CRISPR-Cas Dynamics in Carbapenem-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Clinical Isolates from a Croatian Tertiary Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Bacterial Isolates and Antimicrobial Susceptibility Testing
2.3. Molecular Screening for Carbapenemase Genes and the CRISPR-Cas System
2.4. Pulsed-Field Gel Electrophoresis and Multilocus Sequence Typing
2.5. Statistical Analysis
3. Results
3.1. Antimicrobial Susceptibility of Bacterial Isolates
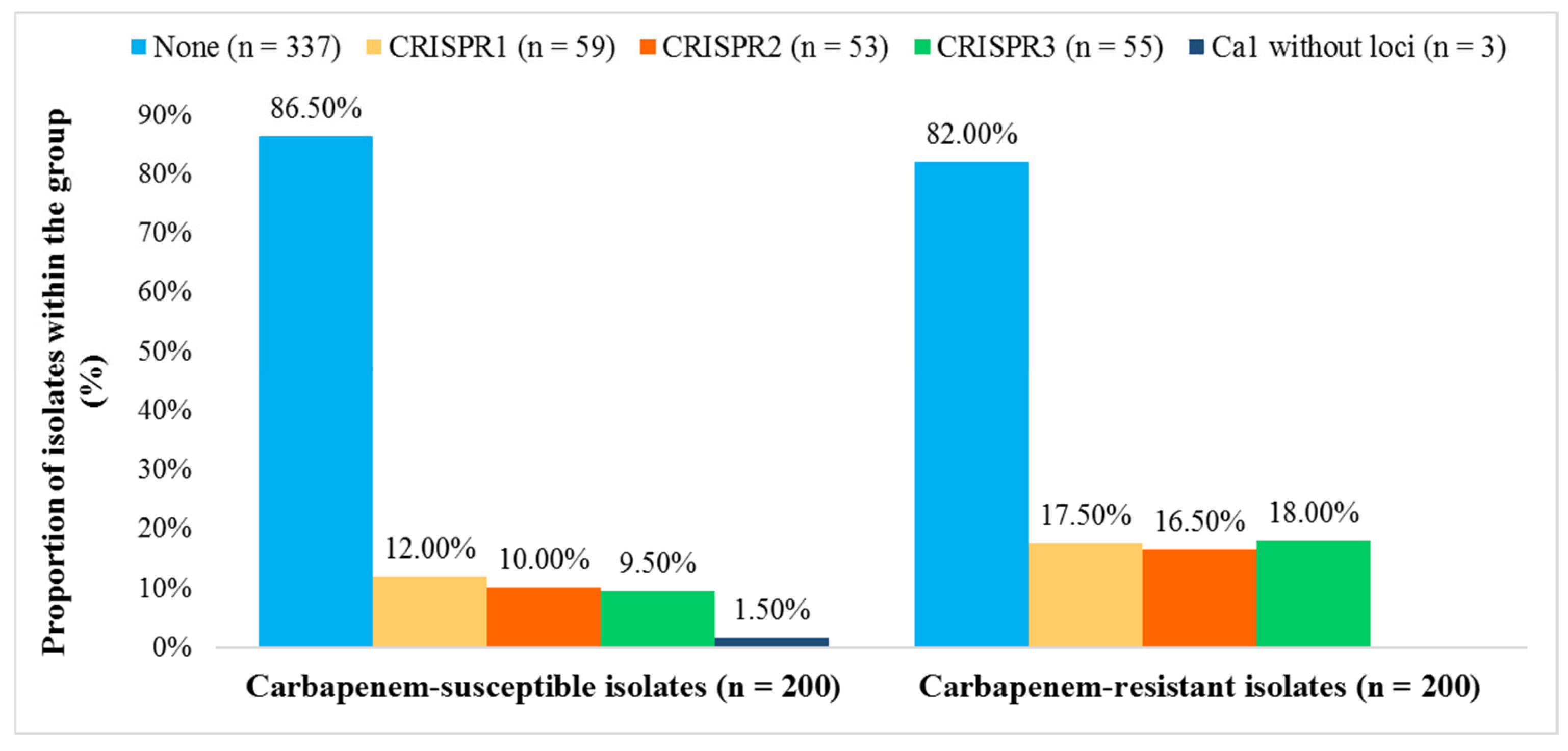
3.2. Distribution of Carbapenemase-Encoding Genes and the CRISPR-Cas System
3.3. Correlation Between Antimicrobial Resistance and the CRISPR-Cas System
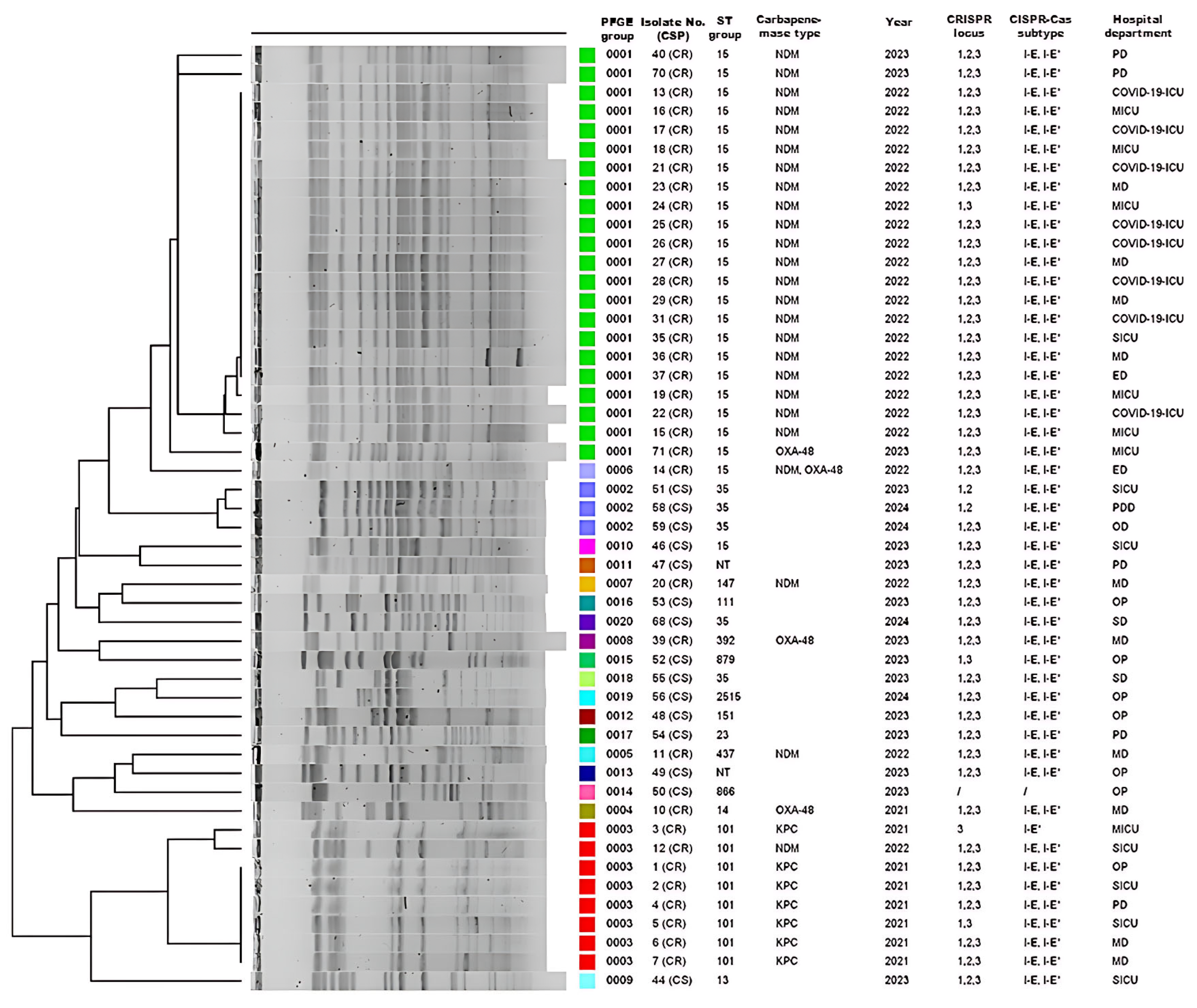
3.4. PFGE and MLST Analysis
3.5. CRISPR Polymorphism and Its Relation with Carbapenem Resistance, MLST, and Hospital Departments
3.6. The Origin of CRISPR Spacers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | Amoxicillin + clavulanic acid |
| AME | Aminoglycoside-modifying enzymes |
| AMK | Amikacin |
| AMP | Ampicillin |
| AMR | Antimicrobial resistance |
| AMX | Amoxicillin |
| BLAST | Basic Local Alignment Search Tool |
| bp | Base pairs |
| CAZ | Ceftazidime |
| CAZ + AVI | Ceftazidime + avibactam |
| CFM | Cefixime |
| CIP | Ciprofloxacin |
| CLX | Cephalexin |
| COL | Colistin |
| CPD | Cefpodoxime |
| CR | Carbapenem resistant |
| CRE | Carbapenem-resistant Enterobacterales |
| CRO | Ceftriaxone |
| CS | Carbapenem susceptible |
| CSP | Carbapenem susceptibility profile |
| CTX | Cefotaxime |
| CXM | Cefuroxime |
| ED | Emergency department |
| ESBL | Extended-spectrum β-lactamase |
| ETP | Ertapenem |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FDC | Cefiderocol |
| FEP | Cefepime |
| GEN | Gentamicin |
| HGT | Horizontal gene transfer |
