Tissue Distribution and Abundance of the Parasitic Dinoflagellate Hematodinium perezi in Naturally Infected Portunus trituberculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Experimental Crabs and Molecular Identification of Hematodinium Species
2.2. Microscopic Diagnosis of H. perezi Infection
2.3. H. perezi and Hemocyte Cell Counts in Hemolymph of P. trituberculatus with Different Infection Levels
2.4. Histopathological Diagnosis of H. perezi Infections in Different P. trituberculatus Tissues
2.5. Quantitative Analysis of H. perezi Infection in Different P. trituberculatus Tissues
2.6. Statistical Analysis
3. Results
3.1. Life Stages and Cell-Count-Based Quantification of H. perezi in Hemolymph of P. trituberculatus with Different Infection Levels
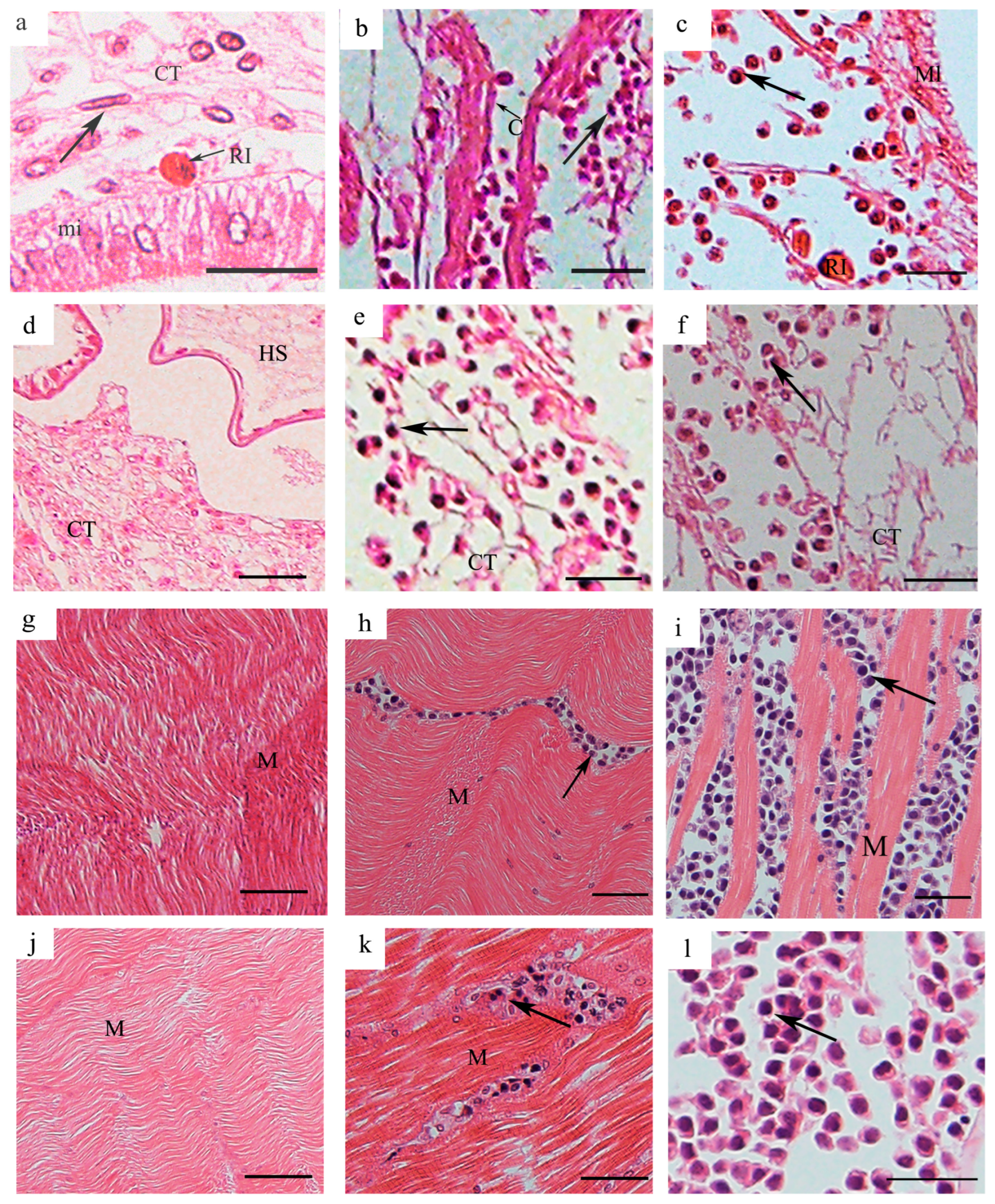
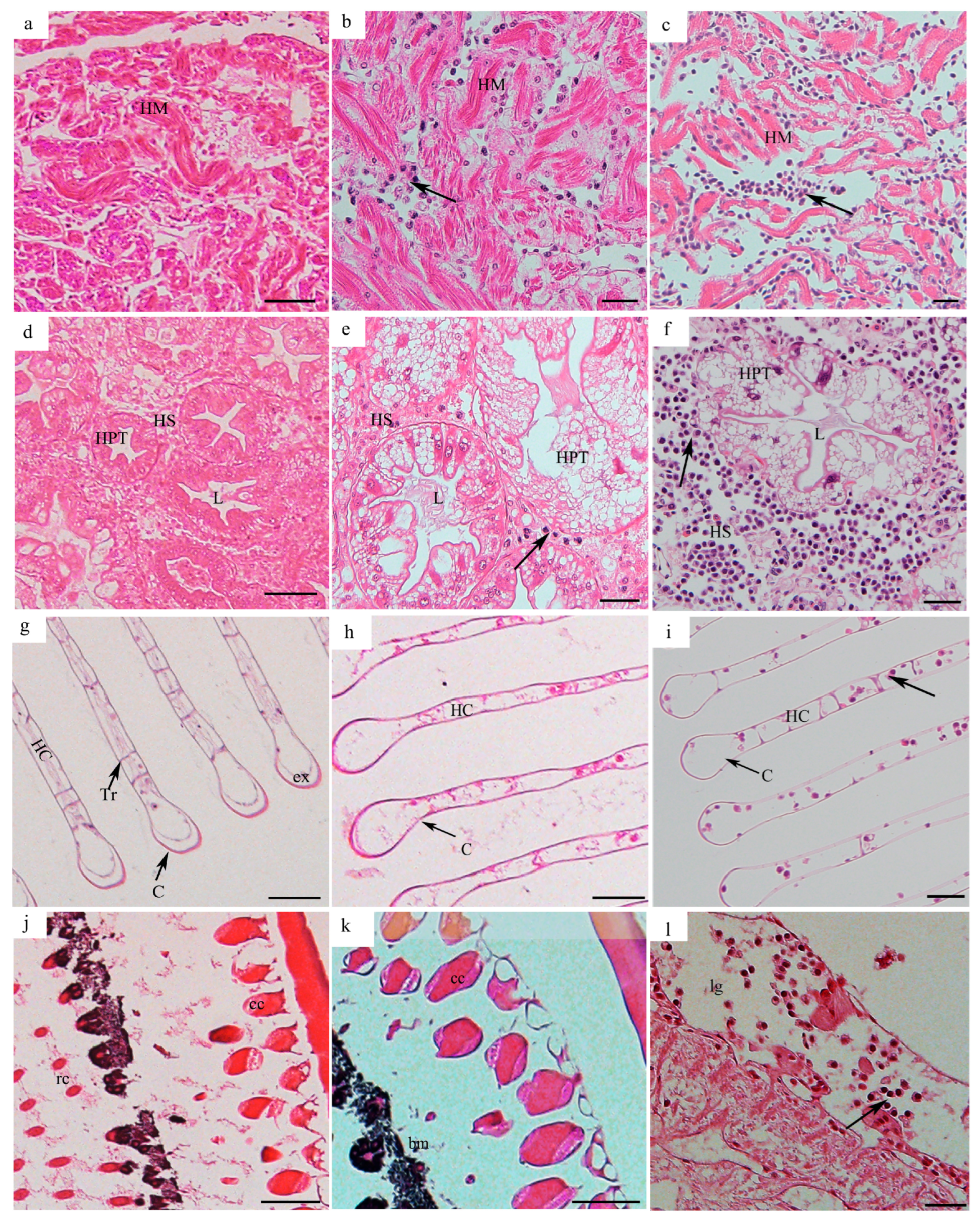
3.2. Tissue Distribution of H. perezi in P. trituberculatus with Different Infection Levels
3.2.1. Tissue Distribution of H. perezi in P. trituberculatus with Infection Level I
3.2.2. Tissue Distribution of H. perezi in P. trituberculatus with Infection Level II
3.2.3. Tissue Distribution of H. perezi in P. trituberculatus with Infection Level III
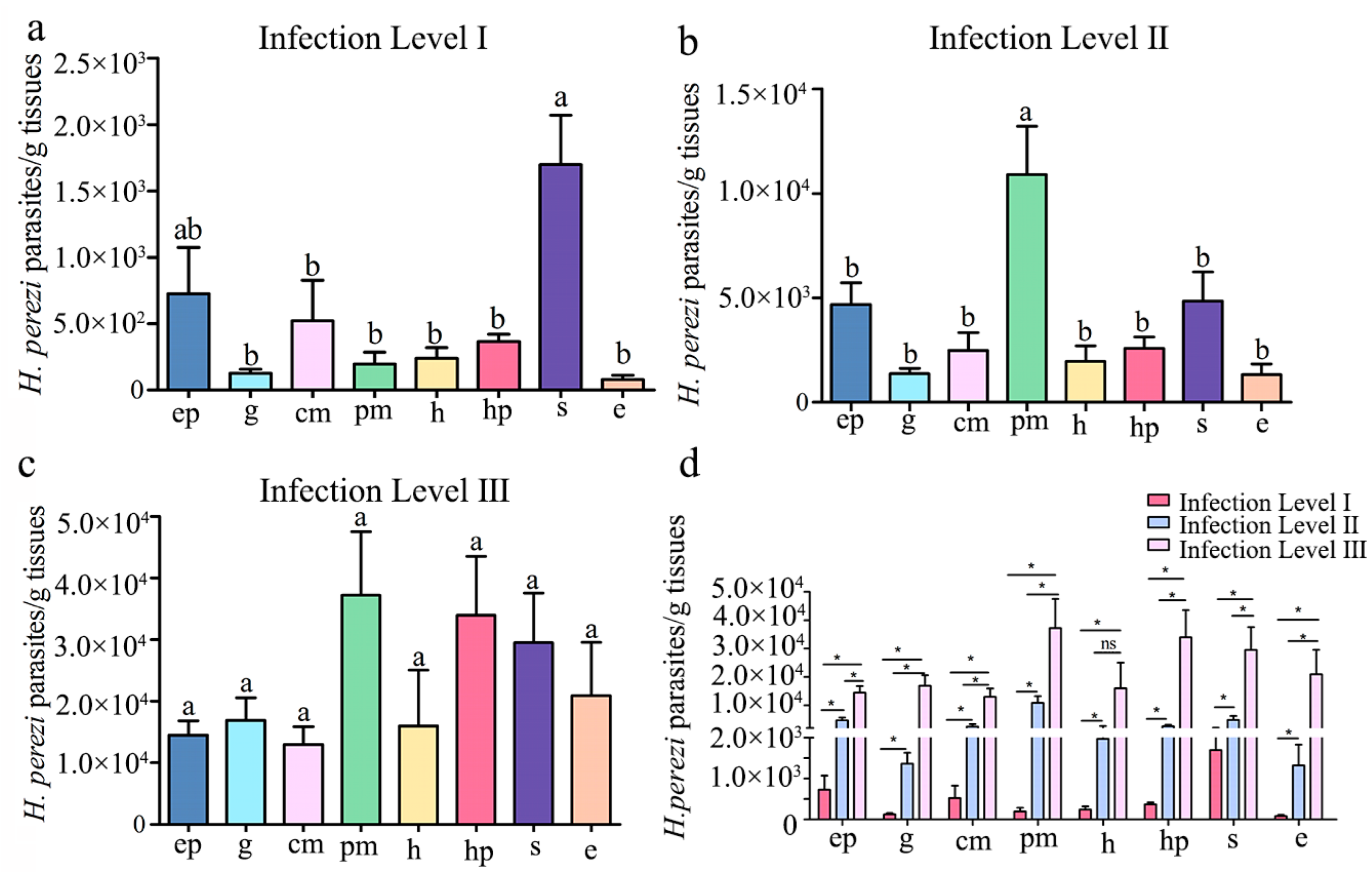
3.3. qPCR Assay of H. perezi Abundance in P. trituberculatus Tissues with Different Infection Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alimin, A.W.F.; Yusoff, N.A.H.; Kadriah, I.A.K.; Anshary, H.; Abdullah, F.; Jabir, N.; Susianingsih, E.; Hassan, M. Parasitic Dinoflagellate Hematodinium in Marine Decapod Crustaceans: A Review on Current Knowledge and Future Perspectives. Parasitol. Res. 2024, 123, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Huang, Q. The Parasitic Dinoflagellate Hematodinium Infects Marine Crustaceans. Mar. Life Sci. Technol. 2021, 3, 313–325. [Google Scholar] [CrossRef]
- Small, H.J. Advances in Our Understanding of the Global Diversity and Distribution of Hematodinium spp.—Significant Pathogens of Commercially Exploited Crustaceans. J. Invertebr. Pathol. 2012, 110, 234–246. [Google Scholar] [CrossRef]
