Coronary Artery Disease in People Living with HIV May Reflect Their Sensitivity to Inflammation Associated with Cytomegalovirus
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort
2.2. Plasma Inflammatory Markers
2.3. CMV-Reactive Antibody
2.4. Statistical Analyses
3. Results
3.1. PLWH with and Without CAD Were Matched by Age, Sex, and Year of Attendance in the HIV Clinic
3.2. A Diagnosis of CAD Did Not Align with Levels of CMV-Reactive Antibodies
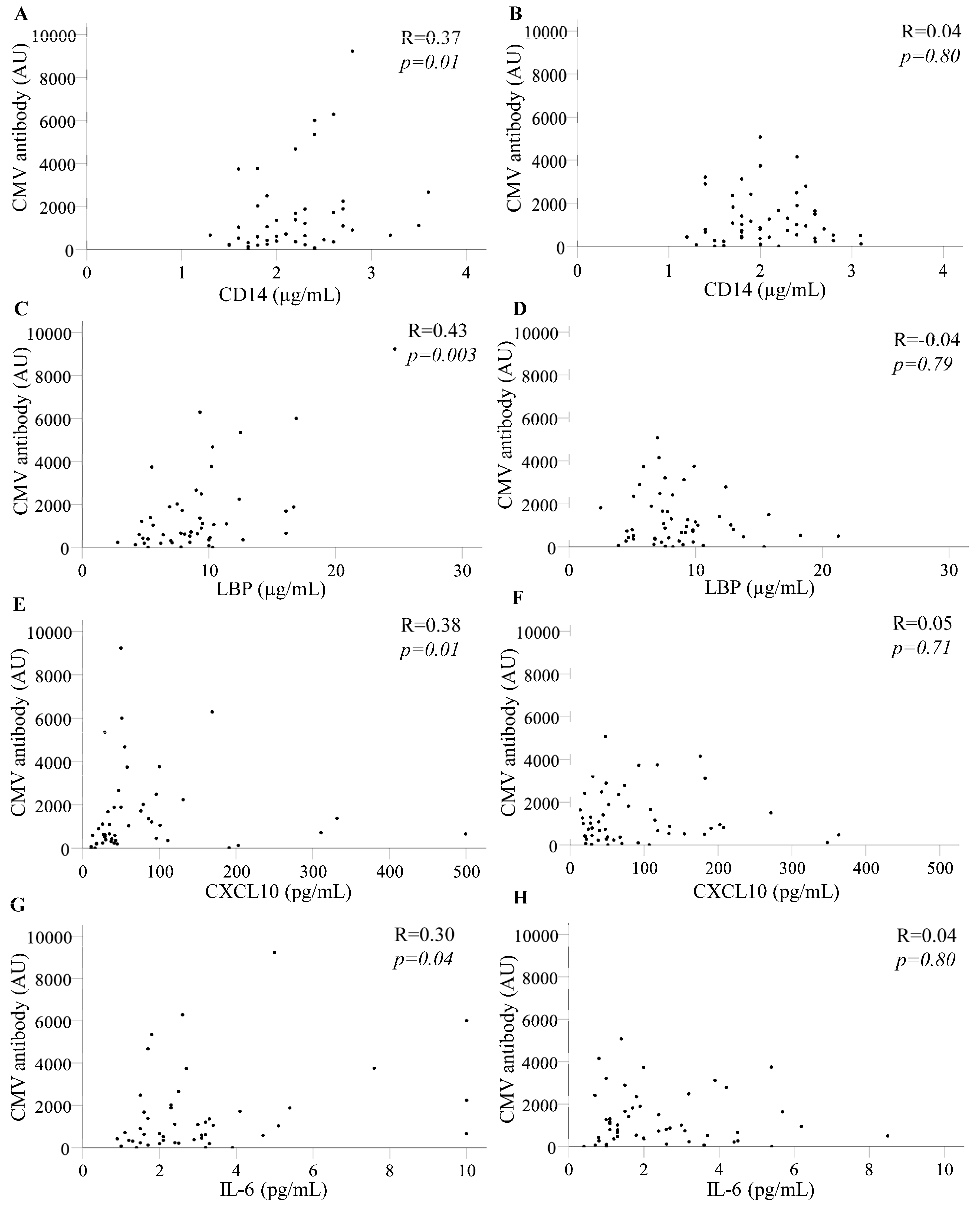
3.3. CMV-Reactive Antibody Levels Correlated Directly with Selected Inflammatory Biomarkers in PLWH Who Developed CAD up to 36 Months Later
3.4. HIV Viraemia Did Not Explain the Correlations Between Levels of CMV-Reactive Antibody and Selected Inflammatory Biomarkers in PLWH Who Developed CAD 12–36 Months Later
3.5. Associations Between CMV Antibodies and Inflammatory Markers in PLWH Who Developed CAD Did Not Reflect Higher Levels of the Markers
3.6. Multivariable Analyses Do Not Identify CMV Antibodies as Predictors of CAD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhuo, C.; Li, Q.; Liu, L. Cytomegalovirus (CMV) infection in HIV/AIDS patients and diagnostic values of CMV-DNA detection across different sample types. Ann. Palliat. Med. 2020, 9, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Affandi, J.S.; Montgomery, J.; Brunt, S.J.; Nolan, D.; Price, P. The immunological footprint of CMV in HIV-1 patients stable on long-term ART. Immun. Ageing 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, G.; Bai, J.; He, B.; Huang, K.; Hu, X.; Liu, D. Cytomegalovirus infection and relative risk of cardiovascular disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A meta-analysis of prospective studies up to 2016. J. Am. Heart Assoc. 2017, 6, e005025. [Google Scholar] [CrossRef]
- Arvanitis, P.; Davis, M.R.; Farmakiotis, D. Cytomegalovirus infection and cardiovascular outcomes in abdominal organ transplant recipients: A systematic review and meta-analysis. Transplant. Rev. 2024, 38, 100860. [Google Scholar] [CrossRef]
- Ibrahim, A.I.; Obeid, M.T.; Jouma, M.J.; Moasis, G.A.; Al-Richane, W.L.; Kindermann, I.; Boehm, M.; Roemer, K.; Mueller-Lantzsch, N.; Gärtner, B.C. Detection of herpes simplex virus, cytomegalovirus and Epstein-Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J. Clin. Virol. 2005, 32, 29–32. [Google Scholar] [CrossRef]