| ICU | Intensive care unit |
| IPM | Imipenem |
| IPM + REL | Imipenem + relebactam |
| LVX | Levofloxacin |
| MD | Medical department |
| MEM | Meropenem |
| MGE | Mobile genetic element |
| MIC | Minimum inhibitory concentration |
| MICU | Medical intensive care units |
| MLST | Multilocus sequence typing |
| n | Number |
| N/A | Not applicable |
| NT | Non-typable |
| OD | Oncology department |
| OP | Outpatients |
| PCR | Polymerase chain reaction |
| PD | Pulmonology departments |
| PFGE | Pulsed-field gel electrophoresis |
| PDD | Pediatric department |
| SD | Surgery departments |
| SICU | Surgical intensive care units |
| ST | Sequence type |
| SXT | Trimethoprim + sulfamethoxazole |
| TOL + TAZ | Ceftolozane + tazobactam |
| TZP | Piperacillin + tazobactab |
| W | Weak |
References
- Tang, M.; Kong, X.; Hao, J.; Liu, J. Epidemiological characteristics and formation mechanisms of multidrug-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2020, 11, 581543. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Henriques, I.; Gomila, M.; Manaia, C.M. Common and distinctive genomic features of Klebsiella pneumoniae thriving in the natural environment or in clinical settings. Sci. Rep. 2022, 12, 10441. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections caused by carbapenem-resistant Enterobacteriaceae: An update on therapeutic options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhao, J.; Chang, K.; Zhuo, X.; Cao, B. “Superbugs” with hypervirulence and carbapenem resistance in Klebsiella pneumoniae: The rise of such emerging nosocomial pathogens in China. Sci. Bull. 2023, 68, 2658–2670. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Alocilja, E.C. A Review of Carbapenem Resistance in Enterobacterales and Its Detection Techniques. Microorganisms 2023, 11, 1491. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pages, J.M. Enterobacter aerogenes and Enterobacter cloacae: Versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef]
