Efficacy of GS-441524 for Feline Infectious Peritonitis: A Systematic Review (2018–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Literature Search
2.3. Inclusion Criteria
2.4. Data Extraction
- Remission: clinical recovery with no recurrence of disease after a single GS-441524 treatment course.
- Remission after relapse: recovery after an initial response, subsequent disease relapse, and then a second course of GS-441524.
- Death after relapse: initial improvement on GS-441524, but relapse occurred and the cat ultimately died or was euthanized despite additional treatment.
- Death without remission: no significant clinical improvement on GS-441524, and the cat died or was euthanized during or after the treatment course.
- Relapse with treatment change: disease relapse that was treated with a different therapy (e.g., another antiviral like Molnupiravir) due to GS-441524 failure.
2.5. Bias and Quality Assessment
2.6. Data Synthesis
- Treatment modality: GS-441524 alone vs. GS-441524 in combination with other antivirals (e.g., GC376, Remdesivir) or immunomodulators (e.g., interferon omega).
- FIP form: effusive vs. non-effusive vs. mixed FIP.
- Presence of aggravating signs: cases with vs. without neurological/ocular involvement.
- Route of administration: oral vs. subcutaneous, with cases that received GS-441524 by more than one route excluded.
3. Results
3.1. Article Selection
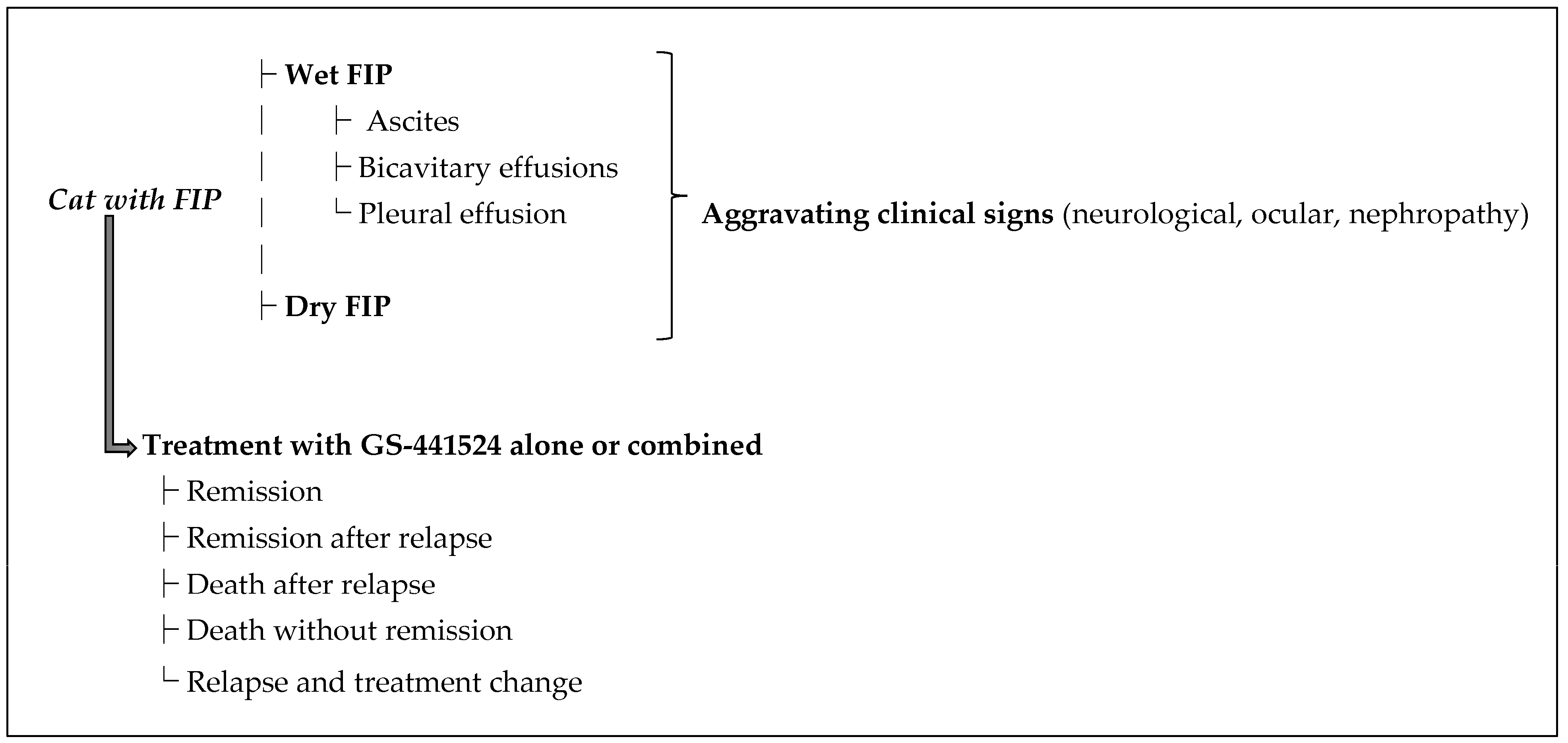
3.2. Summary of Treated Cases and Outcomes
- Effusive FIP: 202 cases (31.1%). Among these, 148 (73.3%) achieved remission, 9 (4.5%) went into remission after relapse, 1 (0.5%) died after relapse, 35 (17.3%) died without remission, and 9 (4.5%) were switched to another treatment after relapse. Success rate for wet FIP was ~77.7%.
- Non-effusive FIP: 187 cases (28.8%). Outcomes: 154 (82.4%) went into remission, 10 (5.3%) went into remission after relapse, 1 (0.5%) died after relapse, 10 (5.3%) died without remission, and 12 (6.4%) switched after relapse. Success rate ~87.7%.
