Converging Transmission Routes of the Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b Virus in Uruguay: Phylogeographic Insights into Its Spread Across South America
Abstract
1. Introduction
2. Materials and Methods
2.1. Uruguayan Strains and New Sample Collection
2.2. AIV Detection and Sequencing HA Subtyping
2.3. Dataset
2.4. Phylogenetic Reconstruction
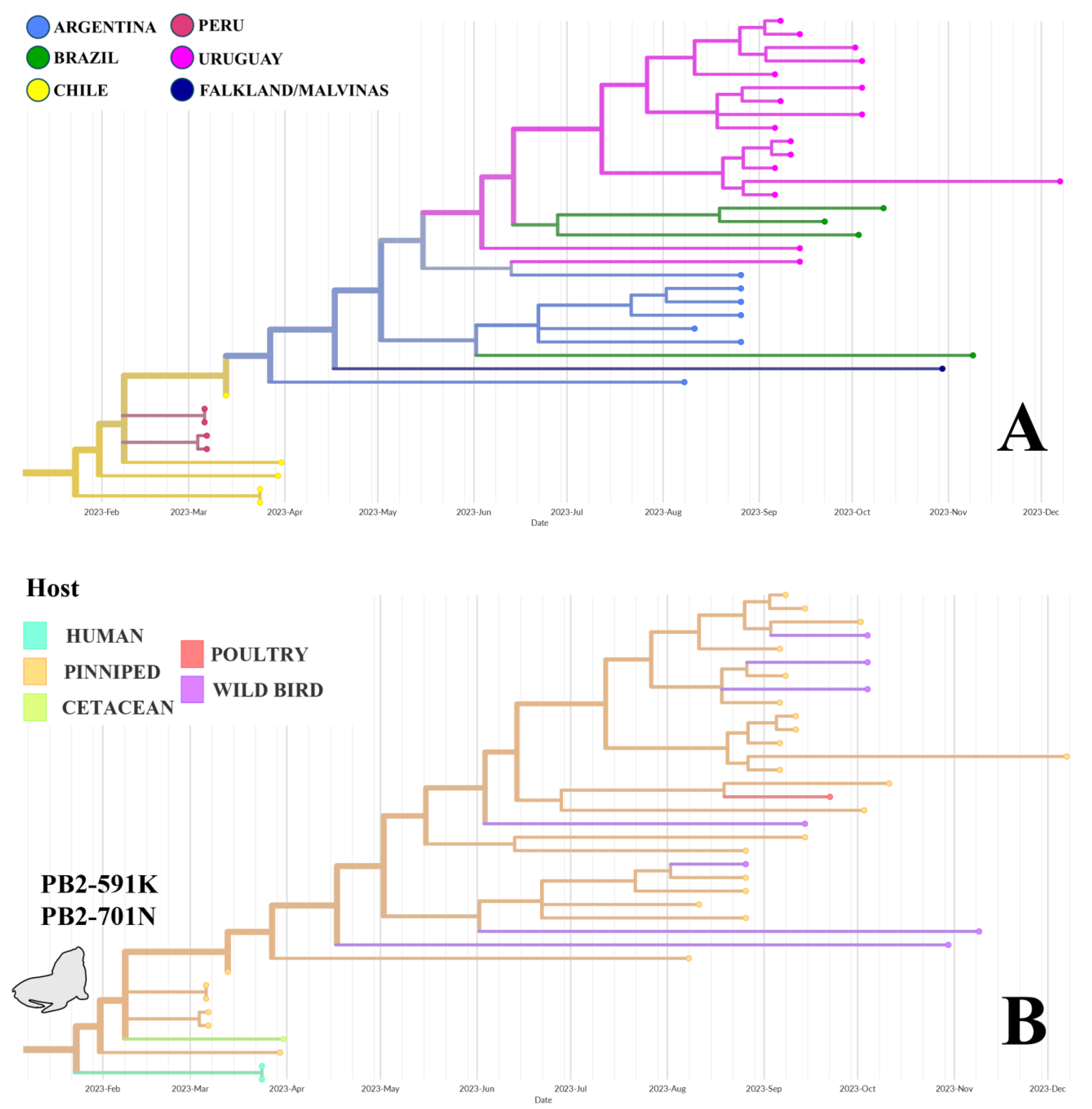
3. Results
3.1. Detection and Sequencing of HPAI H5N1 in a Uruguayan Royal Tern
3.2. Phylogeographic Analysis
3.3. Visualization of Virus Spread
4. Discussion
4.1. Avian-Derived Transmission (Phylogroup A)
4.2. Pinniped-Derived Transmission (Phylogroup B)
4.3. Mammalian Adaptation and One Health Risk
4.4. Uruguayan Dynamics: Role of Pinnipeds and Birds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic Characterization of the Pathogenic Influenza A/Goose/Guangdong/1/96 (H5N1) Virus: Similarity of Its Hemagglutinin Gene to Those of H5N1 Viruses from the 1997 Outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Donis, R.O. Nomenclature Updates Resulting from the Evolution of Avian Influenza A (H5) Virus Clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Terregino, C.; et al. Avian Influenza Overview December 2022–March 2023. EFSA J. 2023, 21, e07917. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Youk, S.; Torchetti, M.K.; Lantz, K.; Lenoch, J.B.; Killian, M.L.; Leyson, C.; Bevins, S.N.; Dilione, K.; Ip, H.S.; Stallknecht, D.E.; et al. H5N1 Highly Pathogenic Avian Influenza Clade 2.3.4.4b in Wild and Domestic Birds: Introductions into the United States and Reassortments, December 2021–April 2022. Virology 2023, 587, 109860. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, Global Spread, and Pathogenicity of Highly Pathogenic Avian Influenza H5Nx Clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global Dissemination of H5N1 Influenza Viruses Bearing the Clade 2.3.4.4b HA Gene and Biologic Analysis of the Ones Detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. [Google Scholar] [CrossRef]
- Koopmans, M.P.G.; Barton Behravesh, C.; Cunningham, A.A.; Adisasmito, W.B.; Almuhairi, S.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; et al. The Panzootic Spread of Highly Pathogenic Avian Influenza H5N1 Sublineage 2.3.4.4b: A Critical Appraisal of One Health Preparedness and Prevention. Lancet Infect. Dis. 2024, 24, e774–e781. [Google Scholar] [CrossRef]
- Lowen, A.C.; Baker, A.L.; Bowman, A.S.; García-Sastre, A.; Hensley, S.E.; Lakdawala, S.S.; Moncla, L.H.; Nelson, M.I.; Pekosz, A.; Poulson, R.L.; et al. Pandemic Risk Stemming from the Bovine H5N1 Outbreak: An Account of the Knowns and Unknowns. J. Virol. 2025, 99, e00052-25. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The Global H5N1 Influenza Panzootic in Mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and Spread of High Pathogenicity Avian Influenza Virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef]