- Coppola, N.; Maraolo, A.E.; Onorato, L.; Scotto, R.; Calò, F.; Atripaldi, L.; Borrelli, A.; Corcione, A.; De Cristofaro, M.G.; Durante-Mangoni, E.; et al. Epidemiology, Mechanisms of Resistance and Treatment Algorithm for Infections Due to Carbapenem-Resistant Gram-Negative Bacteria: An Expert Panel Opinion. Antibiotics 2022, 11, 1263. [Google Scholar] [CrossRef]
- Tambić-Andrašević, A.; Lucić, S.; Tambić, T. Antibiotic resistance in Croatia. Med. Flum. 2018, 54, 312–321. [Google Scholar] [CrossRef]
- Bedenić, B.; Slade, M.; Starčević, L.Ž.; Sardelić, S.; Vranić-Ladavac, M.; Benčić, A.; Zekan, Š.; Plečko, V.; Butić, I.; Papić, N.; et al. Epidemic spread of OXA-48 β-lactamase in Croatia. J. Med. Microbiol. 2018, 67, 1031–1041. [Google Scholar] [CrossRef]
- Kuzina, E.S.; Kislichkina, A.A.; Sizova, A.A.; Skryabin, Y.P.; Novikova, T.S.; Ershova, O.N.; Savin, I.A.; Khokhlova, O.E.; Bogun, A.G.; Fursova, N.K. High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39. Microorganisms 2023, 11, 459. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Iredell, J.R. CRISPR-Cas system in antibiotic resistance plasmids in Klebsiella pneumoniae. Front. Microbiol. 2019, 10, 2934. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Newire, E.; Aydin, A.; Juma, S.; Enne, V.I.; Roberts, A.P. Identification of a Type IV-A CRISPR-Cas system located exclusively on IncHI1B/IncFIB plasmids in Enterobacteriaceae. Front. Microbiol. 2020, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lv, L.; Wang, X.; Xiu, Z.; Chen, G. Comparative analysis of CRISPR-Cas systems in Klebsiella genomes. J. Basic Microbiol. 