Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation
Abstract
1. Introduction
2. Current Treatment Strategies for Helicobacter pylori
- (I)
- Proton pump inhibitors (PPIs) lead to more alkaline pH in the gastric mucosa, necessary to obtain optimal bioavailability of the acid-susceptible antibiotics, by inhibiting the gastric acid pump (H+/K+-ATPase). Moreover, PPIs can affect H. pylori growth by directly inhibiting it, thereby supporting its eradication [20]. Several proton pump inhibitors are available; rabeprazole or esomeprazole 20 to 40 mg twice daily is recommended. Rabeprazole is preferred because it undergoes primarily non-enzymatic metabolism, with minimal involvement of the genetically variable enzyme CYP2C19. This results in more consistent acid suppression that is less affected by patients’ genetic differences [20].
- (II)
- Bismuth subsalicylates are part of some treatment regimens due to their anti-inflammatory and bactericidal properties [21]. However, bismuth mechanism’s of action on H. pylori is still not fully understood, notwithstanding research suggesting that bismuth can cause several abnormalities in bacterial cells by inhibition of enzymes, i.e., urease, catalase, and lipase, binding to the bacterial wall and periplasmic space and causing bacterial cell damage and inhibiting adherence to the surface of epithelial cells. Moreover, bismuth subsalicylates show advantageous gastroduodenal effects, for example, by protecting gastric mucous from peptic luminal degradation [22].
3. Difficulties in H. pylori Treatment
4. Antibiotic Resistance in Helicobacter pylori
5. Primary Pathways of Antibiotic Resistance in H. pylori
5.1. Genetic Mutations
5.2. Efflux Pumps
5.3. Enzymatic Degradation
5.4. Alteration of the Antibiotic Target Sites
| Antibiotic | Resistance Mechanism | Involved Genes/Mechanisms |
|---|---|---|
| Amoxicillin | Decreased affinity of penicillin-binding proteins (PBPs) | Mutations in PBP1 or PBP2 |
| Overexpression of efflux pumps | hp1165, hefA | |
| Tetracycline | Modification of ribosomal binding site | Mutations in 16S rRNA |
| Overexpression of efflux pumps | hp1165, hefA | |
| Rifamycins | Mutations in the β subunit of RNA polymerase | Mutations in rpoB (codons 525–586) |
| Levofloxacin | Mutations in DNA gyrase (QRDR region) | Mutations in gyrA |
| Mutations in gyrB | Less common than gyrA, but may enhance resistance | |
| Overexpression of efflux pumps | hp1165, hefA | |
| Metronidazole | Loss of function of reducing enzymes | Mutations in rdxA (stop codons, deletions) |
| Mutations in frxA (NADPH-dependent reductase) | Stop codons, deletions | |
| Overexpression of efflux pumps | hp1165, hefA | |
| Clarithromycin | Modification of ribosomal binding site | Mutations in 23S rRNA, mainly A2143G, A2144G, A2142G, A2142C |
| Overexpression of efflux pumps | hp1165, hefA |
6. Mechanism of Antibiotic Resistance in H. pylori
6.1. Resistance to Clarithromycin
6.2. Resistance to Metronidazole
6.3. Resistance to Amoxicillin
6.4. Resistance to Levofloxacin
6.5. Resistance to Tetracycline
6.6. Resistance to Rifamycins
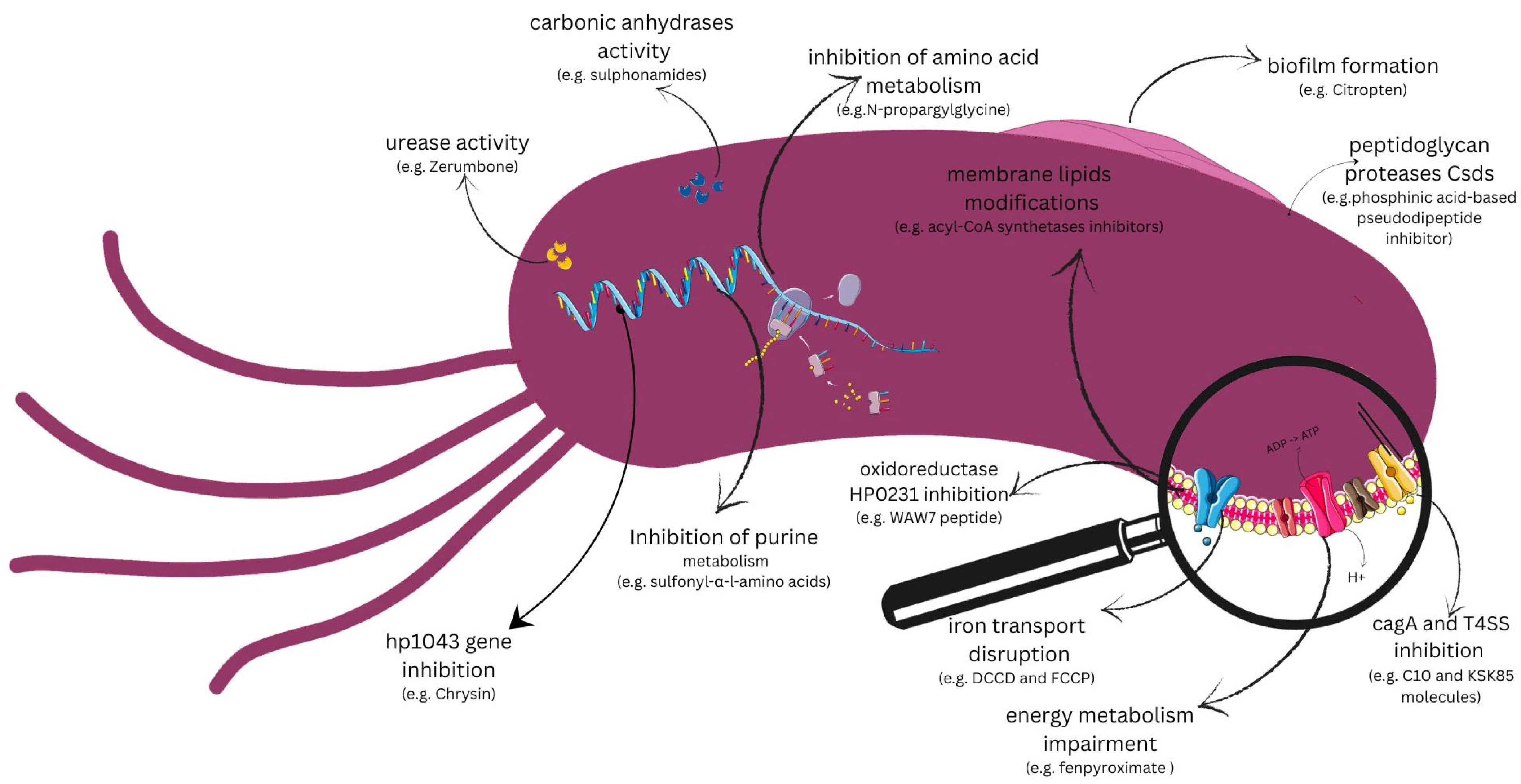
7. Potential New Drug Targets for Helicobacter pylori
7.1. Targets Pointed to Virulence Factors
7.1.1. Urease
7.1.2. Carbonic Anhydrases
7.1.3. Biofilm Formation
7.1.4. hp1043 Gene
7.1.5. Peptidoglycan Proteases
7.1.6. CagA Toxin and Type IV Secretion System (T4SS)
7.1.7. HP0231 Oxidoreductase
7.2. Targets Pointed out on Metabolic Pathways
7.2.1. Energy Metabolism in H. pylori
7.2.2. Inhibition of Amino Acid Metabolism
7.2.3. Fatty Acid Metabolism
7.2.4. Purine Metabolism
7.2.5. Metabolic Pathways of Iron in H. pylori
8. Vaccine Against Helicobacter pylori
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Öztekin, M.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Overview of Helicobacter pylori Infection: Clinical Features, Treatment, and Nutritional Aspects. Diseases 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori Infection and the Risk of Gastric Carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef]
