Comparison of the Serodiagnostic Accuracy Tests for Lyme Disease in Adults and Children: A Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategies
2.3. Screening and Data Extraction
2.4. Evaluation of Literature Quality
2.5. Statistical Analyses
2.5.1. Traditional Meta-Analysis
2.5.2. NMA
3. Results
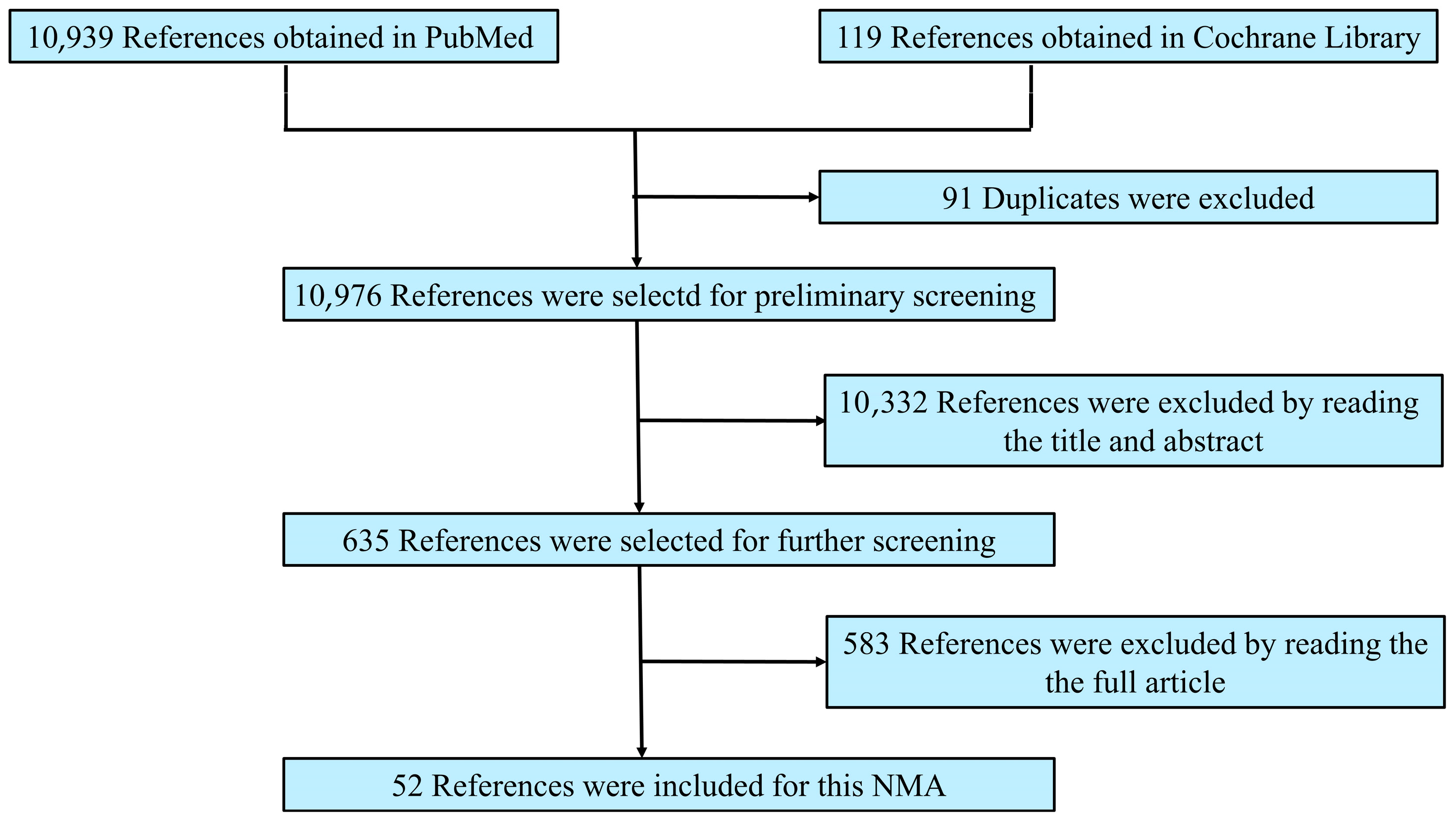
3.1. Process and Results of Literature Screening
3.2. Characteristics and Risk of Bias of Included Literature
3.3. Outcomes
3.3.1. Traditional Meta-Analysis Results
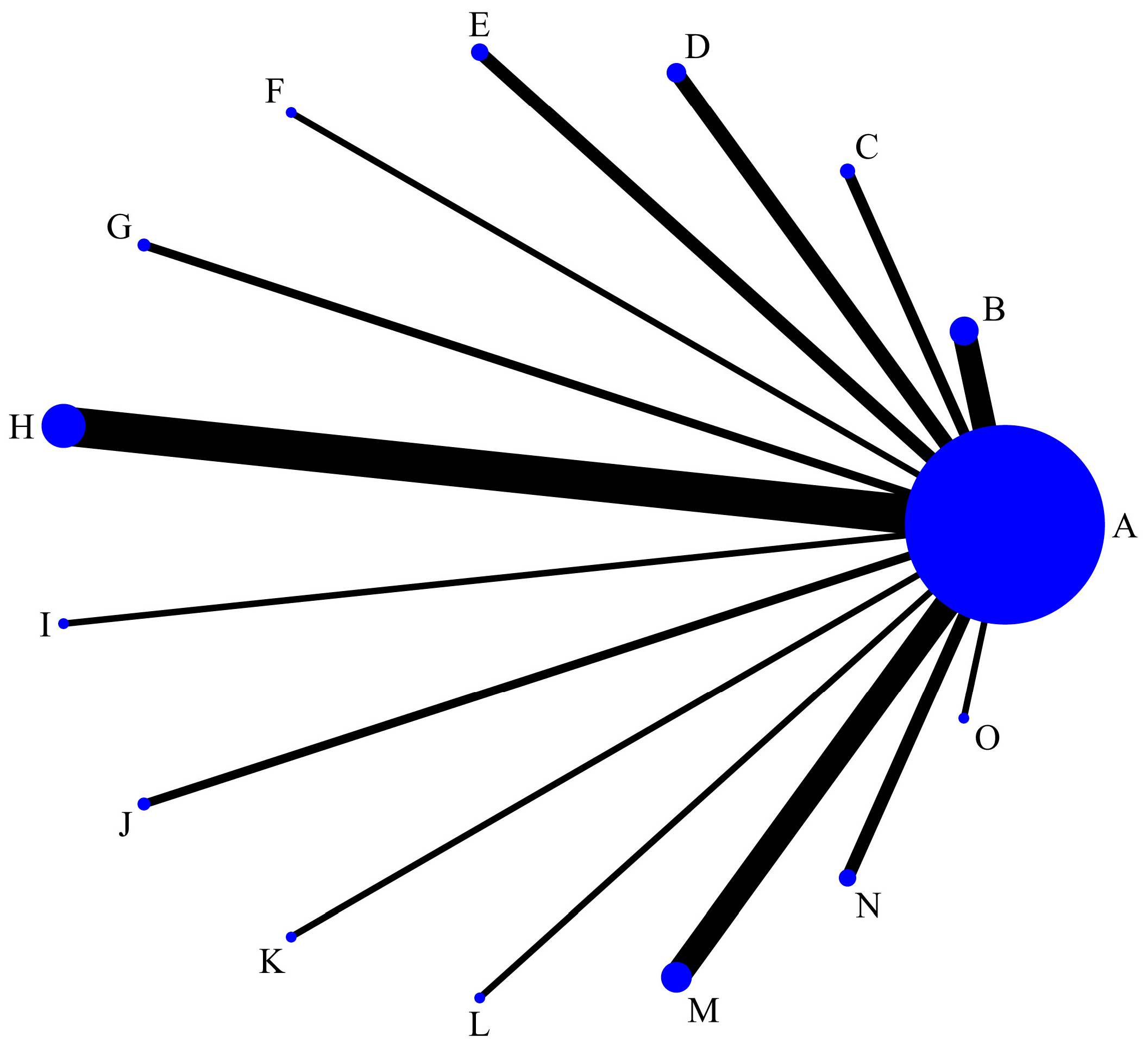
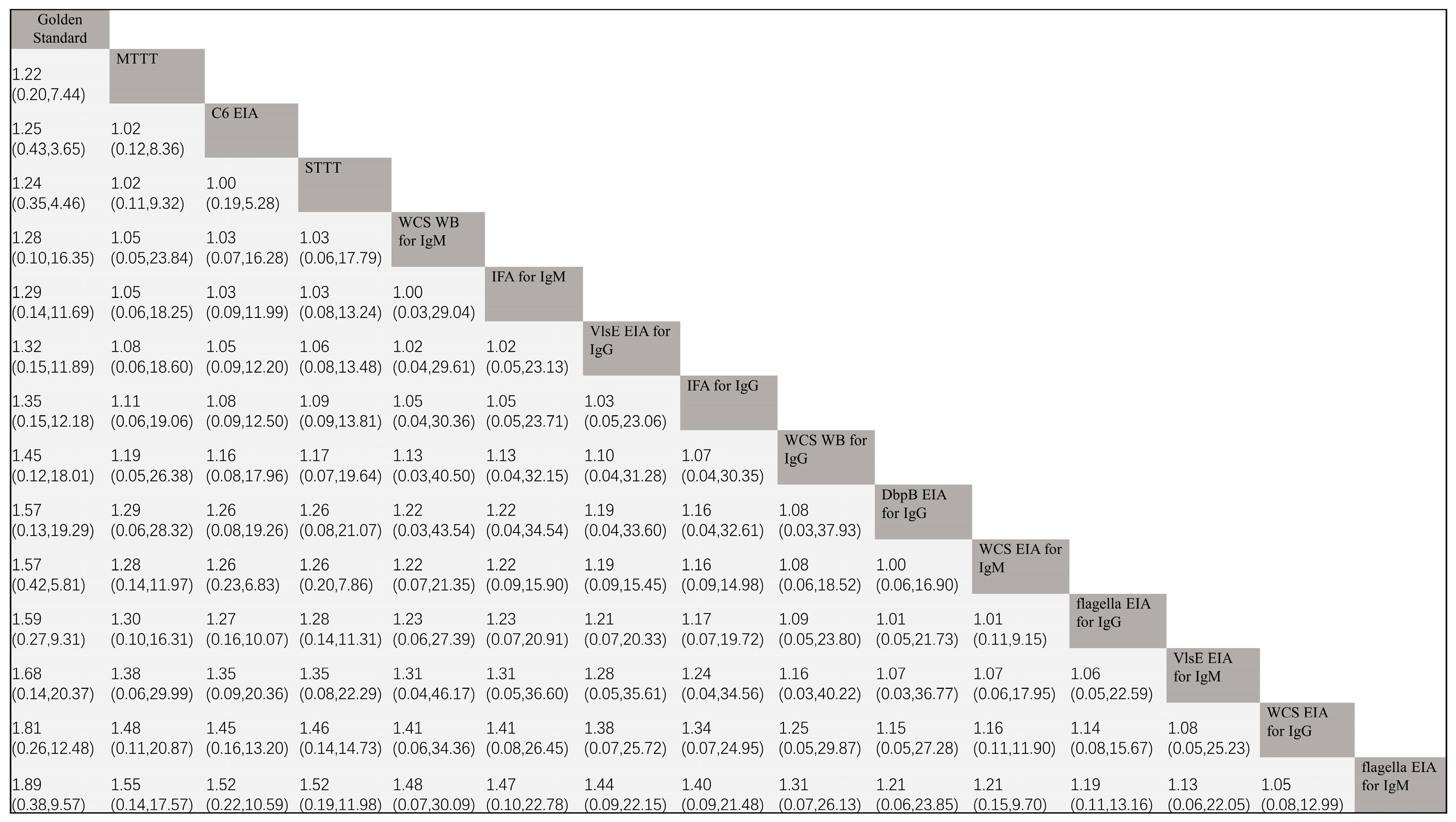
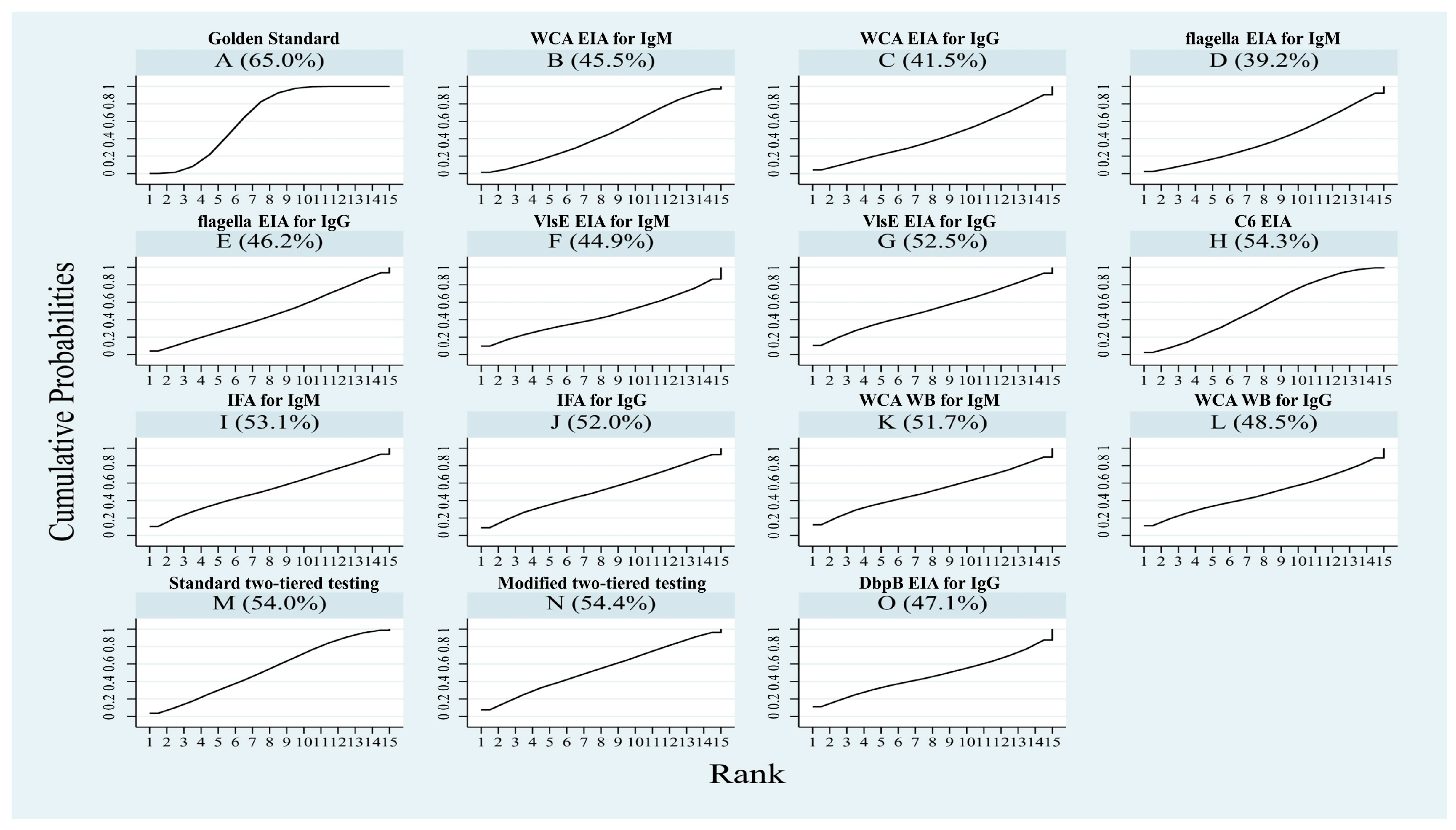
3.3.2. NMA
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steere, A.C. Lyme disease. N. Engl. J. Med. 2001, 345, 115–125. [Google Scholar] [CrossRef]
- Talbot, N.C.; Spillers, N.J.; Luther, P.; Flanagan, C.; Soileau, L.G.; Ahmadzadeh, S.; Viswanath, O.; Varrassi, G.; Shekoohi, S.; Cornett, E.M.; et al. Lyme Disease and Post-treatment Lyme Disease Syndrome: Current and Developing Treatment Options. Cureus 2023, 15, e43112. [Google Scholar] [CrossRef] [PubMed]
- Besant, G.; Wan, D.; Yeung, C.; Blakely, C.; Branscombe, P.; Suarez-Fuster, L.; Redfearn, D.; Simpson, C.; Abdollah, H.; Glover, B.; et al. Suspicious index in Lyme carditis: Systematic review and proposed new risk score. Clin. Cardiol. 2018, 41, 1611–1616. [Google Scholar] [CrossRef]
- Chou, E.; Lin, Y.P.; Cady, N.C. Recent strategies for the diagnosis of early Lyme disease. Sci. Prog. 2018, 101, 311–331. [Google Scholar] [CrossRef]
- Lohr, B.; Fingerle, V.; Norris, D.E.; Hunfeld, K.P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef]
- Grąźlewska, W.; Holec-Gąsior, L.; Sołowińska, K.; Chmielewski, T.; Fiecek, B.; Contreras, M. Epitope Mapping of BmpA and BBK32 Borrelia burgdorferi Sensu Stricto Antigens for the Design of Chimeric Proteins with Potential Diagnostic Value. ACS Infect. Dis. 2023, 9, 2160–2172. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yang, B.; Mallett, S.; Takwoingi, Y.; Davenport, C.F.; Hyde, C.J.; Whiting, P.F.; Deeks, J.J.; Leeflang, M.M.G. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann. Intern. Med. 2021, 174, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Plana, M.N.; Arevalo-Rodriguez, I.; Fernández-García, S.; Soto, J.; Fabregate, M.; Pérez, T.; Roqué, M.; Zamora, J. Meta-DiSc 2.0: A web application for meta-analysis of diagnostic test accuracy data. BMC. Med. Res. Methodol. 2022, 22, 306. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Diagnostic tests 3: Receiver operating characteristic plots. BMJ 1994, 309, 188. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Wu, J.; Hu, L.; Zhang, G.; Wu, F.; He, T. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133057. [Google Scholar] [CrossRef]