- Mixed FIP (both effusive and non-effusive components): 162 cases (24.9%). Outcomes: 134 (82.7%) went into remission, 3 (1.9%) went into remission after relapse, 0 died after relapse, 24 (14.8%) died without remission, and 1 (0.6%) switched after relapse. Success rate ~84.6%.
3.3. GS-441524 Monotherapy vs. Combination Therapy
- GS-441524 + Interferon omega: One study (12 cats total) reported using feline interferon-ω alongside GS-441524 in primarily dry FIP cases. Notably, all 12 cats survived (1 required an extra GS-441524 cycle) for an effective success rate of ~100% in that small sample. This regimen was associated with prolonged interferon therapy (up to 1 year) following a standard GS-441524 course [21]. Due to the small number of felines in these studies, no failure was reported in this treatment group, which means that the effectiveness of the treatment can not be assessed statistically.
- GS-441524 + Remdesivir: Two studies (37 cats) combined GS-441524 (oral) with injectable Remdesivir [8,22]. Remdesivir was either given initially for a few days or concurrently. Outcome: 35/37 survived (94.6% success). Statistically, this was not different from GS-441524 alone (p = 0.066) and we can not exclude that the better outcome in this drug’s combination is not due to chance. All cats with neurological signs in this group survived, but numbers were too small to obtain a small p-value and draw firm statistical conclusions.
- GS-441524 + GC376: Two studies (48 cats) combined GS-441524 with GC376 [9,23]. Outcome: 45/48 survived (93.8% success). Again, not statistically superior to monotherapy (p = 0.054). Among those with non-effusive FIP and concurrent GC376, success was 94% (including two relapses that were retreated successfully). The addition of GC376 did not clearly overcome the challenge of neurologic disease (one cat with CNS signs relapsed).