- Gravina, A.G.; Zagari, R.M.; Musis, C.D.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and Extragastric Diseases: A Review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Santos, M.L.C.; Brito, B.B.D.; Silva, F.A.F.D.; Sampaio, M.M.; Marques, H.S.; Silva, N.O.E.; Queiroz, D.M.D.M.; Melo, F.F.D. Helicobacter pylori Infection: Beyond Gastric Manifestations. World J. Gastroenterol. 2020, 26, 4076–4093. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and Its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori Outer Membrane Proteins: An Updated Review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-Date Overview on the Virulence and Pathogenesis Mechanisms. Braz. J. Microbiol. 2022, 53, 33–50. [Google Scholar] [CrossRef]
- Davis, G.S.; Mobley, H.L.T. Contribution of dppA to Urease Activity in Helicobacter pylori 26695. Helicobacter 2005, 10, 416–423. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; He, W.; Li, X. Efficacy and Safety of Bismuth Quadruple Regimens Containing Tetracycline or Furazolidone for Initial Eradication of Helicobacter pylori. Medicine 2021, 100, e28323. [Google Scholar] [CrossRef]
- Chuah, S.-K.; Tai, W.-C.; Lee, C.-H.; Liang, C.-M.; Hu, T.-H. Quinolone-Containing Therapies in the Eradication of Helicobacter pylori. BioMed Res. Int. 2014, 2014, 151543. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef]
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone Antibiotics and Adverse Events. Aust. Prescr. 2021, 44, 161–164. [Google Scholar] [CrossRef]
- Weir, C.B.; Le, J.K. Metronidazole. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Roberts, L.T.; Issa, P.P.; Sinnathamby, E.S.; Granier, M.; Mayeux, H.; Eubanks, T.N.; Malone, K.; Ahmadzadeh, S.; Cornett, E.M.; Shekoohi, S.; et al. Helicobacter pylori: A Review of Current Treatment Options in Clinical Practice. Life 2022, 12, 2038. [Google Scholar] [CrossRef]
- Lambert, J.R.; Midolo, P. The Actions of Bismuth in the Treatment of Helicobacter pylori Infection. Aliment. Pharmacol. Ther. 1997, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; Van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.H. Pylori Virulence Factors: Genetic Polymorphism and Disease. In Helicobacter pylori; Kim, N., Ed.; Springer Nature: Singapore, 2023; pp. 103–120. ISBN 978-981-97-0012-7. [Google Scholar]
- Yamaoka, Y.; Saruuljavkhlan, B.; Alfaray, R.I.; Linz, B. Pathogenomics of Helicobacter pylori. In Helicobacter pylori and Gastric Cancer; Backert, S., Ed.; Current Topics in Microbiology and Immunology; Springer Nature: Cham, Switzerland, 2023; Volume 444, pp. 117–155. ISBN 978-3-031-47330-2. [Google Scholar]
- González, I.; González, L.; Rojas, A.; Rodríguez, B.L.; Romero, J.; Reyes, O.; Morales, E.; Alonso, J.; Castro, R.P.; Sabatier, C.A.; et al. Pathogenic Potential of Helicobacter pylori Strains Can Explain Differences in H. Pylori Associated Diseases Rates from Chile and Cuba. Bangladesh J. Med. Sci. 2019, 18, 577–585. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S.; Safaralizadeh, R. Helicobacter pylori -related Risk Predictors of Gastric Cancer: The Latest Models, Challenges, and Future Prospects. Cancer Med. 2020, 9, 4808–4822. [Google Scholar] [CrossRef]
- Ita-Balta, Y.; Zegarra-Adanaque, A.; Sanchez-Guillen, J.; Farfán-Delgado, M.; Ortiz-Castro, C.; Murillo Carrasco, A.G.; Miranda Pinto, A.; Manrique-Sam, C. Molecular Detection and Clinical Impact of Helicobacter pylori Virulence Genes in Gastric Diseases: A Study in Arequipa, Peru. Biomedicines 2025, 13, 914. [Google Scholar] [CrossRef]
- Celiberto, F.; Losurdo, G.; Pricci, M.; Girardi, B.; Marotti, A.; Di Leo, A.; Ierardi, E. The State of the Art of Molecular Fecal Investigations for Helicobacter pylori (H. Pylori) Antibiotic Resistances. Int. J. Mol. Sci. 2023, 24, 4361. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori Treatment in the Post-Antibiotics Era—Searching for New Drug Targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Salahi-Niri, A.; Nabavi-Rad, A.; Monaghan, T.M.; Rokkas, T.; Doulberis, M.; Sadeghi, A.; Zali, M.R.; Yamaoka, Y.; Tacconelli, E.; Yadegar, A. Global Prevalence of Helicobacter pylori Antibiotic Resistance among Children in the World Health Organization Regions between 2000 and 2023: A Systematic Review and Meta-Analysis. BMC Med. 2024, 22, 598. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, T.K.T.; Au, N.H.; Phan, B.H. Primary Antibiotic Resistance of Helicobacter pylori in Peptic Ulcer Disease Patients. Vietnam. Med. J. 2024, 544, 158–168. [Google Scholar] [CrossRef]
