NOD2 (Nucleotide-Binding Oligomerization Domain-Containing Protein 2)-Mediated Modulation of the Immune Response Induced by BCG (Bacillus Calmette-Guérin) Bacilli
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Whole-Blood Cultures
2.3. Isolation of DNA and Its Spectrophotometric Evaluation
2.4. Assessment of the NOD2 Gene Polymorphism
2.5. Isolation of RNA and Its Spectrophotometric Evaluation
2.6. Reverse Transcription
2.7. qPCR Reaction
2.8. Peripheral Blood Leukocyte (PBL) Harvest and Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Assessment of the NOD2 Gene Polymorphism
3.2. mRNA Expression of NOD2
3.3. mRNA Expression of IL-1β
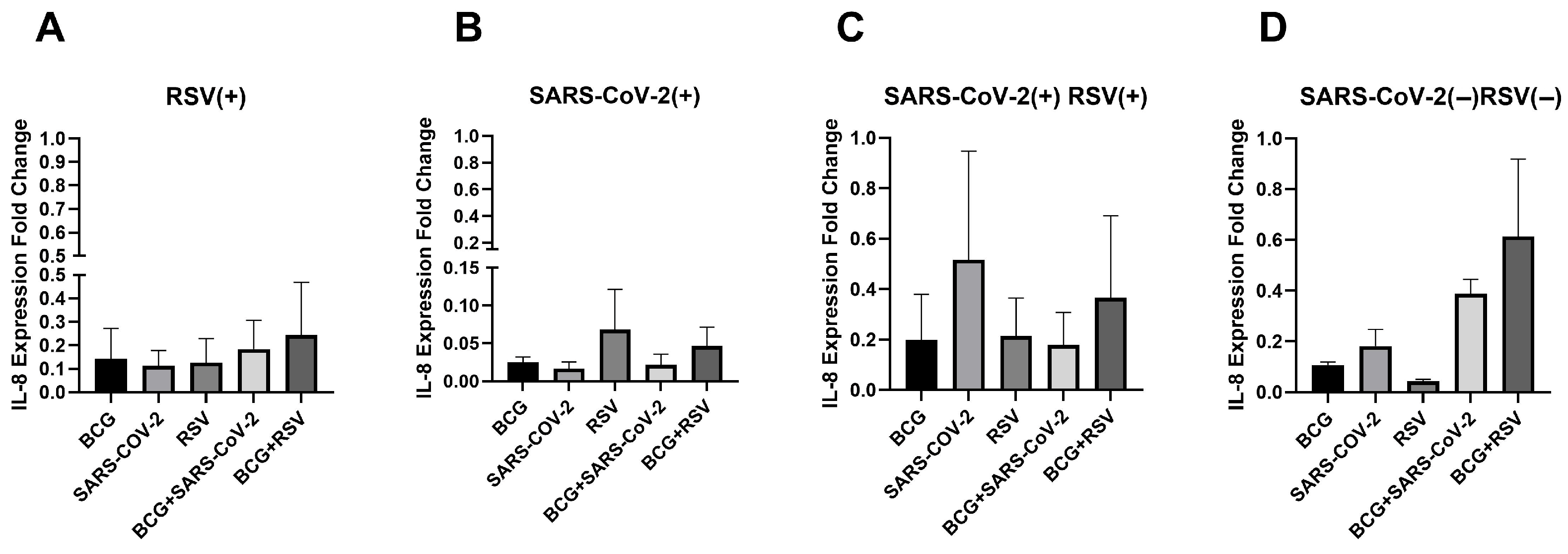
3.4. mRNA Expression of IL-8
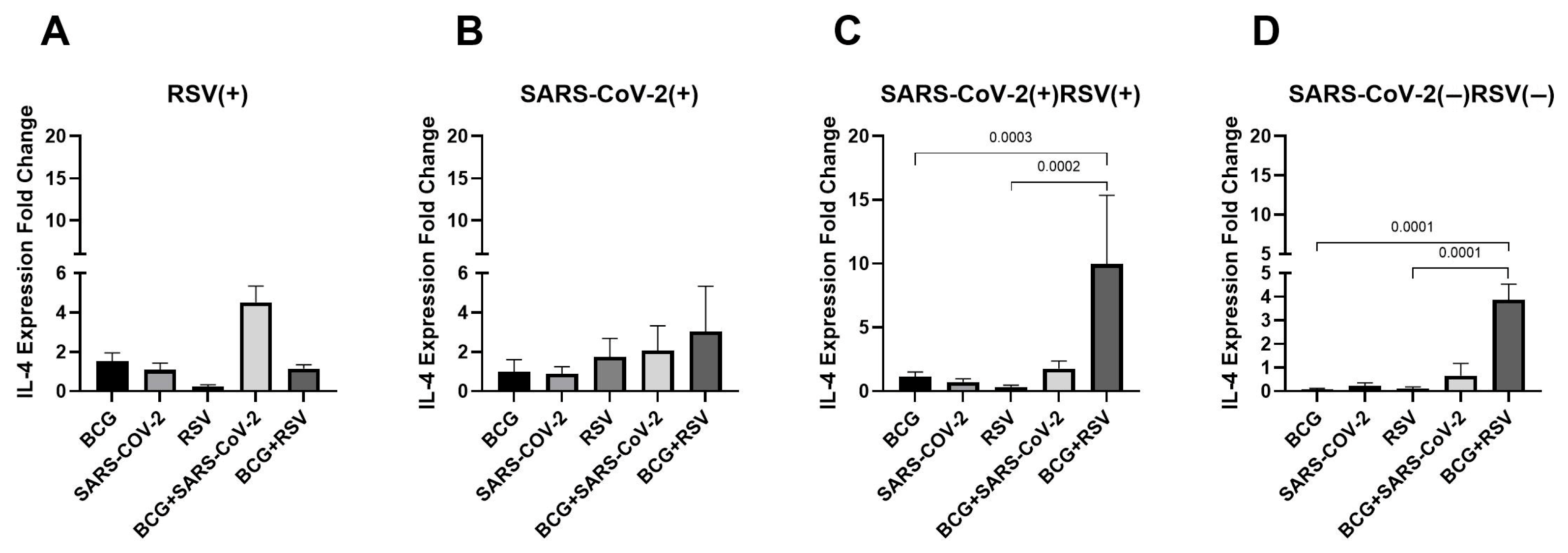
3.5. mRNA Expression of IL-4
3.6. mRNA Expression of TNF
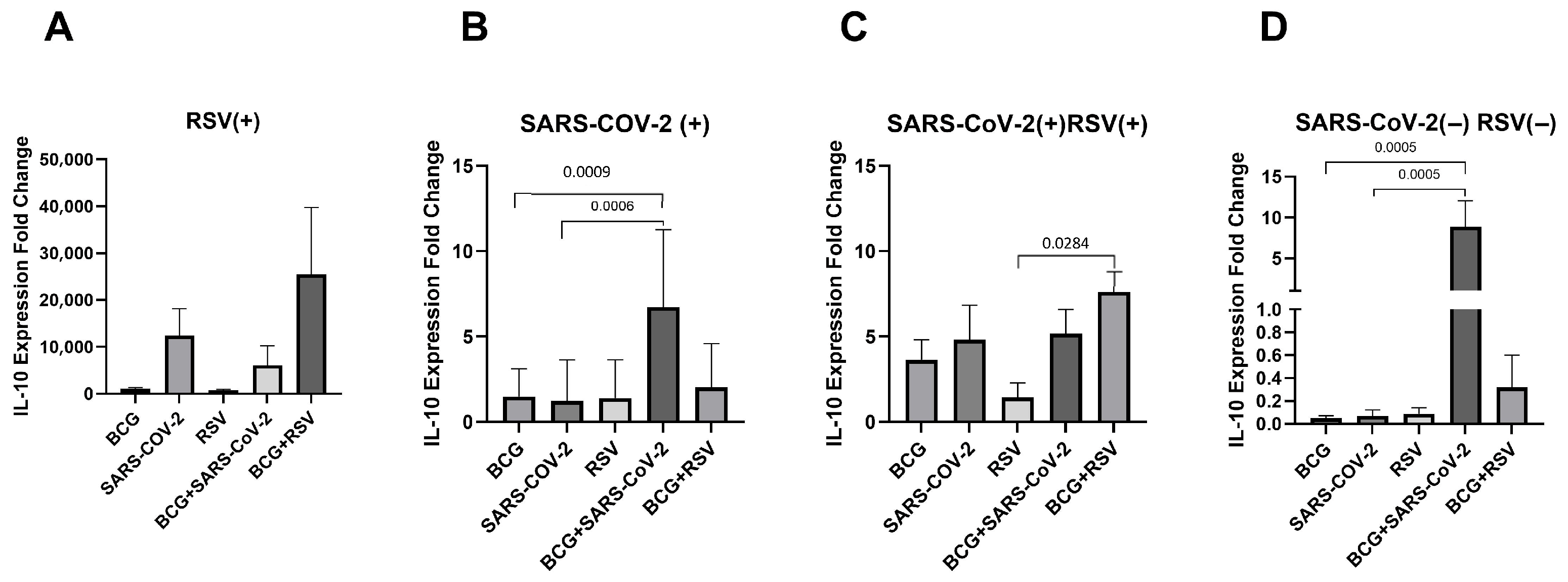
3.7. mRNA Expression of IL-10
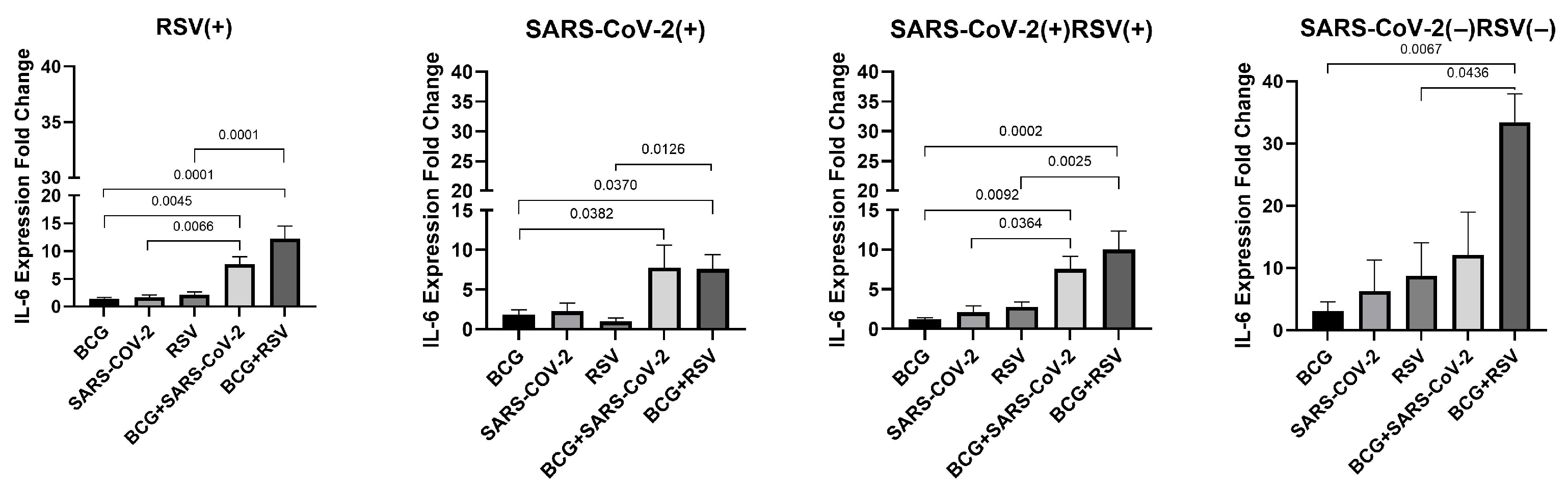
3.8. mRNA Expression of IL-6
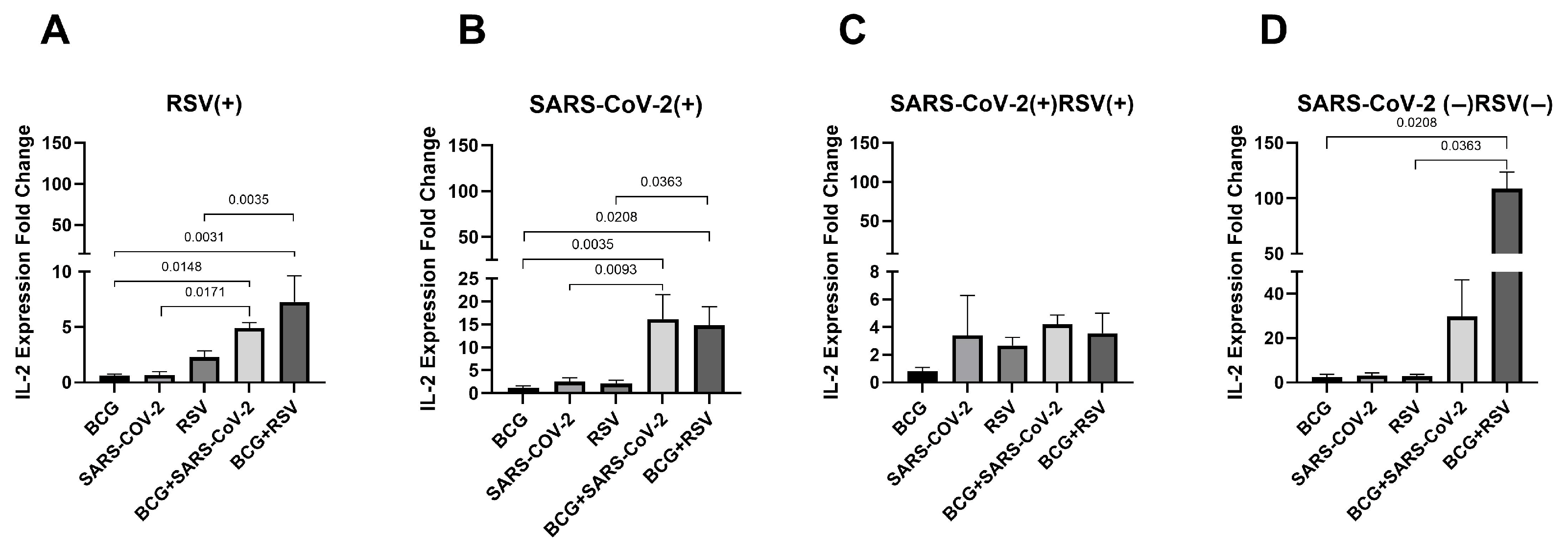
3.9. mRNA Expression of IL-2
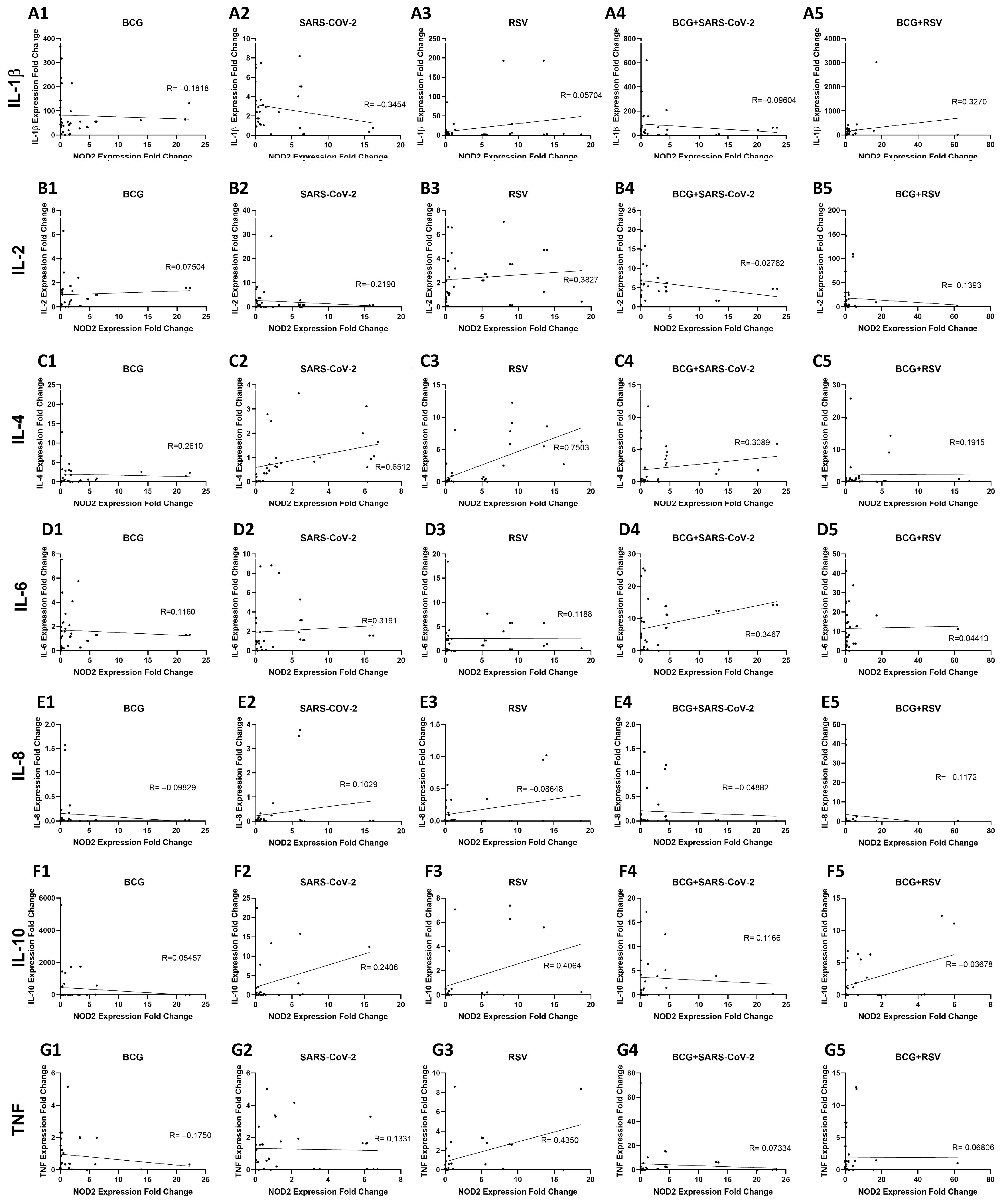
3.10. Relationship Between NOD2 and Cytokine Expression in Whole Blood Cultures Stimulated with Mycobacterium bovis BCG and Viral Antigens
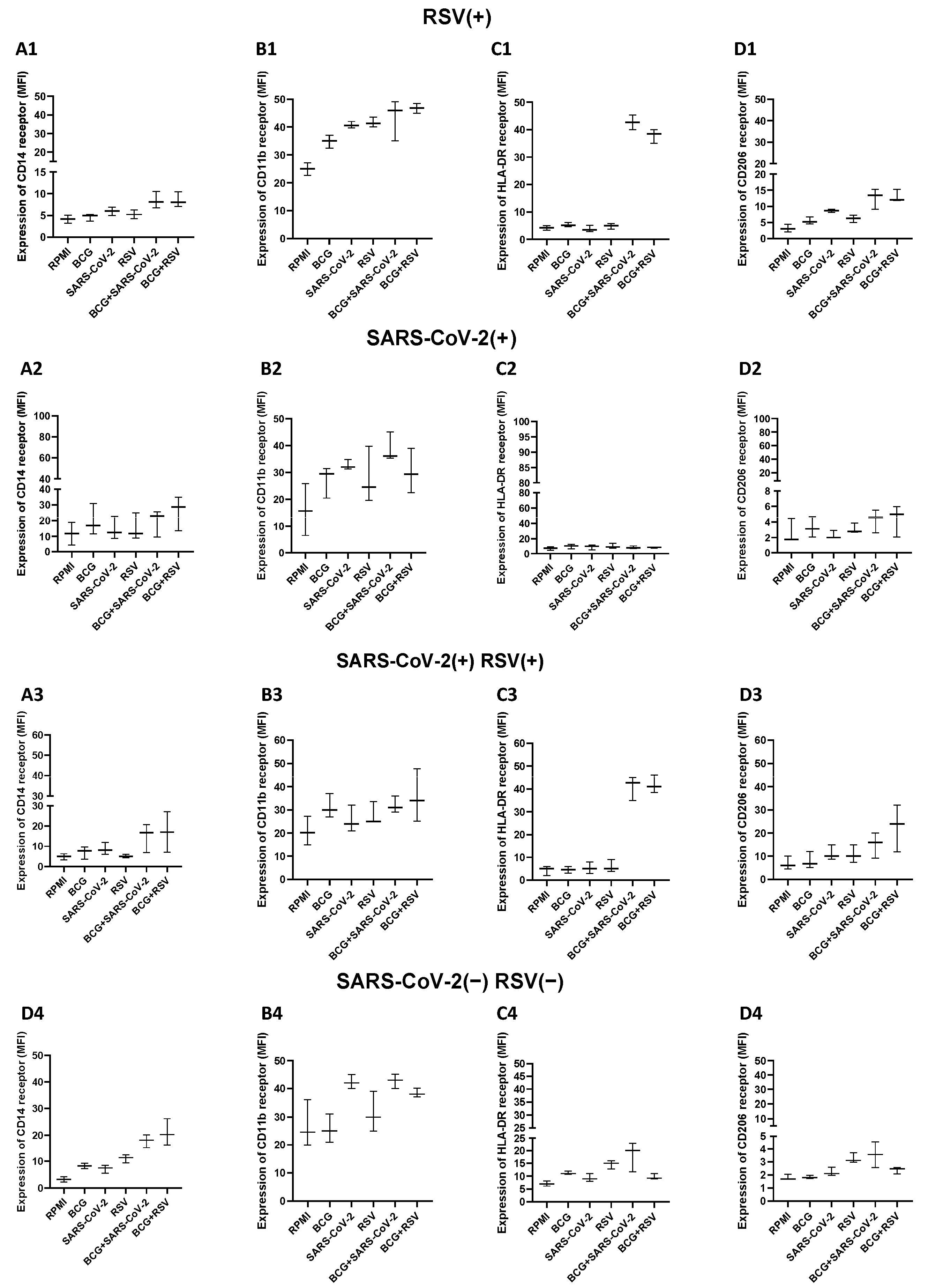
3.11. Expression Levels of CD14, HLA-DR, CD11b, and CD206 on Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette-Guérin |
| SARS-CoV-2 | Directory of open access journals |
| RSV | Three letter acronym |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| H3K4me3 | Histone 3 at lysine 4 |
| IL | Interleukin |
| HPV | Human papillomavirus |
| HSV | Herpes simplex virus |
| Th1 | Type 1 helper lymphocyte |
| IFN | Interferon |
| rBCG-RSV | Recombinant BCG vaccine expressing the RSV nucleoprotein |
| N | number |
References