- Bennett-Laso, B.; Berazay, B.; Muñoz, G.; Ariyama, N.; Enciso, N.; Braun, C.; Krüger, L.; Barták, M.; González-Aravena, M.; Neira, V. Confirmation of Highly Pathogenic Avian Influenza H5N1 in Skuas, Antarctica 2024. Front. Vet. Sci. 2024, 11, 1423404. [Google Scholar] [CrossRef]
- Kuiken, T.; Vanstreels, R.E.T.; Banyard, A.; Begeman, L.; Breed, A.C.; Dewar, M.; Fijn, R.; Serafini, P.P.; Uhart, M.; Wille, M. Emergence, Spread, and Impact of High-pathogenicity Avian Influenza H5 in Wild Birds and Mammals of South America and Antarctica. Conserv. Biol. 2025, 31, e70052. [Google Scholar] [CrossRef]
- Oguzie, J.U.; Marushchak, L.V.; Shittu, I.; Lednicky, J.A.; Miller, A.L.; Hao, H.; Nelson, M.I.; Gray, G.C. Avian Influenza A(H5N1) Virus among Dairy Cattle, Texas, USA. Emerg. Infect. Dis. 2024, 30, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; Aleya, S.; Almagharbeh, W.T.; Aleya, L.; Abdel-Daim, M.M. The Emergence of Highly Pathogenic Avian Influenza H5N1 in Dairy Cattle: Implications for Public Health, Animal Health, and Pandemic Preparedness. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, A.; Gates, A.; Brown, C.; Prezzato, E.; Bernstein, A. Heat-Related Emergency Department Visits—United States, May–September 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanguinetti, G.R.; González-Veliz, R.; Callupe-Leyva, A.; Apaza-Chiara, A.P.; Jara, J.; Silva, W.; Icochea, E.; More-Bayona, J.A. Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b from Peru Forms a Monophyletic Group with Chilean Isolates in South America. Sci. Rep. 2024, 14, 3635. [Google Scholar] [CrossRef]
- Marandino, A.; Tomás, G.; Panzera, Y.; Leizagoyen, C.; Pérez, R.; Bassetti, L.; Negro, R.; Rodríguez, S.; Pérez, R. Spreading of the High-Pathogenicity Avian Influenza (H5N1) Virus of Clade 2.3.4.4b into Uruguay. Viruses 2023, 15, 1906. [Google Scholar] [CrossRef]
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Gonzalez-Reiche, A.S.; Muñoz, G.; Ulloa, M.; Ávila, C.; Navarro, C.; et al. Cross-Species and Mammal-to-Mammal Transmission of Clade 2.3.4.4b Highly Pathogenic Avian Influenza A/H5N1 with PB2 Adaptations. Nat. Commun. 2025, 16, 2232. [Google Scholar] [CrossRef]
- Gamarra-Toledo, V.; Plaza, P.I.; Angulo, F.; Gutiérrez, R.; García-Tello, O.; Saravia-Guevara, P.; Mejía-Vargas, F.; Epiquién-Rivera, M.; Quiroz-Jiménez, G.; Martinez, P.; et al. Highly Pathogenic Avian Influenza (HPAI) Strongly Impacts Wild Birds in Peru. Biol. Conserv. 2023, 286, 110272. [Google Scholar] [CrossRef]