- Chesca, A.A.; Seysenbekova, A.A.; Aliona, L.L.; Laryushina, Y.Y.; Ayatbek, O.O.; Volnovaya, O.O.; Solomadin, M.M.; Yukhnevich, Y.Y. Antibiotic Resistance of Helicobacter pylori: Data from Central Asia. 2024, 2024091357. [Google Scholar] [CrossRef]
- Cabrera, C.; Torres, J.; Serrano, C.A.; Gallardo, P.; Orellana, V.; George, S.; O’Ryan, M.; Lucero, Y. Antimicrobial Resistance of Helicobacter pylori Isolated From Latin American Children and Adolescents (2008–2023): A Systematic Review. Helicobacter 2024, 29, e13101. [Google Scholar] [CrossRef] [PubMed]
- Kouhsari, E.; Roshandel, G.; Hosseinzadeh, S.; Besharat, S.; Khori, V.; Amiriani, T. Molecular Characterization of Antimicrobial Resistance and Virulence Genotyping among Helicobacter pylori-Positive Dyspeptic Patients in North Iran. Infect. Disord. Drug Targets 2025, 25, e090724231788. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaily, A.Y.; Al-Jubori, S.S. Mechanisms of Helicobacter pylori Resistance to Antibiotics. Diyala J. Med. 2024, 26, 163–173. [Google Scholar] [CrossRef]
- Haumaier, F.; Schneider-Fuchs, A.; Backert, S.; Vieth, M.; Sterlacci, W.; Wöhrl, B.M. Rapid Detection of Quinolone Resistance Mutations in gyrA of Helicobacter pylori by Real-Time PCR. Pathogens 2022, 11, 59. [Google Scholar] [CrossRef]
- Lee, S.M. Metronidazole. In Helicobacter pylori; Kim, N., Ed.; Springer Nature: Singapore, 2023; pp. 517–526. ISBN 978-981-97-0012-7. [Google Scholar]
- Krzyżek, P. Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. Int. J. Mol. Sci. 2024, 25, 12222. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; An, Y.; Li, Z.; Liu, D.; Yong, X. The Crosstalk between Efflux Pump and Resistance Gene Mutation in Helicobacter pylori. Gut Microbes 2024, 16, 2379439. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Ge, F.; Sun, C.; Deng, Z.; Yao, W.; He, X. Functional Determination of Site-Mutations in rdxA Involved in Metronidazole Resistance of Helicobacter pylori. Front. Cell Dev. Biol. 2024, 12, 1435064. [Google Scholar] [CrossRef]
- Thorell, K.; Muñoz-Ramírez, Z.Y.; Wang, D.; Sandoval-Motta, S.; Boscolo Agostini, R.; Ghirotto, S.; Torres, R.C.; Falush, D.; Camargo, M.C.; Rabkin, C.S. The Helicobacter pylori Genome Project: Insights into H. pylori population structure from analysis of a worldwide collection of complete genomes. Nat. Commun. 2023, 14, 8184. [Google Scholar] [CrossRef]
- Attaran, B.; Salehi, N.; Ghadiri, B.; Esmaeili, M.; Kalateh, S.; Tashakoripour, M.; Eshagh Hosseini, M.; Mohammadi, M. The Penicillin Binding Protein 1A of Helicobacter pylori, Its Amoxicillin Binding Site and Access Routes. Gut Pathog. 2021, 13, 43. [Google Scholar] [CrossRef]
- Graham, D.Y. Crises in Antimicrobial Stewardship: Misuse of Clarithromycin for Helicobacter pylori Therapy. Pharmacoepidemiology 2024, 3, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Chen, M.-Y.; Chen, J.; Zhang, X.-H.; Feng, Y.; Han, Y.-X.; Li, Y.-L. Success of Susceptibility-Guided Eradication of Helicobacter pylori in a Region with High Secondary Clarithromycin and Levofloxacin Resistance Rates. World J. Gastroenterol. 2024, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ayaş, M.; Oktem-Okullu, S.; Özcan, O.; Kocagöz, T.; Gürol, Y. Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori. Antibiotics 2024, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Khyriem, A.B.; Lynrah, K.G.; Marbaniang, E.; Topno, N. Antimicrobial Resistance Pattern of Helicobacter pylori in Patients Evaluated for Dyspeptic Symptoms in North-Eastern India with Focus on Detection of Clarithromycin Resistance Conferring Point Mutations A2143G and A2142G within Bacterial 23S rRNA Gene. Indian J. Med. Microbiol. 2024, 50, 100652. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 Years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Graham, D.Y.; Rokkas, T. Overcoming the Effects of Increasing Antimicrobial Resistance on Helicobacter pylori Therapy. Expert Rev. Gastroenterol. Hepatol. 2024, 18, 705–711. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Jafari, B.; Khaneshpour, H.; Sadeghi-Deylamdeh, Z.; Shariat, A.; Bannazadeh-Baghi, H.; Nasiri, R. Helicobacter pylori: A Systematic Review of Drug Resistance in Iran. Rev. Res. Med. Microbiol. 2024, 35, 36–41. [Google Scholar] [CrossRef]