- Chatton, É.; Poisson, R. Sur l’existence, Dans Le Sang Des Crabes, de Péridiniens Parasites: Hematodinium perezi n.g., n.sp. (Syndinidae). C. R. Seances Soc. Biol. Paris 1931, 105, 553–557. [Google Scholar]
- Hudson, D.A.; Shields, J.D. Hematodinium Australis n. sp., a Parasitic Dinoflagellate of the Sand Crab Portunus Pelagicus from Moreton Bay, Australia. Dis. Aquat. Organ. 1994, 19, 109–119. [Google Scholar] [CrossRef]
- Huang, Q. The Epizootic Pattern of the Marine Parasitic Dinoflagellate Hematodinium; Institute of Oceanology Chinese Academy of Sciences: Qingdao, China, 2022. [Google Scholar]
- Messick, G.A.; Shields, J.D. Epizootiology of the Parasitic Dinoflagellate Hematodinium sp. in the American Blue Crab Callinectes sapidus. Dis. Aquat. Organ. 2000, 43, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Shields, J.D. A Review of the Parasitic Dinoflagellates Hematodinium Species and Hematodinium-like Infections in Marine Crustaceans. Dis. Aquat. Organ. 2005, 66, 47–70. [Google Scholar] [CrossRef]
- Wheeler, K.; Shields, J.D.; Taylor, D.M. Pathology of Hematodinium Infections in Snow Crabs (Chionoecetes opilio) from Newfoundland, Canada. J. Invertebr. Pathol. 2007, 95, 93–100. [Google Scholar] [CrossRef]
- Messick, G.A. Hematodinium perezi Infections in Adult and Juvenile Blue Crabs Callinectes sapidus from Coastal Bays of Maryland and Virginia, USA. Dis. Aquat. Organ. 1994, 19, 77–82. [Google Scholar] [CrossRef]
- Molto-Martin, I.; Neil, D.M.; Coates, C.J.; MacKenzie, S.A.; Bass, D.; Stentiford, G.D.; Albalat, A. Infection of Norway Lobster (Nephrops norvegicus) by the Parasite Hematodinium sp.: Insights from 30 Years of Field Observations. R. Soc. Open Sci. 2024, 11, 231147. [Google Scholar] [CrossRef]
- Taylor, A.C.; Field, R.H.; Parslow-Williams, P.J. The Effects of Hematodinium sp.-Infection on Aspects of the Respiratory Physiology of the Norway Lobster, Nephrops norvegicus (L.). J. Exp. Mar. Biol. Ecol. 1996, 207, 217–228. [Google Scholar] [CrossRef]