- Kaiser, R.; Rauer, S. Serodiagnosis of neuroborreliosis: Comparison of reliability of three confirmatory assays. Infection 1999, 27, 177–182. [Google Scholar] [CrossRef]
- Wilske, B.; Fingerle, V.; Herzer, P.; Hofmann, A.; Lehnert, G.; Peters, H.; Pfister, H.W.; Preac-Mursic, V.; Soutschek, E.; Weber, K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med. Microbiol. Immunol. 1993, 182, 255–270. [Google Scholar] [CrossRef]
- Qiu, B.; Brunner, M.; Zhang, G.; Sigal, L.; Stein, S. Selection of continuous epitope sequences and their incorporation into poly (ethylene glycol)-peptide conjugates for use in serodiagnostic immunoassays: Application to Lyme disease. Biopolymers 2000, 55, 319–333. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Anderson, J.F. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am. J. Epidemiol. 1988, 127, 818–825. [Google Scholar] [CrossRef]
- Rauer, S.; Spohn, N.; Rasiah, C.; Neubert, U.; Vogt, A. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1998, 36, 857–861. [Google Scholar] [CrossRef]
- Kaiser, R.; Rauer, S. Advantage of recombinant borrelial proteins for serodiagnosis of neuroborreliosis. J. Med. Microbiol. 1999, 48, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Fikrig, E.; Huguenel, E.D.; Berland, R.; Rahn, D.W.; Hardin, J.A.; Flavell, R.A. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J. Infect. Dis. 1992, 165, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.A.; Shapiro, E.D.; Bell, G.L.; Sampieri, A.; Padula, S.J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J. Infect. Dis. 1995, 171, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Lencáková, D.; Fingerle, V.; Stefancíková, A.; Schulte-Spechtel, U.; Petko, B.; Schréter, I.; Wilske, B. Evaluation of recombinant line immunoblot for detection of Lyme disease in Slovakia: Comparison with two other immunoassays. Vector-Borne Zoonotic Dis. 2008, 8, 381–390. [Google Scholar] [CrossRef]
- Berardi, V.P.; Weeks, K.E.; Steere, A.C. Serodiagnosis of early Lyme disease: Analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J. Infect. Dis. 1988, 158, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Padula, S.J.; Dias, F.; Sampieri, A.; Craven, R.B.; Ryan, R.W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1994, 32, 1733–1738. [Google Scholar] [CrossRef]