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-Induced Trained Immunity: History, Mechanisms and Potential Applications. J. Transl. Med. 2023, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Moorlag, S.J.C.F.M.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of Innate Immune Antiviral Responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Trindade, B.C.; Chen, G.Y. NOD1 and NOD2 in Inflammatory and Infectious Diseases. Immunol. Rev. 2020, 297, 139. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Van Loenhout, J.; De Jong, D.; Hendrik, S.; et al. Bacille Calmette-Guérin Induces NOD2-Dependent Nonspecific Protection from Reinfection via Epigenetic Reprogramming of Monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-Mediated Aerobic Glycolysis as Metabolic Basis for Trained Immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Lee, A.; Floyd, K.; Wu, S.; Fang, Z.; Tan, T.K.; Froggatt, H.M.; Powers, J.M.; Leist, S.R.; Gully, K.L.; Hubbard, M.L.; et al. BCG Vaccination Stimulates Integrated Organ Immunity by Feedback of the Adaptive Immune Response to Imprint Prolonged Innate Antiviral Resistance. Nat. Immunol. 2023, 25, 41. [Google Scholar] [CrossRef]
- Abarca, K.; Rey-Jurado, E.; Muñoz-Durango, N.; Vázquez, Y.; Soto, J.A.; Gálvez, N.M.S.; Valdés-Ferrada, J.; Iturriaga, C.; Urzúa, M.; Borzutzky, A.; et al. Safety and Immunogenicity Evaluation of Recombinant BCG Vaccine against Respiratory Syncytial Virus in a Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. eClinicalMedicine 2020, 27, 100517. [Google Scholar] [CrossRef]
- Cautivo, K.M.; Bueno, S.M.; Cortes, C.M.; Wozniak, A.; Riedel, C.A.; Kalergis, A.M. Efficient Lung Recruitment of Respiratory Syncytial Virus-Specific Th1 Cells Induced by Recombinant Bacillus Calmette-Guérin Promotes Virus Clearance and Protects from Infection. J. Immunol. 2010, 185, 7633–7645. [Google Scholar] [CrossRef] [PubMed]