- Field, R.H.; Chapman, C.J.; Taylor, A.C.; Neil, D.M.; Vickerman, K. Infection of the Norway Lobster Nephrops norvegicus by a Hematodinium-like Species of Dinoflagellate on the West Coast of Scotland. Dis. Aquat. Organ. 1992, 13, 1–15. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Feist, S.W.; Bateman, K.S.; Hine, P.M. Haemolymph Parasite of the Shore Crab Carcinus maenas: Pathology, Ultrastructure and Observations on Crustacean Haplosporidians. Dis. Aquat. Organ. 2004, 59, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Green, M.; Bateman, K.; Small, H.J.; Neil, D.M.; Feist, S.W. Infection by a Hematodinium-like Parasitic Dinoflagellate Causes Pink Crab Disease (PCD) in the Edible Crab Cancer pagurus. J. Invertebr. Pathol. 2002, 79, 179–191. [Google Scholar] [CrossRef]
- Smith, A.L.; Hirschle, L.; Vogan, C.L.; Rowley, A.F. Parasitization of Juvenile Edible Crabs (Cancer pagurus) by the Dinoflagellate, Hematodinium sp.: Pathobiology, Seasonality and Its Potential Effects on Commercial Fisheries. Parasitology 2015, 142, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Love, D.; Rice, S.; Moles, D.; Eaton, W. Seasonal Prevalence and Intensity of Bitter Crab Dinoflagellate Infection and Host Mortality in Alaskan Tanner Crabs Chionoecetes bairdi from Auke Bay, Alaska, USA. Dis. Aquat. Org. 1993, 15, 1–7. [Google Scholar] [CrossRef]
- Meyers, T.; Koeneman, T.; Botelho, C.; Short, S. Bitter Crab Disease: A Fatal Dinoflagellate Infection and Marketing Problem for Alaskan Tanner Crabs Chionoecetes bairdi. Dis. Aquat. Organ. 1987, 3, 195–216. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xia, X.A.; Wu, Q.Y.; Liu, W.H.; Lin, Y.S. Infection with Hematodinium sp. in Mud Crabs Scylla serrata Cultured in Low Salinity Water in Southern China. Dis. Aquat. Organ. 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, M.; Xiao, J.; Xu, W.J.; Li, C.W. Hematodinium spp. Infections in Wild and Cultured Populations of Marine Crustaceans along the Coast of China. Dis. Aquat. Organ. 2017, 124, 181–191. [Google Scholar] [CrossRef]
- Li, C.; Song, S.; Liu, Y.; Chen, T. Hematodinium Infections in Cultured Chinese Swimming Crab, Portunus trituberculatus, in Northern China. Aquaculture 2013, 396–399, 59–65. [Google Scholar] [CrossRef]
- Xu, W.; Shi, H.; Xu, H. Preliminary Study on the Hematodinium Infection in Cultured Portunus trituberculatus. Acta Hydrobiol. Sin. 2007, 31, 637–642. [Google Scholar]