3.4. Influence of Clinical Factors on Efficacy
3.5. Safety and Adverse Effects
3.6. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3CLPRO | 3-Cysteine-like Protein |
| EFCOV | Enteric Feline Coronavirus |
| EMA | European Medicines Agency |
| FCOV | Feline Coronavirus |
| FDA | Food and Drug Administration |
| FeLV | Feline Leukemia Virus |
| FIP | Feline Infectious Peritonitis |
| FIPV | Feline Infectious Peritonitis Virus |
| FIV | Feline Immunodeficiency Virus |
| GRADE | Grating of Recommendations, Assessment, Development, and Evaluation |
| MPRO | Main Protein |
| Nsp5 | Non-structural Protein 5 |
| Nsps | Non-structural Proteins |
| OR | Odds Ratio |
| PICO | Population, Interventions, Comparisons, Outcomes |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| RD | Risk Difference |
| RR | Relative Risk |
| RT-qPCR | Reverse Transcription-Quantitative Polymerase Chain Reaction |
References
- Delaplace, M.; Huet, H.; Gambino, A.; Le Poder, S. Feline Coronavirus Antivirals: A Review. Pathogens 2021, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An update on feline infectious peritonitis: Diagnostics and therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Wang, Q.; Liang, X.Y.; Zhang, S.; Bao, D.; Zhao, H.; Li, S.B.; Wang, K.; Hu, G.X.; Gao, F.S. An updated review of feline coronavirus: Mind the two biotypes. Virus Res. 2023, 326, 199059. [Google Scholar] [CrossRef] [PubMed]
- Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Feline infectious peritonitis. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Thayer, V.; Gogolski, S.; Felten, S.; Hartmann, K.; Kennedy, M.; Olah, G.A. 2022 AAFP/EveryCat Feline Infectious Peritonitis Diagnosis Guidelines. J. Feline Med. Surg. 2022, 24, 905–933. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S.; Addie, D.D.; Egberink, H.; Hofmann-Lehmann, R.; Hosie, M.J.; Truyen, U.; Belak, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines. Viruses 2023, 15, 1847. [Google Scholar] [CrossRef] [PubMed]
- Izes, A.M.; Yu, J.; Norris, J.M.; Govendir, M. Current status on treatment options for feline infectious peritonitis and SARS-CoV-2 positive cats. Vet. Q. 2020, 40, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.J.; Norris, J.M.; Malik, R.; Govendir, M.; Hall, E.J.; Kimble, B.; Thompson, M.F. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS-441524. J. Vet. Intern. Med. 2023, 37, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Jacque, N.; Novicoff, W.; Li, E.; Negash, R.; Evans, S.J.M. Unlicensed Molnupiravir is an Effective Rescue Treatment Following Failure of Unlicensed GS-441524-like Therapy for Cats with Suspected Feline Infectious Peritonitis. Pathogens 2022, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Coggins, S.; Barker, E.N.; Gunn-Moore, D.; Jeevaratnam, K.; Norris, J.M.; Hughes, D.; Stacey, E.; MacFarlane, L.; O’Brien, C.; et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J. Feline Med. Surg. 2023, 25, 1098612x231194460. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Donato, H.; Donato, M. Stages for Undertaking a Systematic Review. Acta. Med. Port. 2019, 32, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Uemura, Y. Therapeutic Effects of Mutian((R)) Xraphconn on 141 Client-Owned Cats with Feline Infectious Peritonitis Predicted by Total Bilirubin Levels. Vet. Sci. 2021, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Uemura, Y. Prognostic Prediction for Therapeutic Effects of Mutian on 324 Client-Owned Cats with Feline Infectious Peritonitis Based on Clinical Laboratory Indicators and Physical Signs. Vet. Sci. 2023, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. Neurological and Ocular FIP. 2023. Available online: https://www.sockfip.org/2021-dr-pedersen-new-years-update-neurological-ocular-fip/ (accessed on 19 May 2025).