- Rodríguez, S.; Marandino, A.; Tomás, G.; Panzera, Y.; Wallau, G.L.; Zimmer Dezordi, F.; Carrazco-Montalvo, A.; Cassarino, M.; Russi, V.; Pérez, R.; et al. Infection of South American Coatis (Nasua Nasua) with Highly Pathogenic Avian Influenza H5N1 Virus Displaying Mammalian Adaptive Mutations. Microb. Pathog. 2024, 195, 106895. [Google Scholar] [CrossRef]
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.L.; Dezordi, F.Z.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Pinnipeds and Seabirds in Uruguay: Implications for Bird-Mammal Transmission in South America. Virus Evol. 2024, 10, veae031. [Google Scholar] [CrossRef]
- Ulloa, M.; Fernández, A.; Ariyama, N.; Colom-Rivero, A.; Rivera, C.; Nuñez, P.; Sanhueza, P.; Johow, M.; Araya, H.; Torres, J.C.; et al. Mass Mortality Event in South American Sea Lions (Otaria Flavescens) Correlated to Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreak in Chile. Vet. Q. 2023, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.I.; Gamarra-Toledo, V.; Euguí, J.R.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg. Infect. Dis. 2024, 30, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise Alignment for Nucleotide Sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; McCauley, J. GISAID: Global Initiative on Sharing All Influenza Data–from Vision to Reality. Eurosurveillance 2017, 22, 30494. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-Time Tracking of Pathogen Evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef]
- Huddleston, J.; Hadfield, J.; Sibley, T.; Lee, J.; Fay, K.; Ilcisin, M.; Harkins, E.; Bedford, T.; Neher, R.; Hodcroft, E. Augur: A Bioinformatics Toolkit for Phylogenetic Analyses of Human Pathogens. JOSS 2021, 6, 2906. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Sagulenko, P.; Puller, V.; Neher, R.A. TreeTime: Maximum-Likelihood Phylodynamic Analysis. Virus Evol. 2018, 4, vex042. [Google Scholar] [CrossRef] [PubMed]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly Pathogenic Avian Influenza A (H5N1) in Marine Mammals and Seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- Uhart, M.M.; Vanstreels, R.E.T.; Nelson, M.I.; Olivera, V.; Campagna, J.; Zavattieri, V.; Lemey, P.; Campagna, C.; Falabella, V.; Rimondi, A. Epidemiological Data of an Influenza A/H5N1 Outbreak in Elephant Seals in Argentina Indicates Mammal-to-Mammal Transmission. Nat. Commun. 2024, 15, 9516. [Google Scholar] [CrossRef]
- Ariyama, N.; Pardo-Roa, C.; Muñoz, G.; Aguayo, C.; Ávila, C.; Mathieu, C.; Almonacid, L.I.; Medina, R.A.; Brito, B.; Johow, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Wild Birds, Chile. Emerg. Infect. Dis. 2023, 29, 1842–1845. [Google Scholar] [CrossRef]