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Jwair, N.A.; Al-Ouqaili, M.T.S.; Al-Marzooq, F. Inverse association between the existence of CRISPR/Cas systems and antibiotic resistance, extended spectrum β-lactamase, and carbapenemase production in multidrug-, extensive drug-, and pandrug-resistant Klebsiella pneumoniae. Antibiotics 2023, 12, 980. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Chen, C.; Li, J.; Du, F.; Long, D.; Zhang, W. Distribution of CRISPR-Cas Systems in Clinical Carbapenem-Resistant Klebsiella pneumoniae Strains in a Chinese Tertiary Hospital and Its Potential Relationship with Virulence. Microb. Drug Resist. 2020, 26, 630–636. [Google Scholar] [CrossRef]
- Wang, G.; Song, G.; Xu, Y. Association of CRISPR/Cas system with the drug resistance in Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 1929–1935. [Google Scholar] [CrossRef]
- Kadkhoda, H.; Gholizadeh, P.; Ghotaslou, R.; Pirzadeh, T.; Ahangarzadeh Rezaee, M.; Nabizadeh, E.; Feizi, H.; Samadi Kafil, H.; Aghazadeh, M. Prevalence of the CRISPR-cas system in Klebsiella pneumoniae isolates. BMC Infect. Dis. 2024, 24, 554. [Google Scholar] [CrossRef]
- Li, H.Y.; Kao, C.Y.; Lin, W.H.; Zheng, P.X.; Yan, J.J.; Wang, M.C.; Teng, C.H.; Tseng, C.C.; Wu, J.J. Characterization of CRISPR-Cas systems in clinical Klebsiella pneumoniae isolates uncovers its potential association with antibiotic susceptibility. Front. Microbiol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Alkompoz, A.K.; Hamed, S.M.; Zaid, A.S.A.; Almangour, T.A.; Al-Agamy, M.H.; Aboshanab, K.M. Correlation of CRISPR/Cas and antimicrobial resistance in Klebsiella pneumoniae clinical isolates. Microorganisms 2023, 11, 1948. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0 2024. Available online: http://www.eucast.org (accessed on 28 May 2025).
