5%) | |
| Mean age (SD) (years) | 44.38 (17.6) | 48.03 (17.6) | 35.69 (14.3) | 0.001 |
| Pulmonary TB | 81 (82%) | 57 (82%) | 24 (82%) | 1 |
| Extrapulmonary TB | 17 (17%) | 12 (17%) | 5 (17%) | |
| COPD | 10 (10%) | 9 (13%) | 1 (3.45%) | 0.273 |
| Heart failure | 1 (1%) | 1 (1.4%) | 0 | 1 |
| AMI/Stroke | 3 (3.5%) | 3 (4.35%) | 0 | 0.553 |
| Cancer/Autoimmune | 14 (14.2%) | 12 (17.3%) | 2 (6.9%) | 0.22 |
| Neurologic disease | 9 (9.18%) | 6 (8.7%) | 3 (10.3%) | 1 |
| HIV infection | 6 (6.1%) | 5 (7.25%) | 1 (3.45%) | 0.667 |
| Diabetes Mellitus | 7 (7.1%) | 5 (7.25%) | 2 (6.9%) | 1 |
| Toxic habits | ||||
| Tobacco use | 56 (57%) | 46 (66%) | 10 (34%) | 0.007 |
| Alcohol consumption | 35 (35%) | 32 (46%) | 3 (10.3%) | 0.001 |
| IV drug use | 7 (7.1%) | 7 (10%) | 0 | 0.1 |
| Albumin (g/dL, SD) | 3.91 (0.66) | 3.91 (0.66) | 3.90 (0.68) | 0.997 |
| CRP (mg/L, SD) | 59.71 (64.5) | 56.73 (60.6) | 66.8 (73.4) | 0.929 |
| Educational level | ||||
| Higher education (University degree or equivalent post-secondary qualification) | 5 (5%) | 5 (7.24%) | 0 | 0.33 |
| Secondary education (Completion of high school or equivalent vocational training) | 50 (50%) | 35 (50%) | 15 (51%) | |
| Primary education (Completion of primary/elementary school only) | 35 (35%) | 25 (36%) | 10 (34%) | |
| Unknown (Educational level not reported or unavailable) | 8 (8%) | 4 (5.7%) | 4 (13%) | |
| Employment status | ||||
| Employed | 57 (58%) | 42 (60.8%) | 15 (51.7%) | 0.595 |
| Unemployed | 31 (31%) | 19 (27.5%) | 12 (41%) |
| Variable | Unfavourable Outcome: Yes | Unfavourable Outcome: No | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 11 (100%) | 55 (63%) | 0.01 |
| Female | 0 | — | |
| Mean age (SD), years | 46.3 (20.9) | 44.1 (17.3) | 0.75 |
| Origin | |||
| Migrant | 4 (14%) | 25 (86%) | 0.72 |
| Native | 7 (10%) | 62 (90%) | |
| Comorbidities | |||
| COPD | 2 (18%) | 9 (82%) | 0.31 |
| Heart failure | 0 | 11 (100%) | 1 |
| AMI/Stroke | 0 | 11 (100%) | 1 |
| Cancer/Autoimmune disease | 0 | 11 (100%) | 0.35 |
| Neurologic disease | 2 (18%) | 9 (82%) | 0.26 |
| HIV infection | 1 (9%) | 10 (91%) | 0.52 |
| Toxic habits | |||
| Tobacco use | 6 (55%) | 5 (45%) | 0.89 |
| Alcohol use | 4 (36%) | 7 (64%) | 1 |
| IV drug use | 7 (10%) | 0 | 0.1 |
| Drug resistance | |||
| Isoniazid resistance | 0 | 11 (100%) | 1 |
| Laboratory values | |||
| Albumin (g/dL), median (IQR) | 3.6 (2.78–4.15) | 4.11 (3.5–4.4) | 0.065 |
| CRP (mg/L), median (IQR) | 102 (38.9–150) | 25 (9.95–85.5) | 0.033 |
| BMI (kg/m2), median (IQR) | 18.4 (18.2–18.7) | 22.6 (18.7–26.9) | 0.127 |
| CRP/Albumin ratio | 20.8 (10.5–48.6) | 6.72 (2.13–24.7) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, I.G.; Romero, M.; Gascón, I.; Solves, V.; Pascual, R.; Wikman-Jorgensen, P.E. Human Tuberculosis in Migrant and Autocthonous Patients: A Ten-Year Single-Centre Experience. Pathogens 2025, 14, 824. https://doi.org/10.3390/pathogens14080824
Soriano IG, Romero M, Gascón I, Solves V, Pascual R, Wikman-Jorgensen PE. Human Tuberculosis in Migrant and Autocthonous Patients: A Ten-Year Single-Centre Experience. Pathogens. 2025; 14(8):824. https://doi.org/10.3390/pathogens14080824
Chicago/Turabian StyleSoriano, Isabel García, Mónica Romero, Isabel Gascón, Verónica Solves, Reyes Pascual, and Philip Erick Wikman-Jorgensen. 2025. "Human Tuberculosis in Migrant and Autocthonous Patients: A Ten-Year Single-Centre Experience" Pathogens 14, no. 8: 824. https://doi.org/10.3390/pathogens14080824
APA StyleSoriano, I. G., Romero, M., Gascón, I., Solves, V., Pascual, R., & Wikman-Jorgensen, P. E. (2025). Human Tuberculosis in Migrant and Autocthonous Patients: A Ten-Year Single-Centre Experience. Pathogens, 14(8), 824. https://doi.org/10.3390/pathogens14080824