- Okuda, M.; Lin, Y.; Wang, C.; Kakiuchi, T.; Kikuchi, S. Metronidazole for Helicobacter pylori Eradication Therapy among Children and Adolescents in Japan: Overcoming Controversies and Concerns. Helicobacter 2019, 24, e12575. [Google Scholar] [CrossRef]
- Su, X.; Deng, Y.; Chen, X.; Li, Y.; Hao, Q.; Tang, Y.; Mu, R.; Wu, Y.; Zhou, Y.; Hu, S. Effect of an Individualized Bismuth Quadruple Regimen Guided by 10-Day or 14-Day Antibiotic Susceptibility Testing for First-Line Eradication Treatment of Helicobacter pylori in Ningxia, China. Front. Med. 2025, 11, 1510376. [Google Scholar] [CrossRef]
- Yeh, J.-A.; Huang, H.-K.; Chou, A.-L.; Lin, H.-J.; Feng, C.-L.; Kuo, C.-J.; Lai, C.-H. Helicobacter pylori Eradication with High-Dose Proton Pump Inhibitor-Amoxicillin Dual Therapy: A Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2024, 63, 107159. [Google Scholar] [CrossRef]
- Cimuanga-Mukanya, A.; Tshibangu-Kabamba, E.; Kisoko, P.D.J.N.; Fauzia, K.A.; Tshibangu, F.M.; Wola, A.T.; Kashala, P.T.; Ngoyi, D.M.; Ahuka-Mundeke, S.; Revathi, G.; et al. Synergistic Effects of Novel Penicillin-Binding Protein 1A Amino Acid Substitutions Contribute to High-Level Amoxicillin Resistance of Helicobacter pylori. mSphere 2024, 9, e00089-24. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.K.; Chua, K.H.; Kee, B.P.; Chuah, K.H.; Por, L.Y.; Puah, S.M. Genetic Variations of Penicillin-Binding Protein 1A: Insights into the Current Status of Amoxicillin-Based Regimens for Helicobacter pylori Eradication in Malaysia. J. Med. Microbiol. 2024, 73, 001832. [Google Scholar] [CrossRef] [PubMed]
- López-Vidal, Y.; Calva Mercado, J.J.; Amieva-Fernández, R.I.; Majalca-Martínez, C.; Ávila-Vargas, G.; Castillo-Rojas, G. Stable Trend of Antimicrobial Susceptibility Profiles of Helicobacter pylori Isolated from Patients during a 12-Year Period in Mexico. Preprints 2024, 2024071395. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H.; et al. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef]
- Thompson, D.; Xu, J.; Ischia, J.; Bolton, D. Fluoroquinolone Resistance in Urinary Tract Infections: Epidemiology, Mechanisms of Action and Management Strategies. BJUI Compass 2024, 5, 5–11. [Google Scholar] [CrossRef]
- López-Gasca, M.; Peña, J.; García-Amado, M.-A.; Michelangeli, F.; Contreras, M. Point Mutations at gyrA and gyrB Genes of Levofloxacin-Resistant Helicobacter pylori Isolates in the Esophageal Mucosa from a Venezuelan Population. Am. J. Trop. Med. Hyg. 2018, 98, 1051–1055. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Y.; Yan, J.; Ye, G. Antibiotic Resistance in Helicobacter pylori: From Potential Biomolecular Mechanisms to Clinical Practice. Clin. Lab. Anal. 2023, 37, e24885. [Google Scholar] [CrossRef]
- Srisuphanunt, M.; Wilairatana, P.; Kooltheat, N.; Duangchan, T.; Katzenmeier, G.; Rose, J.B. Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Trop. Med. Infect. Dis. 2023, 8, 163. [Google Scholar] [CrossRef]
- Alvarez-Aldana, A.; Fernandez Uribe, P.A.; Mejía Valencia, T.; Guaca-Gonzalez, Y.M.; Santacruz-Ibarra, J.J.; Arturo-Arias, B.L.; Castañeda-Chavez, L.J.; Pacheco-López, R.; Londoño-Giraldo, L.M.; Moncayo-Ortiz, J.I. Antimicrobial susceptibility of clinical Helicobacter pylori isolates and its eradication by standard triple therapy: A study in west central region of Colombia. Microbiol Spectr. 2024, 12, e00401-24. [Google Scholar] [CrossRef]
- Milivojević, V.; Krstić, M.; Medić-Brkić, B. Influence of Antibiotic Resistance in the Treatment of Helicobacter pylori Infection. Med. Podml. 2023, 74, 7–11. [Google Scholar] [CrossRef]