- Bureau of Fisheries of Ministry of Agriculture and Rural Affairs of the P.R. China; National Fisheries Technology Ex-tension Center. China Society of Fisheries China Fishery Statistical Yearbook 2024; Bureau of Fisheries of Ministry of Agriculture and Rural Affairs of the P.R. China: Beijing, China, 2024. [Google Scholar]
- Lyu, X.Y.; Huang, Q.; Li, M.; Liu, W.X.; Li, C.W. Life Cycle of Marine Parasitic Dinoflagellate Hematodinium perezi and Effect of Temperature on Growth. Oceanol. Limnol. Sin. 2022, 53, 1430–1440. [Google Scholar] [CrossRef]
- Appleton, P.L.; Vickerman, K. In Vitro Cultivation and Developmental Cycle in Culture of a Parasitic Dinoflagellate (Hematodinium sp.) Associated with Mortality of the Norway Lobster (Nephrops norvegicus) in British Waters. Parasitology. 1998, 116, 115–130. [Google Scholar] [CrossRef]
- Li, C.; Miller, T.L.; Small, H.J.; Shields, J.D. In Vitro Culture and Developmental Cycle of the Parasitic Dinoflagellate Hematodinium sp. from the Blue Crab Callinectes sapidus. Parasitology 2011, 138, 1924–1934. [Google Scholar] [CrossRef]
- Gaudet, P.H.; Cawthorn, R.J.; Buote, M.A.; Morado, J.F.; Wright, G.M.; Greenwood, S.J. In Vitro Cultivation of Hematodinium sp. Isolated from Atlantic Snow Crab, Chionoecetes opilio: Partial Characterization of Late Developmental Stages. Parasitology. 2015, 142, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Li, C.W.; Li, M.; Song, S.Q. Morphology and histopathology of parasitic dinoflagellate Hematodinium sp. infecting Portunus trituberculatus. Oceanol. Limnol. Sin. 2015, 46, 748–757. [Google Scholar] [CrossRef]
- Eaton, W.D.; Love, D.C.; Botelho, C.; Meyers, T.R.; Imamura, K.; Koeneman, T. Preliminary Results on the Seasonality and Life Cycle of the Parasitic Dinoflagellate Causing Bitter Crab Disease in Alaskan Tanner Crabs (Chionoecetes bairdi). J. Invertebr. Pathol. 1991, 57, 426–434. [Google Scholar] [CrossRef]
- Small, H.J.; Shields, J.D.; Hudson, K.L.; Reece, K.S. Molecular Detection of Hematodinium sp. Infecting the Blue Crab, Callinectes sapidus. J. Shellfish Res. 2007, 26, 131–139. [Google Scholar] [CrossRef]