- Sir Karakus, G.; Tastan, C.; Dilek Kancagi, D.; Yurtsever, B.; Tumentemur, G.; Demir, S.; Turan, R.D.; Abanuz, S.; Cakirsoy, D.; Seyis, U.; et al. Preclinical Efficacy and Safety Analysis of Gamma-Irradiated Inactivated SARS-CoV-2 Vaccine Candidates. Sci. Rep. 2021, 11, 5804. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Berger, M.M.; Brenner, T.; et al. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS-CoV-2 Proteins Is Not Associated with Recovery in Critical COVID-19 Patients. Cell Rep. Med. 2020, 1, 100092. [Google Scholar] [CrossRef]
- Heliö, T.; Halme, L.; Lappalainen, M.; Fodstad, H.; Paavola-Sakki, P.; Turunen, U.; Färkkilä, M.; Krusius, T.; Kontula, K. CARD15/NOD2 Gene Variants Are Associated with Familially Occurring and Complicated Forms of Crohn’s Disease. Gut 2003, 52, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wawrocki, S.; Kielnierowski, G.; Rudnicka, W.; Seweryn, M.; Druszczynska, M. Interleukin-18, Functional IL-18 Receptor and IL-18 Binding Protein Expression in Active and Latent Tuberculosis. Pathogens 2020, 9, 451. [Google Scholar] [CrossRef]
- Aeby, M.; Blanc, P.; Fellay, I.; Oberson, A.; Filgueira, L. The Expression of Fibrogenic Cytokines by Human Peripheral Blood Mononuclear Cells in Response to SARS-CoV-2 Spike Protein. COVID 2023, 3, 897–913. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Villanueva, P.; Wadia, U.; Crawford, N.W.; Messina, N.L.; Kollmann, T.R.; Lucas, M.; Manning, L.; Richmond, P.; Pittet, L.F.; Curtis, N. The Safety of Co-Administration of Bacille Calmette-Guérin (BCG) and Influenza Vaccines. PLoS ONE 2022, 17, e0268042. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.L.; Bewley, K.; Churchward, C.; Fotheringham, S.; Gkolfinos, K.; Gooch, K.; Handley, A.; et al. Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques. Front. Immunol. 2021, 12, 801799. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, And Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- McNamara, P.S.; Flanagan, B.F.; Selby, A.M.; Hart, C.A.; Smyth, R.L. Pro- and Anti-Inflammatory Responses in Respiratory Syncytial Virus Bronchiolitis. Eur. Respir. J. 2004, 23, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Levitz, R.; Wattier, R.; Phillips, P.; Solomon, A.; Lawler, J.; Lazar, I.; Weibel, C.; Kahn, J.S. Induction of IL-6 and CCL5 (RANTES) in human respiratory epithelial (A549) cells by clinical isolates of respiratory syncytial virus is strain specific. Virol. J. 2012, 10, 190. [Google Scholar] [CrossRef]
- Sardarabadi, P.; Lee, K.Y.; Sun, W.L.; Kojabad, A.A.; Liu, C.H. Investigating T Cell Immune Dynamics and IL-6’s Duality in a Microfluidic Lung Tumor Model. ACS Appl. Mater. Interfaces 2024, 17, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
- Pyle, C.J.; Uwadiae, F.I.; Swieboda, D.P.; Harker, J.A. Early IL-6 Signalling Promotes IL-27 Dependent Maturation of Regulatory T Cells in the Lungs and Resolution of Viral Immunopathology. PLoS Pathog. 2017, 13, e1006640. [Google Scholar] [CrossRef]
- Besteman, S.B.; Phung, E.; Raeven, H.H.M.; Amatngalim, G.D.; Rumpret, M.; Crabtree, J.; Schepp, R.M.; Rodenburg, L.W.; Siemonsma, S.G.; Verleur, N.; et al. Recurrent Respiratory Syncytial Virus Infection in a CD14-Deficient Patient. J. Infect. Dis. 2022, 226, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, M.; Kaczmarek, J.; Kowalewska-Pietrzak, M.; Druszczynska, M. Immunomodulatory Effect of the Bacillus Calmette-Guérin (BCG) Vaccine on the In Vitro Interferon Response Induced by Respiratory Syncytial Virus (RSV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigens. Arch. Immunol. Ther. Exp. 2025, 73, 1–16. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Legg, J.P.; Hussain, I.R.; Warner, J.A.; Johnston, S.L.; Warner, J.O. Type 1 and Type 2 Cytokine Imbalance in Acute Respiratory Syncytial Virus Bronchiolitis. Am. J. Respir. Crit. Care Med. 2003, 168, 633–639. [Google Scholar] [CrossRef]
- Wan, Z.; Zhou, Z.; Liu, Y.; Lai, Y.; Luo, Y.; Peng, X.; Zou, W. Regulatory T Cells and T Helper 17 Cells in Viral Infection. Scand. J. Immunol. 2020, 91, e12873. [Google Scholar] [CrossRef]
- Yamada, Y.; Matsumoto, K.; Hashimoto, N.; Saikusa, M.; Homma, T.; Yoshihara, S.; Saito, H. Effect of Th1/Th2 Cytokine Pretreatment on RSV-Induced Gene Expression in Airway Epithelial Cells. Int. Arch. Allergy Immunol. 2011, 154, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.A.; Arredondo, S.M.; Bono, M.R.; Gaggero, A.A.; Díaz, P.V. T Helper 1/T Helper 2 Cytokine Imbalance in Respiratory Syncytial Virus Infection Is Associated with Increased Endogenous Plasma Cortisol. Pediatrics 2006, 117, e878–e886. [Google Scholar] [CrossRef]
- Caballero, M.T.; Serra, M.E.; Acosta, P.L.; Marzec, J.; Gibbons, L.; Salim, M.; Rodriguez, A.; Reynaldi, A.; Garcia, A.; Bado, D.; et al. TLR4 Genotype and Environmental LPS Mediate RSV Bronchiolitis through Th2 Polarization. J. Clin. Investig. 2015, 125, 571–582. [Google Scholar] [CrossRef]
- Lagranderie, M.; Balazuc, A.M.; Abolhassani, M.; Chavarot, P.; Nahori, M.A.; Thouron, F.; Milon, G.; Marchal, G. Development of Mixed Th1/Th2 Type Immune Response and Protection against Mycobacterium Tuberculosis after Rectal or Subcutaneous Immunization of Newborn and Adult Mice with Mycobacterium Bovis BCG. Scand. J. Immunol. 2002, 55, 293–303. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Wang, F.; Liu, R.; Yang, J.; Chen, B. New Insights into Genetic Characteristics between Multiple Myeloma and COVID-19: An Integrative Bioinformatics Analysis of Gene Expression Omnibus Microarray and the Cancer Genome Atlas Data. Int. J. Lab. Hematol. 2021, 43, 1325. [Google Scholar] [CrossRef]