- Mathiesen, M.J.; Christiansen, M.; Hansen, K.; Holm, A.; Asbrink, E.; Theisen, M. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 1998, 36, 3474–3479. [Google Scholar] [CrossRef] [PubMed]
- Smismans, A.; Goossens, V.J.; Nulens, E.; Bruggeman, C.A. Comparison of five different immunoassays for the detection of Borrelia burgdorferi IgM and IgG antibodies. Clin. Microbiol. Infect. 2006, 12, 648–655. [Google Scholar] [CrossRef][Green Version]
- Panelius, J.; Lahdenne, P.; Saxen, H.; Heikkilä, T.; Seppälä, I. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 2001, 39, 4013–4019. [Google Scholar] [CrossRef]
- Karlsson, M. Western immunoblot and flagellum enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 1990, 28, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Magnarelli, L.A.; Ijdo, J.W.; Padula, S.J.; Flavell, R.A.; Fikrig, E. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 2000, 38, 1735–1739. [Google Scholar] [CrossRef]
- Hansen, K.; Pii, K.; Lebech, A.M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J. Clin. Microbiol. 1991, 29, 166–173. [Google Scholar] [CrossRef]
- Barstad, B.; Tveitnes, D.; Noraas, S.; Selvik Ask, I.; Saeed, M.; Bosse, F.; Vigemyr, G.; Huber, I.; Øymar, K. Cerebrospinal Fluid B-lymphocyte Chemoattractant CXCL13 in the Diagnosis of Acute Lyme Neuroborreliosis in Children. Pediatr. Infect. Dis. J. 2017, 36, e286–e292. [Google Scholar] [CrossRef]
- Marangoni, A.; Moroni, A.; Accardo, S.; Cevenini, R. Borrelia burgdorferi VlsE antigen for the serological diagnosis of Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 349–354. [Google Scholar] [CrossRef]
- Bacon, R.M.; Biggerstaff, B.J.; Schriefer, M.E.; Gilmore, R.D., Jr.; Philipp, M.T.; Steere, A.C.; Wormser, G.P.; Marques, A.R.; Johnson, B.J. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 2003, 187, 1187–1199. [Google Scholar] [CrossRef]
- Peltomaa, M.; McHugh, G.; Steere, A.C. The VlsE (IR6) peptide ELISA in the serodiagnosis of Lyme facial paralysis. Otol. Neurotol. 2004, 25, 838–841. [Google Scholar] [CrossRef]
- Liang, F.T.; Steere, A.C.; Marques, A.R.; Johnson, B.J.; Miller, J.N.; Philipp, M.T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 1999, 37, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Pomelova, V.G.; Korenberg, E.I.; Kuznetsova, T.I.; Bychenkova, T.A.; Bekman, N.I.; Osin, N.S. C6 Peptide-Based Multiplex Phosphorescence Analysis (PHOSPHAN) for Serologic Confirmation of Lyme Borreliosis. PLoS ONE 2015, 10, e0130048. [Google Scholar] [CrossRef]