- Ruiz-Saenz, J.; Martinez-Gutierrez, M.; Pujol, F.H. Multiple Introductions of Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b into South America. Travel Med. Infect. Dis. 2023, 53, 102591. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Nelson, M.I.; Artuso, M.C.; Marchione, V.D.; Piccini, L.E.; Benedetti, E.; Crespo-Bellido, A.; Pierdomenico, A.; Wolff, T.; Uhart, M.; et al. Novel Highly Pathogenic Avian Influenza (A)H5N1 Triple Reassortant in Argentina. bioRXiv 2025, bioRXiv:2025.05.23.655175. [Google Scholar]
- Piaggio, A.J.; Shriner, S.A.; VanDalen, K.K.; Franklin, A.B.; Anderson, T.D.; Kolokotronis, S.-O. Molecular Surveillance of Low Pathogenic Avian Influenza Viruses in Wild Birds across the United States: Inferences from the Hemagglutinin Gene. PLoS ONE 2012, 7, e50834. [Google Scholar] [CrossRef] [PubMed]
- Artuso, M.C.; Marchione, V.D.; Benedetti, E.; Bonastre, P.; Alvarez, A.M.; Piccini, L.; Ponde, A.; Barrios Benito, E.; Fabeiro, M.; Waisman, K.; et al. Detection and Characterization of Highly Pathogenic Avian Influenza A (H5N1) Clade 2.3.4.4b Virus Circulating in Argentina in 2023. Rev. Argent. Microbiol. 2024, in press. [Google Scholar] [CrossRef]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in Farmed Minks, Spain, October 2022. Eurosurveillance 2023, 28, 2300109. [Google Scholar] [CrossRef]
- Halwe, N.J.; Cool, K.; Breithaupt, A.; Schön, J.; Trujillo, J.D.; Nooruzzaman, M.; Kwon, T.; Ahrens, A.K.; Britzke, T.; McDowell, C.D.; et al. H5N1 Clade 2.3.4.4b Dynamics in Experimentally Infected Calves and Cows. Nature 2025, 637, 903–912. [Google Scholar] [CrossRef]
- Hu, X.; Saxena, A.; Magstadt, D.R.; Gauger, P.C.; Burrough, E.R.; Zhang, J.; Siepker, C.; Mainenti, M.; Gorden, P.J.; Plummer, P.J.; et al. Genomic Characterization of Highly Pathogenic Avian Influenza A H5N1 Virus Newly Emerged in Dairy Cattle. Emerg. Microbes Infect. 2024, 13, 2380421. [Google Scholar] [CrossRef]
- Li, W.; Lee, H.H.Y.; Li, R.F.; Zhu, H.M.; Yi, G.; Peiris, J.S.M.; Yang, Z.F.; Mok, C.K.P. The PB2 Mutation with Lysine at 627 Enhances the Pathogenicity of Avian Influenza (H7N9) Virus Which Belongs to a Non-Zoonotic Lineage. Sci. Rep. 2017, 7, 2352. [Google Scholar] [CrossRef]
- De Araújo, A.C.; Silva, L.M.N.; Cho, A.Y.; Repenning, M.; Amgarten, D.; De Moraes, A.P.; Malta, F.; Miller, M.; Dorlass, E.G.; Palameta, S.; et al. Incursion of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus, Brazil, 2023. Emerg. Infect. Dis. 2024, 30, 619. [Google Scholar] [CrossRef]
- Rivetti, A.V.; Reischak, D.; De Oliveira, C.H.S.; Otaka, J.N.P.; Domingues, C.S.; Freitas, T.D.L.; Cardoso, F.G.; Montesino, L.O.; Da Silva, A.L.S.; Camillo, S.C.A.; et al. Phylodynamics of Avian Influenza A(H5N1) Viruses from Outbreaks in Brazil. Virus Res. 2024, 347, 199415. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Kalthoff, D.; Veits, J.; Pavlova, S.; Schwemmle, M.; Mänz, B.; Mettenleiter, T.C.; Stech, J. Reversion of PB2-627E to -627K during Replication of an H5N1 Clade 2.2 Virus in Mammalian Hosts Depends on the Origin of the Nucleoprotein. J. Virol. 