- Losurdo, G.; Ierardi, E.; Di Leo, A. Helicobacter pylori Antibiotic Resistance: Stewardship, Tailored Therapies, and Future Perspectives. Gastroenterology 2021, 161, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Narimani, T.; Tabesh, E.; Shafiee, F.; Soltani, R. Antibiotic Resistance Pattern of Helicobacter pylori Strains Isolated from Patients in Isfahan, Iran. J. Res. Med. Sci. 2022, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-R.; Wang, X.-J.; Zhu, M.-J.; Chen, A.-L.; Zhang, D.; Du, Q.; Kim, J.J.; Hu, W.-L. Efficacy and Safety of Low-Dose Tetracycline, Amoxicillin Quadruple Therapy in Helicobacter pylori Infection: A Retrospective Single Center Study. World J. Gastroenterol. 2024, 30, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Benejat, L.; Mujica, H.; Peña, J.; García-Amado, M.-A.; Michelangeli, F.; Lehours, P. Real-Time PCR Detection of a 16S rRNA Single Mutation of Helicobacter pylori Isolates Associated with Reduced Susceptibility and Resistance to Tetracycline in the Gastroesophageal Mucosa of Individual Hosts. J. Med. Microbiol. 2019, 68, 1287–1291. [Google Scholar] [CrossRef]
- Hamouche, L.; Poljak, L.; Carpousis, A.J. Ribosomal RNA Degradation Induced by the Bacterial RNA Polymerase Inhibitor Rifampicin. RNA 2021, 27, 946–958. [Google Scholar] [CrossRef]
- Gugnani, J.S.; Abhishek, F.; Agarwal, Y.; Damera, A.R.; Kaur, H.; Taleb, B.; Mane, R.; Soni, U.; Nayar, K.D. Effectiveness of Rifabutin-Based Regimens in Treating Helicobacter pylori Infections. Cureus 2023, 15, e50541. [Google Scholar] [CrossRef]
- Hays, C.; Burucoa, C.; Lehours, P.; Tran, C.T.; Leleu, A.; Raymond, J. Molecular Characterization of Helicobacter pylori Resistance to Rifamycins. Helicobacter 2018, 23, e12451. [Google Scholar] [CrossRef]
- Uthansingh, K.; Kumari, R.; Pati, G.K.; Behera, M.K.; Sahu, M.C.; Narayan, J.; Patnaik, S.K.; Mallick, P.; Sahu, M.K. Molecular Docking of Anti Helicobacter pylori Antibiotics and Proton Pump Inhibitor: A Single Center Survey. J. Pure Appl. Microbiol. 2021, 15, 2103–2116. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors—Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Campanale, M.; Nucera, E.; Ojetti, V.; Cesario, V.; Di Rienzo, T.A.; D’Angelo, G.; Pecere, S.; Barbaro, F.; Gigante, G.; De Pasquale, T.; et al. Nickel Free-Diet Enhances the Helicobacter pylori Eradication Rate: A Pilot Study. Dig. Dis. Sci. 2014, 59, 1851–1855. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Benoit, S.L.; Misra, S.K.; Sharp, J.S.; Maier, R.J. Noncatalytic Antioxidant Role for Helicobacter pylori Urease. J. Bacteriol. 2018, 200, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Yang, J.Y.; Lee, P.; Kim, J.-B.; Kim, S.-H. Zerumbone Inhibits Helicobacter pylori Urease Activity. Molecules 2021, 26, 2663. [Google Scholar] [CrossRef] [PubMed]
- Maślanka, M.; Tabor, W.; Krzyżek, P.; Grabowiecka, A.; Berlicki, Ł.; Mucha, A. Inhibitory Activity of Catecholic Phosphonic and Phosphinic Acids against Helicobacter pylori Ureolysis. Eur. J. Med. Chem. 2023, 257, 115528. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Hsieh, P.-L.; Tsai, G.-J. Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells. Mar. Drugs 2020, 18, 542. [Google Scholar] [CrossRef]
- Supuran, C.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites 2017, 7, 56. [Google Scholar] [CrossRef]
- Bury-Moné, S.; Mendz, G.L.; Ball, G.E.; Thibonnier, M.; Stingl, K.; Ecobichon, C.; Avé, P.; Huerre, M.; Labigne, A.; Thiberge, J.-M.; et al. Roles of α and β Carbonic Anhydrases of Helicobacter pylori in the Urease-Dependent Response to Acidity and in Colonization of the Murine Gastric Mucosa. Infect. Immun. 2008, 76, 497–509. [Google Scholar] [CrossRef]
- Modakh, J.K.; Liu, Y.C.; Machuca, M.A.; Supuran, C.T.; Roujeinikova, A. Structural Basis for the Inhibition of Helicobacter pylori α-Carbonic Anhydrase by Sulfonamides. PLoS ONE 2015, 10, e0127149. [Google Scholar] [CrossRef]
- Modak, J.K.; Tikhomirova, A.; Gorrell, R.J.; Rahman, M.M.; Kotsanas, D.; Korman, T.M.; Garcia-Bustos, J.; Kwok, T.; Ferrero, R.L.; Supuran, C.T.; et al. Anti- Helicobacter pylori Activity of Ethoxzolamide. J. Enzym. Inhib. Med. Chem. 2019, 34, 1660–1667. [Google Scholar] [CrossRef]