- Wanjalla, C.N.; Mashayekhi, M.; Bailin, S.; Gabriel, C.L.; Meenderink, L.M.; Temu, T.; Fuller, D.T.; Guo, L.; Kawai, K.; Virmani, R.; et al. Anti-cytomegalovirus CD4+ T Cells are associated with Subclinical Atherosclerosis in Persons with HIV. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1459–1473. [Google Scholar] [CrossRef]
- Affandi, J.S.; Lee, S.; Chih, H.; Brook, E.; Waters, S.; Howson, P.; Reid, C.M.; Irish, A.; Price, P. Cytomegalovirus burden improves a predictive model identifying measures of vascular risk in renal transplant recipients and healthy adults. J. Med. Virol. 2020, 92, 3650–3657. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Baker, J.V. Cardiovascular Disease Among Persons Living with HIV: New Insights into Pathogenesis and Clinical Manifestations in a Global Context. Circulation 2023, 147, 83–100. [Google Scholar] [CrossRef]
- Suarez-Zdunek, M.A.; Knudsen, A.D.; Fuchs, A.; Kirkby, N.S.; Benfield, T.; Gerstoft, J.; Trøseid, M.; Ostrowski, S.R.; Køber, L.V.; Kofoed, K.F.; et al. Cytomegalovirus Antibodies and Coronary Artery Disease in People with HIV: A Cohort Study. Viruses 2025, 17, 231. [Google Scholar] [CrossRef]
- Brunt, S.J.; Lee, S.; D’Orsogna, L.; Bundell, C.; Burrows, S.; Price, P. The use of humoral responses as a marker of CMV burden in HIV patients on ART requires consideration of T-cell recovery and persistent B-cell activation. Dis. Markers 2014, 2014, 947432. [Google Scholar] [CrossRef] [PubMed]
- Mushin, A.S.; Trevillyan, J.M.; Lee, S.J.; Hearps, A.C.; Hoy, J.F. Factors associated with the development of coronary artery disease in people with HIV. Sex Health 2023, 20, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Cheng, J.; Herrington, D.M.; Kandula, N.R.; Kanaya, A.M. Adipose tissue-derived metabolite risk scores and risk for type 2 diabetes in South Asians. Int. J. Obes. 2024, 48, 668–673. [Google Scholar] [CrossRef]
- Schnittman, S.R.; Lu, M.T.; Mayrhofer, T.; Burdo, T.H.; Fitch, K.V.; McCallum, S.; Fulda, E.S.; Zanni, M.V.; Foldyna, B.; Malvestutto, C.; et al. Cytomegalovirus immunoglobulin G (IgG) titer and coronary artery disease in people with Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2023, 76, e613–e621. [Google Scholar] [CrossRef]
- Parrinello, C.M.; Sinclair, E.; Landay, A.L.; Lurain, N.; Sharrett, A.R.; Gange, S.J.; Xue, X.; Hunt, P.W.; Deeks, S.G.; Hodis, H.N.; et al. Cytomegalovirus Immunoglobulin G Antibody is associated with Subclinical Carotid Artery Disease among HIV-Infected Women. J. Infect. Dis. 2012, 205, 1788–1796. [Google Scholar] [CrossRef]