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Watahiki, M.; Kawahara, R.; Suzuki, M.; Aoki, M.; Uchida, K.; Matsumoto, Y.; Kumagai, Y.; Noda, M.; Masuda, K.; Fukuda, C.; et al. Single-Tube Multiplex Polymerase Chain Reaction for the detection of genes encoding Enterobacteriaceae carbapenemase. Jpn. J. Infect. Dis. 2020, 73, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Gong, J.; Yuan, X.; Lu, H.; Jiang, L. Drug Resistance Genes and Molecular Epidemiological Characteristics of Carbapenem-Resistant Klebsiella pneumoniae. Infect. Drug Resist. 2023, 16, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Pan, Y.J.; Hsieh, P.F.; Hsu, C.R.; Wu, M.C.; Wang, J.T. Imipenem represses CRISPR-Cas interference of DNA acquisition through H-NS stimulation in Klebsiella pneumoniae. Sci. Rep. 2016, 6, 31644. [Google Scholar] [CrossRef]
- Kitchel, B.; Rasheed, J.K.; Patel, J.B.; Srinivasan, A.; Navon-Venezia, S.; Carmeli, Y.; Brolund, A.; Giske, C.G. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: Clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 2009, 53, 3365–3370. [Google Scholar] [CrossRef]
- Han, H.; Zhou, H.; Li, H.; Gao, Y.; Lu, Z.; Hu, K.; Xu, B. Optimization of pulse-field gel electrophoresis for subtyping of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2013, 10, 2720–2731. [Google Scholar] [CrossRef]
- Jelic, M.; Butic, I.; Plecko, V.; Cipris, I.; Jajic, I.; Bejuk, D.; Koscak, I.; Marinkovic, S.; Pal, M.P.; Tambic Andrasevic, A. KPC-producing Klebsiella pneumoniae isolates in Croatia: A nationwide survey. Microb. Drug Resist. 2016, 22, 662–667. [Google Scholar] [CrossRef]
- Brisse, S.; Fèvre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent clones of Klebsiella pneumoniae: Identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef]
- Spratt, B.G.; Hanage, W.P.; Li, B.; Aanensen, D.M.; Feil, E.J. Displaying the relatedness among isolates of bacterial species—The eBURST approach. FEMS Microbiol. Lett. 2004, 241, 129–134. [Google Scholar] [CrossRef]
- Russo, A.; Fusco, P.; Morrone, H.L.; Trecarichi, E.M.; Torti, C. New advances in management and treatment of multidrug-resistant Klebsiella pneumoniae. Expert Rev. Anti Infect. Ther. 2023, 21, 41–55. [Google Scholar] [CrossRef]
- Owaid, H.A.; Al-Ouqaili, M.T.S. Molecular and bacteriological investigations for the coexistence CRISPR/Cas system and β-lactamases of types extended-spectrum and carbapenemases in Multidrug, extensive drug and Pandrug-Resistant Klebsiella pneumoniae. Saudi J. Biol. Sci. 2024, 31, 104022. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, E.A.; Saki, M.; Savari, M.; Meghdadi, H.; Akrami, S. Association between the presence of CRISPR-Cas system genes and antibiotic resistance in Klebsiella pneumoniae isolated from patients admitted in Ahvaz teaching hospitals. BMC Infect. Dis. 2024, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, J.; Wang, D.; Guo, Q.; Wang, M. Coexistence of blaKPC-IncFII plasmids and type I-E* CRISPR-Cas systems in ST15 Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1125531. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. The basic building blocks and evolution of CRISPR-Cas systems. Biochem. Soc. Trans. 2013, 41, 1392–1400. [Google Scholar] [CrossRef]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef]
- Kannadasan, A.B.; Sumantran, V.N.; Vaidyanathan, R. A Global Comprehensive Study of the Distribution of Type I-E and Type I-E* CRISPR-Cas Systems in Klebsiella pneumoniae. Indian J. Community Med. 2023, 48, 567–572. [Google Scholar] [CrossRef]
- Berger, S.; Alauzet, C.; Aissa, N.; Hénard, S.; Rabaud, C.; Bonnet, R.; Lozniewski, A. Characterization of a new blaOXA-48-carrying plasmid in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 4064–4067. [Google Scholar] [CrossRef]
- Kadkhoda, H.; Gholizadeh, P.; Ghotaslou, R.; Nabizadeh, E.; Pirzadeh, T.; Ahangarzadeh Rezaee, M.; Feizi, H.; Samadi Kafil, H.; Aghazadeh, M. Role of CRISPR-cas system on virulence traits and carbapenem resistance in clinical Klebsiella pneumoniae isolates. Microb. Pathog. 2025, 199, 107151. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Lin, J.; Gu, Y.; Liu, Z.; Hu, J. Detection of CRISPR—Cas and type I R-M systems in Klebsiella pneumoniae of human and animal origins and their relationship to antibiotic resistance and virulence. Microbiol. Spectr. 2025, 13, e0000924. [Google Scholar] [CrossRef]

| Department | Number of Samples (%) |
|---|---|
| Medical Departments | 64 (16.0) |
| Medical Intensive Care Units | 57 (14.3) |
| Pediatric Intensive Care Units | 51 (12.8) |
| Surgical Intensive Care Units | 45 (11.3) |
| Surgery Departments | 44 (11.0) |
| Outpatients | 40 (10.0) |
| Hematology Departments | 25 (6.3) |
| Pulmonology Departments | 23 (5.8) |
| Emergency Departments | 21 (5.3) |
| COVID-19 Intensive Care Units | 12 (3.0) |
| Pediatric Departments | 9 (2.3) |
| Oncology Departments | 6 (1.5) |
| COVID-19 Departments | 3 (0.8) |
| Gene/CRISPR Locus | Primer Sequence (5′-3′) | Annealing Temperature (°C) | PCR Product Size (bp) |
|---|---|---|---|
| cas1 | F-GCTGTTTGTCAAAGTTACCCGCGAACTC | 58 | 150 |
| R-GGTTTTGATCGCCTCATGAGTCACAGTTG | |||
| rpoB | F-GGCGAAATGGCWGAGAACCA | 54 | 1100 |
| R-GAGTCTTCGAAGTTGTAACC | |||
| I-E CRISPR1 | F-GACGGTGGTTATATGGTGAC | 52 | Variable (400–900) |
| R-CATTGATGCCTCTACGTCAG | |||
| I-E* CRISPR2 | F-GTAGCGAAACCCTGATCAAGCG | 57 | Variable (900–1300) |