- Vissers, M.; Remijn, T.; Oosting, M.; de Jong, D.J.; Diavatopoulos, D.A.; Hermans, P.W.M.; Ferwerda, G. Respiratory Syncytial Virus Infection Augments NOD2 Signaling in an IFN-β-Dependent Manner in Human Primary Cells. Eur. J. Immunol. 2012, 42, 2727–2735. [Google Scholar] [CrossRef]
- Yamazaki, K.; Takazoe, M.; Tanaka, T.; Kazumori, T.; Nakamura, Y. Absence of Mutation in the NOD2/CARD15 Gene among 483 Japanese Patients with Crohn’s Disease. J. Hum. Genet. 2002, 47, 469–472. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Blok, B.A.; Aaby, P.; Joosten, L.A.B.; de Jong, D.; van der Meer, J.W.M.; Benn, C.S.; van Crevel, R.; Netea, M.G. Long-Term in Vitro and in Vivo Effects of γ-Irradiated BCG on Innate and Adaptive Immunity. J. Leukoc. Biol. 2015, 98, 995–1001. [Google Scholar] [CrossRef]
- Ahmed, A.; Tripathi, H.; van Meijgaarden, K.E.; Kumar, N.C.; Adiga, V.; Rakshit, S.; Parthiban, C.; Eveline, J.S.; D’Souza, G.; Dias, M.; et al. BCG Revaccination in Adults Enhances Pro-Inflammatory Markers of Trained Immunity along with Anti-Inflammatory Pathways. iScience 2023, 26, 107889. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.; Sarfas, C.; Morrison, A.L.; Williams, J.; Gkolfinos, K.; Mabbutt, A.; Eckworth, W.; Lawrence, S.; Dennis, M.; White, A.; et al. Immune Cell Population Dynamics Following Neonatal BCG Vaccination and Aerosol BCG Revaccination in Rhesus Macaques. Sci. Rep. 2024, 14, 16993. [Google Scholar] [CrossRef]