- Van Gorkom, T.; van Arkel, G.H.J.; Heron, M.; Voet, W.; Thijsen, S.F.T.; Kremer, K. The Usefulness of Two CXCL13 Assays on Cerebrospinal Fluid for the Diagnosis of Lyme Neuroborreliosis: A Retrospective Study in a Routine Clinical Setting. J. Clin. Microbiol. 2021, 59, e0025521. [Google Scholar] [CrossRef]
- Pegalajar-Jurado, A.; Fitzgerald, B.L.; Islam, M.N.; Belisle, J.T.; Wormser, G.P.; Waller, K.S.; Ashton, L.V.; Webb, K.J.; Delorey, M.J.; Clark, R.J. Identification of Urine Metabolites as Biomarkers of Early Lyme Disease. Sci. Rep. 2018, 8, 12204. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.B.; Breneman, J.W., 4th; Liu, J.; Rabkina, S.; Zheng, W.; Zhou, S.; Walker, R.P.; Kaul, R. A Fully Automated Multiplex Assay for Diagnosis of Lyme Disease with High Specificity and Improved Early Sensitivity. J. Clin. Microbiol. 2020, 58, e01785-19. [Google Scholar] [CrossRef]
- Marangoni, A.; Sparacino, M.; Cavrini, F.; Storni, E.; Mondardini, V.; Sambri, V.; Cevenini, R. Comparative evaluation of three different ELISA methods for the diagnosis of early culture-confirmed Lyme disease in Italy. J. Med. Microbiol. 2005, 54, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, L.E.; Bennett, J.E.; Balamuth, F.; Levas, M.N.; Neville, D.; Lyons, T.W.; Branda, J.A.; Maulden, A.B.; Lewander, D.; Garro, A. Diagnostic Performance of C6 Enzyme Immunoassay for Lyme Arthritis. Pediatrics 2020, 145, e20190593. [Google Scholar] [CrossRef]
- Rouhiainen, M.; Pietikäinen, A.; Kortela, E.; Kanerva, M.J.; Oksi, J.; Hytönen, J. C6 peptide enzyme immunoassay in Lyme borreliosis serology. J. Microbiol. Methods 2021, 180, 106122. [Google Scholar] [CrossRef]
- Riesbeck, K.; Hammas, B. Comparison of an automated Borrelia indirect chemiluminescent immunoassay (CLIA) with a VlsE/C6 ELISA and Immunoblot. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 517–519. [Google Scholar] [CrossRef][Green Version]
- Ledue, T.B.; Collins, M.F.; Young, J.; Schriefer, M.E. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin. Vaccine Immunol. 2008, 15, 1796–1804. [Google Scholar] [CrossRef]