- Li, C.; Shields, J.D.; Miller, T.L.; Small, H.J.; Pagenkopp, K.M.; Reece, K.S. Detection and Quantification of the Free-Living Stage of the Parasitic Dinoflagellate Hematodinium sp. in Laboratory and Environmental Samples. Harmful Algae. 2010, 9, 515–521. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S.; Ghasemi, A.; Zahediasl, S.; Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Hamilton, K.M.; Morritt, D.; Shaw, P. Molecular and Histological Identification of the Crustacean Parasite Hematodinium sp (Alveolata, Syndinea) in the Shore Crab Carcinus maenas. Acta Protozool. 2007, 46, 183–192. [Google Scholar]
- Davies, C.E.; Batista, F.M.; Malkin, S.H.; Thomas, J.E.; Bryan, C.C.; Crocombe, P.; Coates, C.J.; Rowley, A.F. Spatial and Temporal Disease Dynamics of the Parasite Hematodinium sp. In Shore Crabs, Carcinus maenas. Parasites Vectors 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Reece, K.S.; Shields, J.D. Natural Transmission of Hematodinium Perezi in Juvenile Blue Crabs (Callinectes sapidus) in the Laboratory. J. Invertebr. Pathol. 2023, 198, 107918. [Google Scholar] [CrossRef]
- Huchin-Mian, J.P.; Small, H.J.; Shields, J.D. The Influence of Temperature and Salinity on Mortality of Recently Recruited Blue Crabs, Callinectes sapidus, Naturally Infected with Hematodinium perezi (Dinoflagellata). J. Invertebr. Pathol. 2018, 152, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, M.; Wang, F.; Song, S.; Li, C. Transmission Pattern of the Parasitic Dinoflagellate Hematodinium perezi in Polyculture Ponds of Coastal China. Aquaculture 2021, 538, 736549. [Google Scholar] [CrossRef]
- Huchin-Mian, J.P.; Small, H.J.; Shields, J.D. Patterns in the Natural Transmission of the Parasitic Dinoflagellate Hematodinium perezi in American Blue Crabs, Callinectes sapidus from a Highly Endemic Area. Mar. Biol. 2017, 164, 1–13. [Google Scholar] [CrossRef]