- Knudsen, A.; Kristoffersen, U.; Panum, I.; Hansen YSkottrup, P.; Hasbak, P.; Kjaer, A.; Lebech, A. Coronary artery calcium and intima-media thickness are associated with level of cytomegalovirus immunoglobulin G in HIV-infected patients. HIV Med. 2019, 20, 60–62. [Google Scholar] [CrossRef]
- Quisias, J.; Gill, M.J.; Coburn, S.B.; Krentz, H.B.; Beckthold, B.; Fonseca, K.; Parkins, M.D.; Lang, R. Cytomegalovirus serostatus among people with HIV, characterizing the prevalence, risk factors, and association with immune recovery. HIV Med. 2025, 26, 1074–1085. [Google Scholar] [CrossRef]
- Hoehl, S.; Berger, A.; Ciesek, S.; Rabenau, H.F. Thirty years of CMV seroprevalence—A longitudinal analysis in a German university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1095–1102. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.S.; Nourshargh, S.; Akbar, A.N.; Henson, S.M. Human CD8+ EMRA T cells display a senescence associated secretory phenotype regulated by p38 MAPK. Aging Cell 2018, 17, e12675. [Google Scholar] [CrossRef]
- Pitard, V.; Roumanes, D.; Lafarge, X.; Couzi, L.; Garrigue, I.; Lafon, M.E.; Merville, P.; Moreau, J.F.; Déchanet-Merville, J. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood 2008, 112, 1317–1324. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef]
- Hastie, E.; Moser, C.; Sun, X.; Lennox, J.; Hsue, P.Y.; Bosch, R.J.; Deeks, S.; Meneses, M.V.; Lederman, M.M.; Hunt, P.; et al. Effect of Immune-Modulatory Interventions on Asymptomatic Cytomegalovirus Shedding During Suppressive Antiretroviral Therapy. J. Infect. Dis. 2023, 228, 64–69. [Google Scholar] [CrossRef]
- Waters, S.; Lee, S.; Ariyanto, I.; Kresoje, N.; Leary, S.; Munyard, K.; Gaudieri, S.; Irish, A.; Keil, A.D.; Allcock, R.J.N.; et al. Sequencing of the Viral UL111a Gene Directly from Clinical Specimens reveals variants of HCMV-Encoded IL-10 that are associated with altered immune responses to HCMV. Int. J. Mol. Sci. 2022, 22, 4644. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.; Agostino, M.; Lee, S.; Ariyanto, I.; Kresoje, N.; Leary, S.; Munyard, K.; Gaudieri, S.; Gaff, J.; Irish, A.; et al. Sequencing Directly from Clinical Specimens Reveals Genetic Variations in HCMV-Encoded Chemokine Receptor US28 that may influence antibody levels and interactions with Human Chemokines. Microbiol. Spectrum. 2021, 9, 2. [Google Scholar] [CrossRef]