- Hoeve-Bakker, B.J.A.; Jonker, M.; Brandenburg, A.H.; den Reijer, P.M.; Stelma, F.F.; van Dam, A.P.; van Gorkom, T.; Kerkhof, K.; Thijsen, S.F.T.; Kremer, K. The Performance of Nine Commercial Serological Screening Assays for the Diagnosis of Lyme Borreliosis: A Multicenter Modified Two-Gate Design Study. Microbiol. Spectr. 2022, 10, e0051022. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, I.; Kruger, G.; Eliasson, I. C6 peptide ELISA test in the serodiagnosis of Lyme borreliosis in Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Skarpaas, T.; Ljøstad, U.; Søbye, M.; Mygland, A. Sensitivity and specificity of a commercial C6 peptide enzyme immuno assay in diagnosis of acute Lyme neuroborreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 675–677. [Google Scholar] [CrossRef]
- Branda, J.A.; Strle, K.; Nigrovic, L.E.; Lantos, P.M.; Lepore, T.J.; Damle, N.S.; Ferraro, M.J.; Steere, A.C. Evaluation of Modified 2-Tiered Serodiagnostic Testing Algorithms for Early Lyme Disease. Clin. Infect. Dis. 2017, 64, 1074–1080. [Google Scholar] [CrossRef]
- Wormser, G.P.; Schriefer, M.; Aguero-Rosenfeld, M.E.; Levin, A.; Steere, A.C.; Nadelman, R.B.; Nowakowski, J.; Marques, A.; Johnson, B.J.; Dumler, J.S. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D.; Reed, K.D.; Aspeslet, T.L.; Vandermause, M.F.; Melski, J.W. Comparison of four immunoserologic assays for detection of antibodies to Borrelia burgdorferi in patients with culture-positive erythema migrans. J. Clin. Microbiol. 1994, 32, 1958–1962. [Google Scholar] [CrossRef]
- Craft, J.E.; Grodzicki, R.L.; Steere, A.C. Antibody response in Lyme disease: Evaluation of diagnostic tests. J. Infect. Dis. 1984, 149, 789–795. [Google Scholar] [CrossRef]
- Artsob, H.; Huibner, S. Complement fixation test for the diagnosis of Lyme disease. J. Clin. Microbiol. 1990, 28, 637–638. [Google Scholar] [CrossRef]
- Jain, V.K.; Hilton, E.; Maytal, J.; Dorante, G.; Ilowite, N.T.; Sood, S.K. Immunoglobulin M immunoblot for diagnosis of Borrelia burgdorferi infection in patients with acute facial palsy. J. Clin. Microbiol. 1996, 34, 2033–2035. [Google Scholar] [CrossRef]
- Ledue, T.B.; Collins, M.F.; Craig, W.Y. New laboratory guidelines for serologic diagnosis of Lyme disease: Evaluation of the two-test protocol. J. Clin. Microbiol. 1996, 34, 2343–2350. [Google Scholar] [CrossRef]