- Angeli, A.; Ferraroni, M.; Supuran, C.T. Famotidine, an Antiulcer Agent, Strongly Inhibits Helicobacter pylori and Human Carbonic Anhydrases. ACS Med. Chem. Lett. 2018, 9, 1035–1038. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. BioMed Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, K.A.; Effendi, W.I.; Alfaray, R.I.; Malaty, H.M.; Yamaoka, Y.; Mifthussurur, M. Molecular Mechanisms of Biofilm Formation in Helicobacter pylori. Antibiotics 2024, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Saravanan, V.; Mahendran, M.I.M.S.; Kumar, M.P.N. Helicobacter pylori Biofilm Interference by N-Acyl Homoserine Lactonases: In Vitro and in Silico Approaches. Mol. Biol. Rep. 2024, 51, 1106. [Google Scholar] [CrossRef]
- Rader, B.A.; Campagna, S.R.; Semmelhack, M.F.; Bassler, B.L.; Guillemin, K. The Quorum-Sensing Molecule Autoinducer 2 Regulates Motility and Flagellar Morphogenesis in Helicobacter pylori. J. Bacteriol. 2007, 189, 6109–6117. [Google Scholar] [CrossRef]
- Cammarota, G.; Branca, G.; Ardito, F.; Sanguinetti, M.; Ianiro, G.; Cianci, R.; Torelli, R.; Masala, G.; Gasbarrini, A.; Fadda, G.; et al. Biofilm Demolition and Antibiotic Treatment to Eradicate Resistant Helicobacter pylori: A Clinical Trial. Clin. Gastroenterol. Hepatol. 2010, 8, 817–820.e3. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Ibrahim, M.H.; Abdel-Aziz, M.M.; Ghareeb, M.A. Anti-Helicobacter pylori, Anti-Biofilm Activity, and Molecular Docking Study of Citropten, Bergapten, and Its Positional Isomer Isolated from Citrus sinensis L. Leaves. Heliyon 2024, 10, e25232. [Google Scholar] [CrossRef]
- Olekhnovich, I.N.; Vitko, S.; Valliere, M.; Hoffman, P.S. Response to Metronidazole and Oxidative Stress Is Mediated through Homeostatic Regulator HsrA (HP1043) in Helicobacter pylori. J. Bacteriol. 2014, 196, 729–739. [Google Scholar] [CrossRef]
- González, A.; Salillas, S.; Velázquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, Á. Identifying Potential Novel Drugs against Helicobacter pylori by Targeting the Essential Response Regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.-B.; Kim, S.-H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Liu, Y.; Frirdich, E.; Taylor, J.A.; Chan, A.C.K.; Blair, K.M.; Vermeulen, J.; Ha, R.; Murphy, M.E.P.; Salama, N.R.; Gaynor, E.C.; et al. A Bacterial Cell Shape-Determining Inhibitor. ACS Chem. Biol. 2016, 11, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Hilleringmann, M.; Pansegrau, W.; Doyle, M.; Kaufman, S.; MacKichan, M.L.; Gianfaldoni, C.; Ruggiero, P.; Covacci, A. Inhibitors of Helicobacter pylori ATPase Cagα Block CagA Transport and Cag Virulence. Microbiology 2006, 152, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.L.; Good, J.A.D.; Kumar, S.; Krishnan, K.S.; Gaddy, J.A.; Loh, J.T.; Chappell, J.; Almqvist, F.; Cover, T.L.; Hadjifrangiskou, M. Peptidomimetic Small Molecules Disrupt Type IV Secretion System Activity in Diverse Bacterial Pathogens. mBio 2016, 7, e00221-16. [Google Scholar] [CrossRef] [PubMed]
- Roszczenko-Jasińska, P.; Giełdoń, A.; Mazur, D.; Spodzieja, M.; Plichta, M.; Czaplewski, C.; Bal, W.; Jagusztyn-Krynicka, E.K.; Bartosik, D. Exploring the Inhibitory Potential of in Silico-Designed Small Peptides on Helicobacter pylori Hp0231 (DsbK), a Periplasmic Oxidoreductase Involved in Disulfide Bond Formation. Front. Mol. Biosci. 2024, 10, 1335704. [Google Scholar] [CrossRef]
- Lettl, C.; Schindele, F.; Mehdipour, A.R.; Steiner, T.; Ring, D.; Brack-Werner, R.; Stecher, B.; Eisenreich, W.; Bilitewski, U.; Hummer, G.; et al. Selective Killing of the Human Gastric Pathogen Helicobacter pylori by Mitochondrial Respiratory Complex I Inhibitors. Cell Chem. Biol. 2023, 30, 499–512.e5. [Google Scholar] [CrossRef]
- Huang, T.-T.; Liu, Y.-N.; Huang, J.-X.; Yan, P.-P.; Wang, J.-J.; Cao, Y.-X.; Cao, L. Sodium Sulfite-Driven Helicobacter pylori Eradication: Unraveling Oxygen Dynamics through Multi-Omics Investigation. Biochem. Pharmacol. 2024, 222, 116055. [Google Scholar] [CrossRef]
- Chen, M.; Andersen, L.P.; Zhai, L.; Kharazmi, A. Characterization of the Respiratory Chain of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 1999, 24, 169–174. [Google Scholar] [CrossRef]