- Waters, S.; Lee, S.; Ariyanto, I.; Leary, S.; Munyard, K.; Gaudieri, S.; Irish, A.; Allcock, R.J.N.; Price, P. Variants of HCMV UL18 Sequenced Directly from Clinical Specimens Associate with Antibody and T-Cell Responses to HCMV. Int. J. Mol. Sci. 2022, 23, 12911. [Google Scholar] [CrossRef]
| CAD | No CAD | p-Value | |
|---|---|---|---|
| Time of diagnosis or census point | |||
| n | 48 | 48 | |
| Age (years) | 52.5 (33–68) | 53 (35–69) | 0.90 |
| Sex (male/female) | 45/3 | 46/2 | 1.0 |
| Nadir CD4 T-cells/µL | 110 (5–618) | 137 (1–494) | 0.08 |
| Current CD4 T-cells/µL | 442 (8–1359) | 468 (125–1131) | 0.39 |
| >200 copies/mL HIV RNA (yes/no) | 9/39 | 7/41 | 0.78 |
| Time on ART (years) | 11 (1.8–28) | 9.3 (1.5–22) | 0.03 |
| Ever received abacavir (yes/no) | 34/14 | 24/24 | 0.06 |
| 12 months before diagnosis or census point | |||
| n | 47 | 52 | |
| Age (years) | 52 (32–68) | 52 (35–68) | 0.88 |
| Sex (male/female) | 45/2 | 50/2 | 1.0 |
| Nadir CD4 T-cells/µL | 110 (5–618) | 132 (1–494) | 0.48 |
| Current CD4 T-cells/µL | na | na | |
| >200 copies/mL HIV RNA (yes/no) | 12/35 | 10/42 | 0.48 |
| Time on ART (years) | 9.8 (1.1–28) | 8.5 (1.3–25) | 0.23 |
| Ever received abacavir (yes/no) | 29/18 | 20/32 | 0.03 |
| CAD | No CAD | p-Value | |
|---|---|---|---|
| At the time of diagnosis or census point | |||
| CMV serostatus (pos/neg) | 44/4 | 46/2 | 0.68 |
| CMV antibody (AU) | 974 (0–8598) | 838 (0–5311) | 0.94 |
| 6 months before diagnosis or census point | |||
| CMV serostatus (pos/neg) | 48/3 | 51/4 | 1.0 |
| CMV antibody (AU) | 811 (0–8305) | 603 (0–4827) | 0.85 |
| 12 months before diagnosis or census point | |||
| CMV serostatus (pos/neg) | 45/2 | 50/2 | 1.0 |
| CMV antibody (AU) | 713 (0–9230) | 800 (0–5073) | 0.92 |
| 24 months before diagnosis or census point | |||
| CMV serostatus (pos/neg) | 40/1 | 50/2 | 1.0 |
| CMV antibody (AU) | 1043 (0–6050) | 665 (0–5602) | 0.58 |
| 36 months before diagnosis or census point | |||
| CMV serostatus (pos/neg) | 34/1 | 44/2 | 1.0 |
| CMV antibody (AU) | 894 (0–6674) | 783 (0–6528) | 0.99 |
| PLWH with CAD | Diagnosis (T0) | T-6 Months | T-12 Months | T-24 Months | T-36 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | |
| n | 48 | 51 | 47 | 41 | 35 | |||||
| Nadir CD4 T-cells/µL | −0.09 | 0.55 | −0.12 | 0.40 | −0.43 | 0.002 | −0.28 | 0.08 | −0.24 | 0.16 |
| Time on ART (years) | 0.05 | 0.75 | 0.32 | 0.02 | 0.27 | 0.07 | 0.37 | 0.02 | 0.18 | 0.30 |
| CD14 (μg/mL) | 0.34 | 0.02 | 0.38 | 0.01 | 0.37 | 0.01 | 0.15 | 0.35 | 0.33 | 0.05 |
| LBP (μg/mL) | 0.27 | 0.07 | 0.28 | 0.05 | 0.43 | 0.003 | 0.22 | 0.17 | 0.35 | 0.04 |