- Shields, J.D.; Huchin-Mian, J.P.; O’Leary, P.A.; Small, H.J. New Insight into the Transmission Dynamics of the Crustacean Pathogen Hematodinium perezi (Dinoflagellata) Using a Novel Sentinel Methodology. Mar. Ecol. Prog. Ser. 2017, 573, 73–84. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Stamelos, A.; Karagiannis, D. Histopathology and Phylogeny of the Dinoflagellate Hematodinium perezi and the Epibiotic Peritrich Ciliate Epistylis Sp. Infecting the Blue Crab Callinectes sapidus in the Eastern Mediterranean. Microorganisms 2024, 12, 456. [Google Scholar] [CrossRef]
- Sun, S.; Gu, Z.; Fu, H.; Zhu, J.; Ge, X.; Wu, X. Hypoxia Induces Changes in Amp-Activated Protein Kinase Activity and Energy Metabolism in Muscle Tissue of the Oriental River Prawn Macrobrachium Nipponense. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Štrus, J.; Žnidaršič, N.; Mrak, P.; Bogataj, U.; Vogt, G. Structure, Function and Development of the Digestive System in Malacostracan Crustaceans and Adaptation to Different Lifestyles. Cell Tissue Res. 2019, 377, 415–443. [Google Scholar] [CrossRef]
- Vogt, G. Functional Cytology of the Hepatopancreas of Decapod Crustaceans. J. Morphol. 2019, 280, 1405–1444. [Google Scholar] [CrossRef]
- Ceccaldi, H.J. A Synopsis of the Morphology and Physiology of the Digestive System of Some Crustacean Species Studied in France. Rev. Fish. Sci. 1998, 6, 13–39. [Google Scholar] [CrossRef]
- Ohiolei, J.A.; Yan, H.-B.; Odeniran, P.O.; Li, L.; Shumuye, N.A.; Qurishi, S.A.; Isaac, C.; Fu, B.Q.; Jia, W.Z. Echinococcus Granulosus Sensu Lato in Animal Intermediate Hosts: What Is with the Organ Location? Vet. Parasitol. 2022, 304, 109695. [Google Scholar] [CrossRef] [PubMed]
- Bauchau, A.G. Crustaceans. In Invertebrate Blood Cells; Arthropods to Urochordates, Invertebrates and Vertebrates Compared; Ratcliffe, N.A., Rowley, A.F., Eds.; Academic Press: London, UK, 1997; Volume 2. [Google Scholar]
- Wahyul, A.; Alimin, F.; Asma, N.; Yusoff, H.; Ayu, I.; Kadriah, K. Hemolymph as a Biomarker to Access the Health of Decapod Crustaceans: A Review. Int. Aquat. Res. 2024, 16, 327–342. [Google Scholar] [CrossRef]
- Chen, F.; Wang, K. Characterization of the Innate Immunity in the Mud Crab Scylla paramamosain. Fish Shellfish Immunol. 2019, 93, 436–448. [Google Scholar] [CrossRef]