- Roe, M.M.; Do, T.; Turner, S.; Jevitt, A.M.; Chlebicz, M.; White, K.; Oomens, A.G.P.; Rankin, S.; Kovats, S.; Gappa-Fahlenkamp, H. Blood Myeloid Cells Differentiate to Lung Resident Cells and Respond to Pathogen Stimuli in a 3D Human Tissue-Engineered Lung Model. Front. Bioeng. Biotechnol. 2023, 11, 1212230. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Total | Groups | |||
|---|---|---|---|---|---|
| RSV(+) | SARS-CoV-2(+) | RSV(+) SARS- CoV-2(+) | RSV(−) SARS- CoV-2(−) | ||
| Ethnicity | Caucasia | Caucasian | Caucasian | Caucasian | Caucasian |
| N | 48 | 14 | 17 | 11 | 6 |
| Sex | |||||
| Girls, n (%) | 23 (48%) | 8 (57%) | 5 (30%) | 6 (55%) | 4 (66%) |
| Boys, n (%) | 25 (52%) | 6 (43%) | 12 (70%) | 5 (45%) | 2 (33%) |
| Age median | 8 | 9 | 9 | 8 | 8 |
| Age range | 6–12 | 7–12 | 6–12 | 7–12 | 7–12 |
| BCG vaccination | 100% | 100% | 100% | 100% | 100% |
| Compliance with the Polish vaccination schedule | Yes | Yes | Yes | Yes | Yes |
| General health status | Good | Good | Good | Good | Good |
| Chronic respiratory diseases | No | No | No | No | No |
| Form of immunodeficiency | No | No | No | No | No |
| Acute infection <30 days prior to blood collection | No | No | No | No | No |
| Antibiotics <30 days prior to blood collection | No | No | No | No | No |
| Hospitalized for severe RSV or SARS-CoV-2 | No | No | No | No | No |
| Sequence | Temperature of Annealing | Source [Reference] | |
|---|---|---|---|
| R702W | forward 5′-AGATCACAGCAGCCTTCCTG-3′, reverse 5′-CACGCTCTCTTGGCCTCACC-3′ | 62 | [14] |
| G908R | forward 5′-CTCTTGGCCTTTCAGATTCTG-3′ reverse 5′-CAGCTCCTCCCTCTTCACCT-3 | 54 | [14] |
| 1007fs | forward 5′-GGCAGAAGCCCTCCTGCAGGCC-3′ reverse 5′-CCTCAAAATTCTGCCATTCC-3′ | 60 | [14] |
| IL-2 | forward 5′-AGAATCCCAAACTCACCAGGA-3′ reverse 5′-TGCTGATTAAGTCCCTGGGT-3′ | 60 | [16] |
| IL-4 | forward 5′-GCAGTTCTACAGCCACCATG-3′ reverse 5′-ACTCTGGTTGGCTTCCTTCA-3′ | 60 | [16] |
| IL-6 | forward 5′-CCTTCCAAAGATGGCTGAAA-3′ reverse 5′-CAGGGGTGGTTATTGCATCT-3′ | 60 | [16] |