- Snyder, J.L.; Giese, H.; Bandoski-Gralinski, C.; Townsend, J.; Jacobson, B.E.; Shivers, R.; Schotthoefer, A.M.; Fritsche, T.R.; Green, C.; Callister, S.M. T2 Magnetic Resonance Assay-Based Direct Detection of Three Lyme Disease-Related Borrelia Species in Whole-Blood Samples. J. Clin. Microbiol. 2017, 55, 2453–2461. [Google Scholar] [CrossRef]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for Lyme disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Baarsma, M.E.; Schellekens, J.; Meijer, B.C.; Brandenburg, A.H.; Souilljee, T.; Hofhuis, A.; Hovius, J.W.; van Dam, A.P. Diagnostic parameters of modified two-tier testing in European patients with early Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2143–2152. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Crowder, C.C.; Rebman, A.W.; Rounds, M.A.; Matthews, H.E.; Picuri, J.M.; Soloski, M.J.; Ecker, D.J.; Schutzer, S.E.; Aucott, J.N. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS ONE 2012, 7, e36825. [Google Scholar] [CrossRef]
- Molins, C.R.; Delorey, M.J.; Sexton, C.; Schriefer, M.E. Lyme Borreliosis Serology: Performance of Several Commonly Used Laboratory Diagnostic Tests and a Large Resource Panel of Well-Characterized Patient Samples. J. Clin. Microbiol. 2016, 54, 2726–2734. [Google Scholar] [CrossRef]
- Lipsett, S.C.; Branda, J.A.; McAdam, A.J.; Vernacchio, L.; Gordon, C.D.; Gordon, C.R.; Nigrovic, L.E. Evaluation of the C6 Lyme Enzyme Immunoassay for the Diagnosis of Lyme Disease in Children and Adolescents. Clin. Infect. Dis. 2016, 63, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Branda, J.A.; Strle, F.; Strle, K.; Sikand, N.; Ferraro, M.J.; Steere, A.C. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin. Infect. Dis. 2013, 57, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Pegalajar-Jurado, A.; Schriefer, M.E.; Welch, R.J.; Couturier, M.R.; MacKenzie, T.; Clark, R.J.; Ashton, L.V.; Delorey, M.J.; Molins, C.R. Evaluation of Modified Two-Tiered Testing Algorithms for Lyme Disease Laboratory Diagnosis Using Well-Characterized Serum Samples. J. Clin. Microbiol. 2018, 56, e01943-17. [Google Scholar] [CrossRef]
- Arnaboldi, P.M.; Sambir, M.; Dattwyler, R.J. Decorin binding proteins A and B in the serodiagnosis of Lyme disease in North America. Clin. Vaccine Immunol. 2014, 21, 1426–1436. [Google Scholar] [CrossRef]
- Heikkilä, T.; Seppälä, I.; Saxen, H.; Panelius, J.; Peltomaa, M.; Huppertz, H.I.; Lahdenne, P. Cloning of the gene encoding the decorin-binding protein B (DbpB) in Borrelia burgdorferi sensu lato and characterisation of the antibody responses to DbpB in Lyme borreliosis. J. Med. Microbiol. 2002, 51, 641–648. [Google Scholar] [CrossRef][Green Version]