2011, 85, 10691–10698. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Dauber, B.; Wolff, T.; Planz, O.; Klenk, H.-D.; Stech, J. The Viral Polymerase Mediates Adaptation of an Avian Influenza Virus to a Mammalian Host. Proc. Natl. Acad. Sci. USA 2005, 102, 18590–18595. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of Amino Acids in HA and PB2 Critical for the Transmission of H5N1 Avian Influenza Viruses in a Mammalian Host. PLoS Pathog. 2009, 5, e1000709. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Yen, H.L.; Yu, M.Y.M.; Yuen, K.M.; Sia, S.F.; Chan, M.C.W.; Qin, G.; Tu, W.W.; Peiris, J.S.M. Amino Acid Residues 253 and 591 of the PB2 Protein of Avian Influenza Virus A H9N2 Contribute to Mammalian Pathogenesis. J. Virol. 2011, 85, 9641–9645. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Yan, Z.; Gan, T.; Li, L.; Chen, R.; Chen, R.; Zheng, Z.; Hong, W.; Wang, J.; et al. Dual E627K and D701N Mutations in the PB2 Protein of A(H7N9) Influenza Virus Increased Its Virulence in Mammalian Models. Sci. Rep. 2015, 5, 14170. [Google Scholar] [CrossRef] [PubMed]
- Palù, G.; Roggero, P.F.; Calistri, A. Could H5N1 Bird Flu Virus Be the Cause of the next Human Pandemic? Front. Microbiol. 2024, 15, 1477738. [Google Scholar] [CrossRef]
- PAHO/WHO. Epidemiological Update Avian Influenza A (H5N1) in the Americas Region; PAHO/WHO: Washington, DC, USA, 2025. [Google Scholar]
- Royal Tern (Thalasseus maximus). In Birds of the World; Billerman, S.M., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2021. [Google Scholar]
- South American Tern (Sterna hirundinacea). In Birds of the World; Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D., De Juana, E., Medrano, F., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2024. [Google Scholar]



| Strain | Date | Host Species | Phylogroup | PB2-591 | PB2-701 | PB2-627 |
|---|---|---|---|---|---|---|
| 014-M3 | Feb 2023 | Cygnus melancoryphus | A | Q | D | E |
| 040-M5 | Mar 2023 | Gallus gallus | A | Q | D | E |
| 040-M7 | Mar 2023 | Gallus gallus | A | Q | D | E |
| 047-M1 | Mar 2023 | Gallus gallus | A | Q | D | E |
| 047-M3 | Mar 2023 | Gallus gallus | A | Q | D | E |
| 078-M2 | Mar 2023 | Cygnus melancoryphus | A | Q | D | E |
| 124-M1 | Apr 2023 | Gallus gallus | A | Q | D | E |
| 124-M3 | Apr 2023 | Meleagrus gallopavo | A | Q | D | E |
| 124-M6 | Apr 2023 | Meleagrus gallopavo | A | Q | D | E |
| 127-M4 | Apr 2023 | Gallus gallus | A | Q | D | E |
| 127-M1 | Apr 2023 | Gallus gallus | A | Q | D | E |
| 144-M3 | May 2023 | Gallus gallus | A | Q | D | E |
| 146-M1 | May 2023 | Nasua nasua | A | Q | D | E |
| 145-M2 | May 2023 | Nasua nasua | A | Q | D | K |