| R-GCGCTACGTTCTGGGGATG | |||
| I-E* CRISPR3 | F-GACGCTGGTGCGATTCTTGAG R-CGCAGTATTCCTCAACCGCCT | 58 | Variable (1300–2100) |
| Carbapenemase Gene | CRISPR-Cas System | p-Value | ||||
|---|---|---|---|---|---|---|
| cas1 Without Loci (n = 3) | Type I-E (n = 3) | Type I-E* (n = 1) | Types I-E and I-E* (n = 56) | Absent (n = 337) | ||
| blaNDM (n = 53) | 0 | 0 | 0 | 25 (44.6%) | 28 (8.3%) | <0.0001 |
| blaKPC (n = 10) | 0 | 0 | 1 (100%) | 6 (10.7%) | 3 (0.9%) | <0.0001 |
| blaOXA-48 (n = 123) | 0 | 0 | 0 | 3 (5.3%) | 120 (35.6%) | <0.0001 |
| blaOXA-48 and blaNDM (n = 14) | 0 | 0 | 0 | 1 (1.8%) | 13 (3.9%) | 0.9290 |
| without carbapenemase gene (n = 200) | 3 (100%) | 3 (100%) | 0 | 21 (37.5%) | 173 (51.3%) | 0.0296 |
| CRISPR Locus | Spacer Sequence (5′ to 3′) | Isolate No. (CSP) | ST Group | Hospital Department |
|---|---|---|---|---|
| CRISPR3 | GCAGTCGATTCGTCTCGACAGTGACAGGGGC | 12 (CR) | ST101 | Surgical intensive care units |
| CRISPR1 | TCTTGCTTGGAGAGGATTCTACAGTCTCACCAT | 40 (CR) | ST15 | Pulmonology departments |
| CRISPR1 | TACGTCCCGAAGACCACTGCCGGGGGATCAAGG | |||
| CRISPR1 | CTGGGGGCAGGACCCCGATATGACGGAGGTGA | |||
| CRISPR3 | TGTGTGACAAAGCCACGTCCGGGAAGAACAAT | 41 (CS) | ST23 | Surgery departments |
| CRISPR3 | AAACTGCGCATCGTTCGACGCGAGCGACATCGA | 42 (CS) | ST35 | Outpatients |
| CRISPR1 | CGTGTTTTTGTGTTTAATGGTCATTTATGATTT | 52 (SC) | ST879 | Outpatients |
| CRISPR3 | ACACGAAATGACGGGGTTTCGCCGGTCGTCTCA | 56 (CS) | ST2515 | Outpatients |
| CRISPR3 | CCGGCTACAATGCGATCGGTGGGCAGTGGTTGC | |||
| CRISPR3 | AAAACCCAGTAGACGGGGATAGAGACAAAAG | 65 (CS) | ST35 | Surgery departments |
| CRISPR3 | TACGTGGAATACCGTGTTGCACCAATGAATATG | |||
| CRISPR3 | AAATTCAGCAGGTCGCGGGATGCCGTGGTTGT | 68 (CS) | ST35 | Surgery departments |
| CRISPR3 | ACCGCGATCCGTTCCGGCTTAGGCCGTTTAT | |||
| CRISPR3 | CCGTCATTCATATTTCCGGGGAAACTGGGTT | |||
| CRISPR3 | CAACGAAGTAAACGGGGATCGTCCGTCCAAGA | |||
| CRISPR3 | GGCACCCCTTCCGCCCAAAGGGGCCCTATTCTA | 71(CR) | ST15 | Medical intensive care units |
| CRISPR3 | AAACCAATCAAAATTCTTCCCCACATGGAAAGTGGCCA |
| Spacer Sequence (5′ to 3′) | Isolate No. | Phage (Accession No.) | Plasmid (Accession No.) | Homology Type |
|---|---|---|---|---|
| CRISPR1 Locus | ||||
| TGCCTCCAATGCAATCACCGGCCTGCTAACCGG | 8, 40, 49, 71, 46, 28 | BK047705.1 | / | Phage |
| CGTCATCAGCGCCTTGTTCCAGCGGCGACCACC | 8, 40, 49, 54, 71, 46, 28 | NC_071011.1 | / | Phage |
| CCTGCAGCTGGCCGTCGAGCTGACGGATGCCGG | 8, 49, 53, 54, 71, 46, 28 | / | AP024174.1 | Plasmid |
| TGTAGCGCGGCTGGTTGATGCACTGAGGCACTA | 52 | MK422452.1 | / | Phage |
| CAAATGGGAGAAGCTAATCGTTGGGGTGCTGAA | 52 | MK416022.1 | OW848780.1 | Phage and Plasmid |
| TGGTCATCGCGCCCCTTGGCCTGCTCTGCGCTG | 53 | MN013086.1 | / | Phage |