- Chaturvedi, R.; de Sablet, T.; Coburn, L.A.; Gobert, A.P.; Wilson, K.T. Arginine and Polyamines in Helicobacter pylori-Induced Immune Dysregulation and Gastric Carcinogenesis. Amino Acids 2012, 42, 627–640. [Google Scholar] [CrossRef]
- Rosli, N.A.; Al-Maleki, A.R.; Loke, M.F.; Tay, S.T.; Rofiee, M.S.; Teh, L.K.; Salleh, M.Z.; Vadivelu, J. Exposure of Helicobacter pylori to Clarithromycin in Vitro Resulting in the Development of Resistance and Triggers Metabolic Reprogramming Associated with Virulence and Pathogenicity. PLoS ONE 2024, 19, e0298434. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline Metabolism and Redox; Maintaining a Balance in Health and Disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Rivera-Ordaz, A.; Bracher, S.; Sarrach, S.; Li, Z.; Shi, L.; Quick, M.; Hilger, D.; Haas, R.; Jung, H. The Sodium/Proline Transporter PutP of Helicobacter pylori. PLoS ONE 2013, 8, e83576. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.J.; Ji, J.; Bogner, A.N.; Scott, G.K.; Patel, S.M.; Seravalli, J.; Gates, K.S.; Benz, C.C.; Becker, D.F. Noncovalent Inhibition and Covalent Inactivation of Proline Dehydrogenase by Analogs of N-Propargylglycine. Biochemistry 2024, 63, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- McClain, M.S.; Boeglin, W.E.; Algood, H.M.S.; Brash, A.R. Fatty Acids of Helicobacter pylori Lipoproteins CagT and Lpp20. Microbiol. Spectr. 2024, 12, e00470-24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Zeng, L.; Yu, L.; Duan, Y.; Shen, S.; Hu, J.; Zhang, P.; Song, W.; Ruan, X.; et al. Helicobacter pylori FabX Contains a [4Fe-4S] Cluster Essential for Unsaturated Fatty Acid Synthesis. Nat. Commun. 2021, 12, 6932. [Google Scholar] [CrossRef]
- Radka, C.D.; Rock, C.O. Mining Fatty Acid Biosynthesis for New Antimicrobials. Annu. Rev. Microbiol. 2022, 76, 281–304. [Google Scholar] [CrossRef]
- Ong, L.-L.; Jan, H.-M.; Le, H.-H.T.; Yang, T.-C.; Kuo, C.-Y.; Feng, A.-F.; Mong, K.-K.T.; Lin, C.-H. Membrane Lipid Remodeling Eradicates Helicobacter pylori by Manipulating the Cholesteryl 6′-Acylglucoside Biosynthesis. J. Biomed. Sci. 2024, 31, 44. [Google Scholar] [CrossRef]
- Liechti, G.; Goldberg, J.B. Helicobacter pylori Relies Primarily on the Purine Salvage Pathway for Purine Nucleotide Biosynthesis. J. Bacteriol. 2012, 194, 839–854. [Google Scholar] [CrossRef]
- Dilip, H.; Thiruvenkatam, V.; Kirubakaran, S. Studies on Methylpyrazole-Substituted Benzimidazoles to Target Helicobacter pylori Infection through Hp IMPDH Inhibition. ACS Infect. Dis. 2024, 10, 2262–2275. [Google Scholar] [CrossRef]
- Galal, A.M.F.; Mohamed, H.S.; Abdel-Aziz, M.M.; Hanna, A.G. Development, Synthesis, and Biological Evaluation of Sulfonyl-α- l -amino Acids as Potential Anti- Helicobacter pylori and IMPDH Inhibitors. Arch. Der Pharmazie 2021, 354, 2000385. [Google Scholar] [CrossRef]
- Gómez-Garzón, C.; Payne, S.M. Divide and Conquer: Genetics, Mechanism, and Evolution of the Ferrous Iron Transporter Feo in Helicobacter pylori. Front. Microbiol. 2023, 14, 1219359. [Google Scholar] [CrossRef]
- Ye, J.; Feng, T.; Su, L.; Li, J.; Gong, Y.; Ma, X. Interactions between Helicobacter pylori Infection and Host Metabolic Homeostasis: A Comprehensive Review. Helicobacter 2023, 28, e13030. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, J.; Hughes, N.J.; McColm, A.A.; Bagshaw, J.; Clayton, C.L.; Andrews, S.C.; Kelly, D.J. Iron Acquisition and Virulence in Helicobacter pylori: A Major Role for FeoB, a High-affinity Ferrous Iron Transporter. Mol. Microbiol. 2000, 37, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Haley, K.P.; Francis, J.D.; Guevara, M.A.; Doster, R.S.; Craft, K.M.; Moore, R.E.; Chambers, S.A.; Delgado, A.G.; Piazuelo, M.B.; et al. The Innate Immune Glycoprotein Lactoferrin Represses the Helicobacter pylori Cag Type IV Secretion System. ChemBioChem 2021, 22, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.; Yang, Z.; Chen, Z. Lactoferrin: A Glycoprotein That Plays an Active Role in Human Health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef]