| CXCL10 (pg/mL) | 0.26 | 0.08 | 0.30 | 0.03 | 0.38 | 0.01 | 0.39 | 0.01 | 0.06 | 0.73 |
| IL-1RA (ng/mL) | 0.06 | 0.69 | 0.11 | 0.46 | −0.05 | 0.77 | −0.02 | 0.89 | 0.10 | 0.56 |
| D-dimer (ng/mL) | 0.07 | 0.63 | 0.19 | 0.18 | 0.03 | 0.82 | 0.27 | 0.09 | 0.39 | 0.02 |
| LP-PLA2 (μg/mL) | 0.05 | 0.72 | 0.19 | 0.18 | 0.07 | 0.64 | 0.16 | 0.31 | 0.07 | 0.71 |
| VCAM-1 (μg/mL) | 0.17 | 0.25 | 0.36 | 0.01 | 0.23 | 0.13 | 0.09 | 0.57 | 0.14 | 0.44 |
| hsCRP (pg/mL) | 0.08 | 0.60 | 0.11 | 0.44 | 0.17 | 0.25 | 0.19 | 0.23 | 0.24 | 0.16 |
| IL-6 (pg/mL) | 0.22 | 0.13 | 0.31 | 0.03 | 0.30 | 0.04 | 0.25 | 0.11 | 0.18 | 0.31 |
| PLWH without CAD | Selection (T0) | T-6 months | T-12 months | T-24 months | T-36 months | |||||
| n | 48 | 55 | 52 | 52 | 46 | |||||
| Nadir CD4 T-cells/μL | −0.14 | 0.33 | −0.16 | 0.25 | −0.15 | 0.28 | −0.20 | 0.16 | −0.29 | 0.05 |
| Time on ART (years) | 0.25 | 0.08 | 0.21 | 0.12 | 0.13 | 0.37 | 0.17 | 0.23 | 0.25 | 0.10 |
| CD14 (μg/mL) | 0.28 | 0.06 | 0.31 | 0.02 | 0.04 | 0.80 | 0.06 | 0.67 | 0.09 | 0.55 |
| LBP (μg/mL) | 0.23 | 0.11 | 0.33 | 0.01 | −0.04 | 0.79 | −0.05 | 0.71 | −0.08 | 0.59 |
| CXCL10 (pg/mL) | 0.27 | 0.06 | 0.31 | 0.02 | 0.05 | 0.71 | 0.09 | 0.54 | 0.13 | 0.39 |
| IL-1RA (ng/mL) | −0.07 | 0.66 | 0.00 | 0.98 | 0.08 | 0.56 | 0.04 | 0.76 | −0.08 | 0.60 |
| D-dimer (ng/mL) | 0.08 | 0.58 | 0.02 | 0.90 | −0.11 | 0.44 | −0.09 | 0.55 | −0.17 | 0.26 |
| LP-PLA2 (µg/mL) | −0.08 | 0.57 | −0.08 | 0.55 | −0.03 | 0.84 | −0.02 | 0.88 | 0.03 | 0.84 |
| VCAM-1 (μg/mL) | 0.22 | 0.13 | 0.29 | 0.04 | 0.09 | 0.55 | 0.13 | 0.37 | 0.04 | 0.79 |
| hsCRP (pg/mL) | 0.11 | 0.45 | 0.10 | 0.45 | −0.03 | 0.83 | −0.04 | 0.77 | −0.07 | 0.63 |
| IL-6 (pg/mL) | 0.14 | 0.34 | 0.05 | 0.70 | 0.04 | 0.80 | 0.18 | 0.19 | −0.05 | 0.75 |
| PLWH with CAD | T-12 Months | T-24 Months | T-36 Months | |||
|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |
| n | 35 | 33 | 27 | |||
| Nadir CD4 T-cells/μL | −0.24 | 0.16 | −0.24 | 0.18 | −0.11 | 0.59 |
| Time on ART (years) | 0.34 | 0.05 | 0.38 | 0.03 | 0.26 | 0.19 |
| sCD14 (μg/mL) | 0.39 | 0.02 | 0.02 | 0.90 | 0.39 | 0.05 |
| LBP (μg/mL) | 0.60 | <0.001 | 0.25 | 0.17 | 0.51 | 0.007 |
| CXCL10 (pg/mL) | 0.35 | 0.04 | 0.45 | 0.01 | 0.27 | 0.18 |
| IL-6 (pg/mL) | 0.18 | 0.30 | 0.23 | 0.19 | 0.34 | 0.08 |
| PLWH without CAD | T-12 months | T-24 months | T-36 months | |||
| n | 42 | 42 | 38 | |||
| Nadir CD4 T-cells/μL | −0.13 | 0.42 | −0.22 | 0.17 | −0.17 | 0.31 |
| Time on ART (years) | 0.06 | 0.71 | 0.06 | 0.71 | 0.16 | 0.33 |