| IL-1B | qHsaCID0022272 | 60 | Bio-Rad |
| IL-8 | qHsaCED0046633 | 60 | Bio-Rad |
| TNF | qHsaCED0037461 | 60 | Bio-Rad |
| IL-10 | QHsaCED0044704 | 60 | Bio-Rad |
| NOD2 | Genotyping | N (%) |
|---|---|---|
| G908R | G/G | 40 (100) |
| R/R | 0 (0) | |
| G/R | 0 (0) | |
| R702W | R/R | 40 (100) |
| W/W | 0 (0) | |
| R/W | 0 (0) | |
| 1007fs | Leu/Leu | 40 (100) |
| Pro/Pro | 0 (0) | |
| Leu/Pro | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczak, M.; Kaczmarek, J.; Kowalewska-Pietrzak, M.; Stelmach, P.; Druszczynska, M. NOD2 (Nucleotide-Binding Oligomerization Domain-Containing Protein 2)-Mediated Modulation of the Immune Response Induced by BCG (Bacillus Calmette-Guérin) Bacilli. Pathogens 2025, 14, 683. https://doi.org/10.3390/pathogens14070683
Jurczak M, Kaczmarek J, Kowalewska-Pietrzak M, Stelmach P, Druszczynska M. NOD2 (Nucleotide-Binding Oligomerization Domain-Containing Protein 2)-Mediated Modulation of the Immune Response Induced by BCG (Bacillus Calmette-Guérin) Bacilli. Pathogens. 2025; 14(7):683. https://doi.org/10.3390/pathogens14070683
Chicago/Turabian StyleJurczak, Magdalena, Joanna Kaczmarek, Magdalena Kowalewska-Pietrzak, Paulina Stelmach, and Magdalena Druszczynska. 2025. "NOD2 (Nucleotide-Binding Oligomerization Domain-Containing Protein 2)-Mediated Modulation of the Immune Response Induced by BCG (Bacillus Calmette-Guérin) Bacilli" Pathogens 14, no. 7: 683. https://doi.org/10.3390/pathogens14070683
APA StyleJurczak, M., Kaczmarek, J., Kowalewska-Pietrzak, M., Stelmach, P., & Druszczynska, M. (2025). NOD2 (Nucleotide-Binding Oligomerization Domain-Containing Protein 2)-Mediated Modulation of the Immune Response Induced by BCG (Bacillus Calmette-Guérin) Bacilli. Pathogens, 14(7), 683. https://doi.org/10.3390/pathogens14070683