- Dickinson, P.J.; Bannasch, M.; Thomasy, S.M.; Murthy, V.D.; Vernau, K.M.; Liepnieks, M.; Montgomery, E.; Knickelbein, K.E.; Murphy, B.; Pedersen, N.C. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J. Vet. Intern. Med. 2020, 34, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. JBI Evid. Implement. 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Addie, D.D.; Silveira, C.; Aston, C.; Brauckmann, P.; Covell-Ritchie, J.; Felstead, C.; Fosbery, M.; Gibbins, C.; Macaulay, K.; McMurrough, J.; et al. Alpha-1 Acid Glycoprotein Reduction Differentiated Recovery from Remission in a Small Cohort of Cats Treated for Feline Infectious Peritonitis. Viruses 2022, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Syme, H.; Tayler, S. Thirty-two cats with effusive or non-effusive feline infectious peritonitis treated with a combination of remdesivir and GS-441524. J. Vet. Intern. Med. 2023, 37, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bai, Y.; Wang, Y.; Yang, L.; Jin, Y.; Dong, J. Effect of GS-441524 in combination with the 3C-like protease inhibitor GC376 on the treatment of naturally transmitted feline infectious peritonitis. Front. Vet. Sci. 2022, 9, 1002488. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.G.; Perron, M.; Murakami, E.; Bauer, K.; Park, Y.; Eckstrand, C.; Liepnieks, M.; Pedersen, N.C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018, 219, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Krentz, D.; Zenger, K.; Alberer, M.; Felten, S.; Bergmann, M.; Dorsch, R.; Matiasek, K.; Kolberg, L.; Hofmann-Lehmann, R.; Meli, M.L.; et al. Curing Cats with Feline Infectious Peritonitis with an Oral Multi-Component Drug Containing GS-441524. Viruses 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Allinder, M.; Tynan, B.; Martin, C.; Furbish, A.; Austin, G.; Bartges, J.; Lourenco, B.N. Uroliths composed of antiviral compound GS-441524 in 2 cats undergoing treatment for feline infectious peritonitis. J. Vet. Intern. Med. 2024, 38, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. Summary of GS-441524 Treatment for FIP. Sock It to FIP. 2020. Available online: https://www.sockfip.org/2020-dr-pedersen-gs-441524-update/ (accessed on 19 May 2025).
- Ritz, S.; Egberink, H.; Hartmann, K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2007, 21, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.J.; Browning, M.E. Quality assessment and characterization of unregulated antiviral drugs for feline infectious peritonitis: Implications for treatment, safety, and efficacy. Am. J. Vet. Res. 2024, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.M.; Guan, S.; Jacque, N.; Novicoff, W.; Evans, S.J.M. Unlicensed antiviral products used for the at-home treatment of feline infectious peritonitis contain GS-441524 at significantly different amounts than advertised. J. Am. Vet. Med. Assoc. 2024, 262, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Zwicklbauer, K.; Krentz, D.; Bergmann, M.; Felten, S.; Dorsch, R.; Fischer, A.; Hofmann-Lehmann, R.; Meli, M.L.; Spiri, A.M.; Alberer, M.; et al. Long-term follow-up of cats in complete remission after treatment of feline infectious peritonitis with oral GS-441524. J. Feline Med. Surg. 2023, 25, 1098612x231183250. [Google Scholar] [CrossRef] [PubMed]
- Cosaro, E.; Pires, J.; Castillo, D.; Murphy, B.G.; Reagan, K.L. Efficacy of Oral Remdesivir Compared to GS-441524 for Treatment of Cats with Naturally Occurring Effusive Feline Infectious Peritonitis: A Blinded, Non-Inferiority Study. Viruses 2023, 15, 1680. [Google Scholar] [CrossRef] [PubMed]
- Zuzzi-Krebitz, A.M.; Buchta, K.; Bergmann, M.; Krentz, D.; Zwicklbauer, K.; Dorsch, R.; Wess, G.; Fischer, A.; Matiasek, K.; Honl, A.; et al. Short Treatment of 42 Days with Oral GS-441524 Results in Equal Efficacy as the Recommended 84-Day Treatment in Cats Suffering from Feline Infectious Peritonitis with Effusion-A Prospective Randomized Controlled Study. Viruses 2024, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Negash, R.; Li, E.; Jacque, N.; Novicoff, W.; Evans, S.J.M. Owner experience and veterinary involvement with unlicensed GS-441524 treatment of feline infectious peritonitis: A prospective cohort study. Front. Vet. Sci. 2024, 11, 1377207. [Google Scholar] [CrossRef] [PubMed]
- Federation of Veterinarians of Europe. Finally a FIP Treatment Opportunity in the EU! 2024. Available online: https://fve.org/finally-a-fip-treatment-opportunity-in-eu/ (accessed on 19 May 2025).