| 145-M1 | May 2023 | Nasua nasua | A | Q | D | K |
| M27-2023 | Oct 2023 | Thalasseus maximus | A | Q | D | E |
| P6_6923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P7_6923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P5_6923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P4_6923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P10_8923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P8_8923 | Sep 2023 | Arctocephalus australis | B | K | N | E |
| P13_11923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P14_11923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P16_14923 | Sep 2023 | Sterna hirundinacea | B | K | N | E |
| P17_14923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P18_14923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P15_14923 | Sep 2023 | Otaria flavescens | B | K | N | E |
| P26_21023 | Oct 2023 | Arctocephalus australis | B | K | N | E |
| P23_41023 | Oct 2023 | Sterna hirundinacea | B | K | N | E |
| P24_41023 | Oct 2023 | Sterna hirundinacea | B | K | N | E |
| P25_41023 | Oct 2023 | Sterna hirundinacea | B | K | N | E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marandino, A.; Tomás, G.; Panzera, Y.; Williman, J.; Dezordi, F.Z.; Wallau, G.L.; Rodríguez, S.; Pérez, R.; Bassetti, L.; Negro, R.; et al. Converging Transmission Routes of the Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b Virus in Uruguay: Phylogeographic Insights into Its Spread Across South America. Pathogens 2025, 14, 793. https://doi.org/10.3390/pathogens14080793
Marandino A, Tomás G, Panzera Y, Williman J, Dezordi FZ, Wallau GL, Rodríguez S, Pérez R, Bassetti L, Negro R, et al. Converging Transmission Routes of the Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b Virus in Uruguay: Phylogeographic Insights into Its Spread Across South America. Pathogens. 2025; 14(8):793. https://doi.org/10.3390/pathogens14080793
Chicago/Turabian StyleMarandino, Ana, Gonzalo Tomás, Yanina Panzera, Joaquín Williman, Filipe Zimmer Dezordi, Gabriel Luz Wallau, Sirley Rodríguez, Ramiro Pérez, Lucía Bassetti, Raúl Negro, and et al. 2025. "Converging Transmission Routes of the Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b Virus in Uruguay: Phylogeographic Insights into Its Spread Across South America" Pathogens 14, no. 8: 793. https://doi.org/10.3390/pathogens14080793
APA StyleMarandino, A., Tomás, G., Panzera, Y., Williman, J., Dezordi, F. Z., Wallau, G. L., Rodríguez, S., Pérez, R., Bassetti, L., Negro, R., Uriarte, V., Leizagoyen, C., & Pérez, R. (2025). Converging Transmission Routes of the Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b Virus in Uruguay: Phylogeographic Insights into Its Spread Across South America. Pathogens, 14(8), 793. https://doi.org/10.3390/pathogens14080793