- Vazquez, L.; Alpuche, J.; Maldonado, G.; Agundis, C.; Pereyra-Morales, A. Immunity Mechanisms in Crustaceans. Angew. Chemie Int. 2009, 15, 179–188. [Google Scholar] [CrossRef]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-Mediated Immunity in Arthropods: Hematopoiesis, Coagulation, Melanization and Opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef]
- Johansson, M.W.; Keyser, P.; Sritunyalucksana, K.; Söderhäll, K. Crustacean Haemocytes and Haematopoiesis. Aquaculture 2000, 191, 45–52. [Google Scholar] [CrossRef]
- Conneely, E.A.; Coates, C.J. Haematological Deterioration of Hematodinium-Infected Decapod Crustaceans. Dev. Comp. Immunol. 2025, 163, 105307. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Wang, J.; Song, S. Immune Response and Gene Expression in Hemocytes of Portunus trituberculatus Inoculated with the Parasitic Dinoflagellate Hematodinium. Mol. Immunol. 2015, 65, 113–122. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Song, S.; Li, C. Early Transcriptional Response to the Parasitic Dinoflagellate Hematodinium in Hepatopancreas of Portunus trituberculatus. J. Invertebr. Pathol. 2015, 130, 28–36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, M.; Huang, Q.; Hu, L.; Xue, Q.; Wang, J.; Li, C. Tissue Distribution and Abundance of the Parasitic Dinoflagellate Hematodinium perezi in Naturally Infected Portunus trituberculatus. Pathogens 2025, 14, 650. https://doi.org/10.3390/pathogens14070650
Zhang J, Li M, Huang Q, Hu L, Xue Q, Wang J, Li C. Tissue Distribution and Abundance of the Parasitic Dinoflagellate Hematodinium perezi in Naturally Infected Portunus trituberculatus. Pathogens. 2025; 14(7):650. https://doi.org/10.3390/pathogens14070650
Chicago/Turabian StyleZhang, Ju, Meng Li, Qian Huang, Lijun Hu, Qi Xue, Jiayi Wang, and Caiwen Li. 2025. "Tissue Distribution and Abundance of the Parasitic Dinoflagellate Hematodinium perezi in Naturally Infected Portunus trituberculatus" Pathogens 14, no. 7: 650. https://doi.org/10.3390/pathogens14070650
APA StyleZhang, J., Li, M., Huang, Q., Hu, L., Xue, Q., Wang, J., & Li, C. (2025). Tissue Distribution and Abundance of the Parasitic Dinoflagellate Hematodinium perezi in Naturally Infected Portunus trituberculatus. Pathogens, 14(7), 650. https://doi.org/10.3390/pathogens14070650