- Sillanpää, H.; Skogman, B.H.; Sarvas, H.; Seppälä, I.J.; Lahdenne, P. Antibodies to decorin-binding protein B (DbpB) in the diagnosis of Lyme neuroborreliosis in children. Int. J. Infect. Dis. 2014, 28, 160–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alby, K.; Capraro, G.A. Alternatives to Serologic Testing for Diagnosis of Lyme Disease. Clin. Lab. Med. 2015, 35, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 2020, 7, 265. [Google Scholar] [CrossRef]
- Mahajan, V.K. Lyme Disease: An Overview. Indian Dermatol. Online J. 2023, 14, 594–604. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Shapiro, E.D. Clinical practice. Lyme disease. N. Engl. J. Med. 2014, 370, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef]
- Margos, G.; Fedorova, N.; Becker, N.S.; Kleinjan, J.E.; Marosevic, D.; Krebs, S.; Hui, L.; Fingerle, V.; Lane, R.S. Borrelia maritima sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex, occupying a basal position to North American species. Int. J. Syst. Evol. Microbiol. 2020, 70, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, W.; Jia, Z.; Li, J.; Wang, Q.; Deng, H. Meta-analysis of diagnostic value of 18F-FDG PET or PET/CT for detecting lymph node and distant metastases in patients with nasopharyngeal carcinoma. Br. J. Radiol. 2014, 87, 20140296. [Google Scholar] [CrossRef] [PubMed]
- Schriefer, M.E. Lyme Disease Diagnosis: Serology. Clin. Lab. Med. 2015, 35, 797–814. [Google Scholar] [CrossRef]
- Kobayashi, T.; Auwaerter, P.G. Diagnostic Testing for Lyme Disease. Infect. Dis. Clin. N. Am. 2022, 36, 605–620. [Google Scholar] [CrossRef]
- Mead, P.; Petersen, J.; Hinckley, A. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 703. [Google Scholar] [CrossRef]
- Chmielewska-Badora, J.; Cisak, E.; Wójcik-Fatla, A.; Zwoliński, J.; Buczek, A.; Dutkiewicz, J. Correlation of tests for detection of Borrelia burgdorferi sensu lato infection in patients with diagnosed borreliosis. Ann. Agric. Environ. Med. 2006, 13, 307–311. [Google Scholar] [PubMed]

| Test | Number of Studies | Threshold Effect | Heterogeneity | Point Estimate (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| R Value | p Value | I2 | p Value | Pooled Sen | Pooled Spe | Pooled PLR | Pooled NLR | ||
| WCA EIA for IgM | 11 | −0.52 | 0.61 | 98% | 0.00 | 0.57 (0.43, 0.69) | 0.95 (0.86, 0.98) | 11.56 (4.33, 30.86) | 0.46 (0.40, 0.61) |
| WCA EIA for IgG | 5 | 0.60 | 0.28 | 99% | 0.00 | 0.73 (0.65, 0.79) | 0.80 (0.44, 0.96) | 3.67 (0.97, 14.00) | 0.34 (0.22, 0.52) |