| sCD14 (μg/mL) | 0.03 | 0.87 | 0.21 | 0.19 | 0.03 | 0.86 |
| LBP (μg/mL) | −0.13 | 0.42 | −0.03 | 0.87 | −0.19 | 0.26 |
| CXCL10 (pg/mL) | 0.09 | 0.55 | 0.26 | 0.10 | 0.14 | 0.39 |
| IL-6 (pg/mL) | −0.03 | 0.87 | 0.24 | 0.13 | −0.07 | 0.66 |
| CAD | No CAD | p-Value | |
|---|---|---|---|
| 12 months before diagnosis or selection | |||
| n | 47 | 52 | |
| sCD14 (μg/mL) | 2.2 (1.3–3.6) | 2 (1.2–3.1) | 0.27 |
| LBP (μg/mL) | 8.6 (2.8–25) | 8 (2.5–21) | 0.58 |
| CXCL10 (pg/mL) | 45 (11–500) | 51 (14–364) | 0.27 |
| IL-6 (pg/mL) | 2.5 (0.9–10) | 1.8 (0.4–8.5) | 0.02 |
| 24 months before diagnosis or selection | |||
| n | 41 | 52 | |
| sCD14 (μg/mL) | 2.3 (1.5–4.0) | 1.9 (1.2–3.9) | 0.004 |
| LBP (μg/mL) | 8.7 (2.8–32) | 7.5 (2.3–16) | 0.10 |
| CXCL10 (pg/mL) | 49 (18–500) | 52 (15–330) | 0.99 |
| IL-6 (pg/mL) | 2.2 (0.9–10) | 1.7 (0.6–10) | 0.02 |
| 36 months before diagnosis or selection | |||
| n | 35 | 46 | |
| sCD14 (μg/mL) | 2.1 (1.4–3.1) | 2.1 (1.0–3.6) | 0.30 |
| LBP (μg/mL) | 8.2 (3.3–18) | 7.5 (2.3–33) | 0.29 |
| CXCL10 (pg/mL) | 49 (12–391) | 52 (14–200) | 0.69 |
| IL-6 (pg/mL) | 1.6 (0.5–10) | 1.7 (0.6–10) | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veld, L.-f.; Waters, S.; Lee, S.; Hearps, A.C.; Trevillyan, J.; Mushin, A.S.; Foo, D.; Hoy, J.; Price, P. Coronary Artery Disease in People Living with HIV May Reflect Their Sensitivity to Inflammation Associated with Cytomegalovirus. Pathogens 2025, 14, 822. https://doi.org/10.3390/pathogens14080822
Veld L-f, Waters S, Lee S, Hearps AC, Trevillyan J, Mushin AS, Foo D, Hoy J, Price P. Coronary Artery Disease in People Living with HIV May Reflect Their Sensitivity to Inflammation Associated with Cytomegalovirus. Pathogens. 2025; 14(8):822. https://doi.org/10.3390/pathogens14080822
Chicago/Turabian StyleVeld, Luna-faye, Shelley Waters, Silvia Lee, Anna C. Hearps, Janine Trevillyan, Ari S. Mushin, Damien Foo, Jennifer Hoy, and Patricia Price. 2025. "Coronary Artery Disease in People Living with HIV May Reflect Their Sensitivity to Inflammation Associated with Cytomegalovirus" Pathogens 14, no. 8: 822. https://doi.org/10.3390/pathogens14080822
APA StyleVeld, L.-f., Waters, S., Lee, S., Hearps, A. C., Trevillyan, J., Mushin, A. S., Foo, D., Hoy, J., & Price, P. (2025). Coronary Artery Disease in People Living with HIV May Reflect Their Sensitivity to Inflammation Associated with Cytomegalovirus. Pathogens, 14(8), 822. https://doi.org/10.3390/pathogens14080822