| PubMed Central | Google Scholar | Science Direct | Cross-Reference | |
|---|---|---|---|---|
| References retrieved | 182 | 102 | 29 | - |
| References selected after abstract screening and duplicate removal | 36 | 7 | 2 | 10 |
| References selected * after full-text reading | 11 | 0 | 0 | 0 |
| Number of Cases | Remission | Remission After Relapse | Death After Relapse | Relapse Requiring Treatment Change | |
|---|---|---|---|---|---|
| Total | 169 | 116 | 10 | 2 | 13 |
| Neurological signs | 106 | 66 | 7 | 2 | 9 |
| Ocular signs | 48 | 42 | 1 | 0 | 1 |
| Neurological and ocular | 7 | 2 | 1 | 0 | 3 |
| Renal | 8 | 6 | 1 | 0 | 0 |
| Comparison—Efficacy of Treatment in Complicated vs. Non-Complicated FIP | OR [95% CI] | RR [95% CI] | RD [95% CI] | p Value |
|---|---|---|---|---|
| Overall Efficacy | 0.39 [0.25–0.61] | 0.85 [0.77–0.93] | −0.14 [−0.22; −0.07] | <0.0001 |
| Efficacy of GS-441524 Alone | 0.40 [0.25–0.61] | 0.84 [0.77–0.93] | −0.14 [−0.22; −0.06] | <0.0001 |
| Efficacy of GS-441524 with Interferon omega | Not estimable | Not estimable | Not estimable | n.a. |
| Efficacy of GS-441524 with Remdesivir | Not estimable | Not estimable | 0.07 [0.02–0.11] | n.a. |
| Efficacy of GS-441524 with GC376 | 0.09 [0.007–1.13] | 0.80 [0.56–1.14] | −0.20 [−0.48; 0.08] | 0.0281 |
| Comparison—Efficacy of Treatment in Effusive FIP vs. | OR [95% CI] | RR [95% CI] | RD [95% CI] | p Value |
|---|---|---|---|---|
| GS-441524 Monotherapy in Mixed FIP | 0.64 [0.37–1.1] | 0.94 [0.85–1.04] | −0.05 [−0.13; 0.03] | 0.0996 |
| GS-441524 Monotherapy in Non-effusive FIP | 0.49 [0.28–0.85] | 0.89 [0.81–0.97] | −0.10 [−0.17; −0.03] | 0.0096 |
| Comparison—Efficacy of GS-441524 Combined with: | OR [95% CI] | RR [95% CI] | RD [95% CI] | p Value |
|---|---|---|---|---|
| Interferon omega | Not estimable | 1.20 [1.16–1.25] | 0.17 [0.14–0.20] | n.a. a |
| Remdesivir | 3.55 [0.8–15.0] | 1.14 [1.04–1.23] | 0.11 [0.02–0.18] | 0.0664 |
| GC376 | 3.05 [0.9–10.0] | 1.13 [1.03–1.22] | 0.11 [0.03–0.17] | 0.0542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokalsing, E.; Ferrolho, J.; Gibson, M.S.; Vilhena, H.; Anastácio, S. Efficacy of GS-441524 for Feline Infectious Peritonitis: A Systematic Review (2018–2024). Pathogens 2025, 14, 717. https://doi.org/10.3390/pathogens14070717
Gokalsing E, Ferrolho J, Gibson MS, Vilhena H, Anastácio S. Efficacy of GS-441524 for Feline Infectious Peritonitis: A Systematic Review (2018–2024). Pathogens. 2025; 14(7):717. https://doi.org/10.3390/pathogens14070717
Chicago/Turabian StyleGokalsing, Emma, Joana Ferrolho, Mark S. Gibson, Hugo Vilhena, and Sofia Anastácio. 2025. "Efficacy of GS-441524 for Feline Infectious Peritonitis: A Systematic Review (2018–2024)" Pathogens 14, no. 7: 717. https://doi.org/10.3390/pathogens14070717
APA StyleGokalsing, E., Ferrolho, J., Gibson, M. S., Vilhena, H., & Anastácio, S. (2025). Efficacy of GS-441524 for Feline Infectious Peritonitis: A Systematic Review (2018–2024). Pathogens, 14(7), 717. https://doi.org/10.3390/pathogens14070717