| flagella EIA for IgM | 7 | 0.821 | 0.02 | 86% | 0.00 | / | / | / | / |
| flagella EIA for IgG | 6 | −0.79 | 0.62 | 86% | 0.00 | 0.65 (0.56, 0.72) | 0.92 (0.85, 0.96) | 7.98 (4.25, 15.00) | 0.38 (0.31, 0.47) |
| VlsE EIA for IgM | 3 | 0.500 | 0.67 | 92.0% | 0.00 | 0.46 (0.36, 0.56) | 0.94 (0.79, 0.99) | 8.02 (1.94, 33.13) | 0.57 (0.46, 0.70) |
| VlsE EIA for IgG | 4 | −0.28 | 0.40 | 98% | 0.00 | 0.85 (0.52, 0.97) | 0.95 (0.83, 0.99) | 16.89 (4.92, 58.03) | 0.16 (0.04, 0.64) |
| C6 EIA | 17 | −0.58 | 0.01 | 100.0% | 0.00 | / | / | / | / |
| IFA for IgM | 4 | −1.000 | 0.00 | 33% | 0.11 | / | / | / | / |
| IFA for IgG | 4 | −0.200 | 0.800 | 89% | 0.00 | 0.75 (0.68, 0.80) | 0.94 (0.87, 0.95) | 12.56 (2.47, 63.82) | 0.27 (0.21, 0.35) |
| WCA WB for IgM | 3 | 1.000 | 0.000 | 0.0% | 0.71 | / | / | / | / |
| WCA WB for IgG | 3 | −0.500 | 0.667 | 0.0% | 0.42 | 0.44 (0.30, 0.60) | 0.98 (0.89, 1.00) | 24.71 (3.51, 174.01) | 0.57 (0.43, 0.75) |
| STTT | 12 | 0.000 | 1.000 | 92% | 0.000 | 0.66 (0.54, 0.76) | 0.99 (0.98, 0.99) | 63.16 (36.27, 110.01) | 0.35 (0.25, 0.48) |
| MTTT | 6 | −0.200 | 0.704 | 96% | 0.000 | 0.73 (0.61, 0.82) | 0.98 (0.96, 0.99) | 40.81 (19.58, 85.06) | 0.28 (0.19, 0.41) |
| DbpB EIA for IgG | 3 | −1.000 | 0.000 | 80.7% | 0.0057 | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Li, J.; Gao, L.; Wu, X.; Ma, W.; Yang, J.; Zhong, L.; Song, J.; Peng, L.; Bao, F.; et al. Comparison of the Serodiagnostic Accuracy Tests for Lyme Disease in Adults and Children: A Network Meta-Analysis. Pathogens 2025, 14, 784. https://doi.org/10.3390/pathogens14080784
Ma W, Li J, Gao L, Wu X, Ma W, Yang J, Zhong L, Song J, Peng L, Bao F, et al. Comparison of the Serodiagnostic Accuracy Tests for Lyme Disease in Adults and Children: A Network Meta-Analysis. Pathogens. 2025; 14(8):784. https://doi.org/10.3390/pathogens14080784
Chicago/Turabian StyleMa, Weijiang, Jing Li, Li Gao, Xinya Wu, Weijie Ma, Jiaru Yang, Lei Zhong, Jieqin Song, Li Peng, Fukai Bao, and et al. 2025. "Comparison of the Serodiagnostic Accuracy Tests for Lyme Disease in Adults and Children: A Network Meta-Analysis" Pathogens 14, no. 8: 784. https://doi.org/10.3390/pathogens14080784
APA StyleMa, W., Li, J., Gao, L., Wu, X., Ma, W., Yang, J., Zhong, L., Song, J., Peng, L., Bao, F., & Liu, A. (2025). Comparison of the Serodiagnostic Accuracy Tests for Lyme Disease in Adults and Children: A Network Meta-Analysis. Pathogens, 14(8), 784. https://doi.org/10.3390/pathogens14080784