- Imoto, I.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Oka, S.; Misaki, M.; Horiki, N.; Gabazza, E.C. Antimicrobial Effects of Lactoferrin against Helicobacter pylori Infection. Pathogens 2023, 12, 599. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, Y.B.; An, T.J.; Yoo, K.H.; Rhee, C.K. Effect of Broncho-Vaxom (OM-85) on the Frequency of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations. BMC Pulm. Med. 2023, 23, 378. [Google Scholar] [CrossRef]
- Ohm, M.; Hahné, S.J.M.; Van Der Ende, A.; Sanders, E.A.M.; Berbers, G.A.M.; Ruijs, W.L.M.; Van Sorge, N.M.; De Melker, H.E.; Knol, M.J. Vaccine Impact and Effectiveness of Meningococcal Serogroup ACWY Conjugate Vaccine Implementation in the Netherlands: A Nationwide Surveillance Study. Clin. Infect. Dis. 2022, 74, 2173–2180. [Google Scholar] [CrossRef]
- Cen, Q.; Gao, T.; Ren, Y.; Lu, X.; Lei, H. Immune Evaluation of a Saccharomyces Cerevisiae-based Oral Vaccine against Helicobacter pylori in Mice. Helicobacter 2021, 26, e12772. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Ji, Q.; Liu, Q. Evaluation of an Attenuated Listeria Monocytogenes as a Vaccine Vector to Control Helicobacter pylori Infection. Immunol. Lett. 2021, 238, 68–74. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Patil, S.; Lopes, B.S. The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals 2023, 16, 552. [Google Scholar] [CrossRef]
- Espinosa-Ramos, D.; Caballero-Hernández, D.; Gomez-Flores, R.; Trejo-Chávez, A.; Pérez-Limón, L.J.; De La Garza-Ramos, M.A.; Tamez-Guerra, R.; Tamez-Guerra, P.; Rodriguez-Padilla, C. Immunization with a Synthetic Helicobacter pylori Peptide Induces Secretory IgA Antibodies and Protects Mice against Infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8595487. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.H. DNA Vaccines: Roles against Diseases. GERMS 2013, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Doosti, A. Effect of the cagW-Based Gene Vaccine on the Immunologic Properties of BALB/c Mouse: An Efficient Candidate for Helicobacter pylori DNA Vaccine. J. Nanobiotechnol. 2020, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.; Tahmasebi Birgani, M.; Bijanzadeh, M. DNA Vaccine Containing Flagellin A Gene Induces Significant Immune Responses against Helicobacter pylori Infection: An in Vivo Study. Iran. J. Basic Med. Sci. 2021, 24, 796–804. [Google Scholar] [CrossRef]
- Sutton, P.; Boag, J.M. Status of Vaccine Research and Development for Helicobacter pylori. Vaccine 2019, 37, 7295–7299. [Google Scholar] [CrossRef]
- Zeng, M.; Mao, X.-H.; Li, J.-X.; Tong, W.-D.; Wang, B.; Zhang, Y.-J.; Guo, G.; Zhao, Z.-J.; Li, L.; Wu, D.-L.; et al. Efficacy, Safety, and Immunogenicity of an Oral Recombinant Helicobacter pylori Vaccine in Children in China: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef]
- Bugaytsova, J.A.; Piddubnyi, A.; Tkachenko, I.; Rakhimova, L.; Edlund, J.O.; Thorell, K.; Marcotte, H.; Lundquist, A.; Schön, K.; Lycke, N.; et al. Vaccination with Helicobacter pylori attachment proteins protects against gastric cancer. bioRxiv 2023, 4. [Google Scholar] [CrossRef]
- Tu, Z.; Wang, Y.; Liang, J.; Liu, J. Helicobacter pylori-Targeted AI-Driven Vaccines: A Paradigm Shift in Gastric Cancer Prevention. Front. Immunol. 2024, 15, 1500921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakiera, A.; Solarz, A.; Kowalczyk, M.; Cichoż-Lach, H.; Korona-Głowniak, I. Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation. Pathogens 2025, 14, 619. https://doi.org/10.3390/pathogens14070619
Bakiera A, Solarz A, Kowalczyk M, Cichoż-Lach H, Korona-Głowniak I. Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation. Pathogens. 2025; 14(7):619. https://doi.org/10.3390/pathogens14070619
Chicago/Turabian StyleBakiera, Adrian, Anita Solarz, Marika Kowalczyk, Halina Cichoż-Lach, and Izabela Korona-Głowniak. 2025. "Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation" Pathogens 14, no. 7: 619. https://doi.org/10.3390/pathogens14070619
APA StyleBakiera, A., Solarz, A., Kowalczyk, M., Cichoż-Lach, H., & Korona-Głowniak, I. (2025). Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation. Pathogens, 14(7), 619. https://doi.org/10.3390/pathogens14070619