| TCCTTCATTAAGTGAGCAATTGCTTCCTTTTTT | 53 | PP934564.1 | / | Phage |
| CGGCTCTTTTTTATCTCCTTCATCCTTCGCTAT | 54 | BK055976.1 | / | Phage |
| TGATCGGCGTGCCGTTTGTTGGACCCGAAATAG | 54 | BK031684.1 | / | Phage |
| CGAGCTCATCGCCTCCCTGGAGACGGCGGGCGA | 63, 65 | BK031364.1 | / | Phage |
| CAAGACACCTGCAAACGGTATATCTTTGGAGTG | 63, 65 | OP617741.1 | / | Phage |
| CRISPR2 Locus | ||||
| AGGATAGAGCCAAATCCGCTCACACGTGATGA | 15, 48, 8, 12, 71, 41, 28, 46 | / | CP079158.1 | Plasmid |
| CCGGCATCCGTCAGCTCGACGGCCAGCTGCAG | 15, 48, 8, 12, 71, 41, 28, 46 | / | CP081815.1 | Plasmid |
| ACGTGATCGCCCTGGCGCGGACGCCGGGAGGT | 15, 48, 8, 12, 71, 41, 28, 46 | NC_071011.1 | CP079158.1 | Phage and Plasmid |
| ATGGTGCGACTGTAGAATCCTCACCATGCACG | 15, 48, 8, 12, 71, 41, 28, 46 | NC_071011.1 | CP079158.1 | Phage and Plasmid |
| GATAATCCCGTCAGGTTGTGACTCTGCACGAT | 15, 48, 8, 12, 71, 41, 28, 46 | NC_071011.1 | CP079158.1 | Phage and Plasmid |
| TCGAGGACATTACCGAGGACTACGACGACTGG | 15, 48, 8, 12, 71, 41, 28, 46 | BK019580.1 | / | Phage |
| GGTGGTCGCCGCTGGAACAAGGCGCTGATGAC | 15, 48, 8, 12, 71, 41, 28, 46 | NC_071011.1 | CP079158.1 | Phage and Plasmid |
| GACTGGCTCGGCTACGAGGTGCGCTTCGACAC | 15, 8, 12, 71, 28, 46 | / | CP079158.1 | Plasmid |
| CCGGTTAGCAGGCCGGTGATTGCATTGGAGGC | 15, 8, 12, 71, 28, 46 | BK047705.1 | CP079158.1 | Phage and Plasmid |
| CTATTTCGGGTCCAACAAACGGCACGCCGATC | 41 | BK031684.1 | CP093490.1 | Phage and Plasmid |
| ATAGCGAAGGATGAAGGAGATAAAAAAGAGCC | 41 | BK055976.1 | / | Phage |
| CCACTCCAAAGATATACCGTTTGCAGGTGTCTTG | 56, 65, 63 | OP617741.1 | / | Phage |
| TCGCCCGCCGTCTCCAGGGAGGCGATGAGCTCG | 56, 65, 63 | BK031364.1 | / | Phage |
| CRISPR3 Locus | ||||
| TCCAGTACGCCAATGCTGGTAGACCCCTCACA | 8, 10, 12, 13, 28, 41, 42, 46, 63, 68, 70, 71 | / | CP183011.1 | Plasmid |
| AGAACGAATGCCCGCGCTGGTACGGCGCGTCGTGGATTCC | 8, 10, 12, 13, 28, 46, 63, 70, 71 | / | CP079158.1 | Plasmid |
| ATTCAGCTGAAAACTGCCAGTATCGCGGCGGT | 8, 10 | / | CP079675.1 | Plasmid |
| TCGCCGTCGAAGTGCTGCGCGATAGGGATGAT | 8, 10 | / | CP079675.1 | Plasmid |
| TGCCGGATATCATCACCGCGATTAAACGGCGG | 12, 70, 13, 28, 46 | / | CP067552.1 | Plasmid |
| GCTAACCAGTGGATAGAGCACTATGTGACGAC | 12, 70, 13, 28, 46, 71 | BK049466.1 | / | Phage |
| GCTACTGCATCCACGGCGTACATGCTCAGTGT | 12, 70, 13, 28, 46, 71 | BK052889.1 | / | Phage |
| CTTCGACACCAACCCAAACAGATCTGGCCTGGA | 41 | BK049466.1 | / | Phage |
| TGGAATTTTCGCGTCTCCAAAAACTGCGCATC | 42 | BK052641.1 | / | Phage |
| GCGGACGCGCTCCATGAAGTAGTCCCGCAGGT | 42 | BK056588.1 | / | Phage |
| CCGCAGCCGCGGCGGCTTTTGCCGGTGCTGAC | 63 | MN013086.1 | / | Phage |
| TGCCTGCTGATCTGCGGCGTTATCTGGACAGCAG | 63 | OR532892.1 | / | Phage |
| TGTCCCGGATACGCTTTCCGCCATTGATGCGC | 63 | OU509537.1 | / | Phage |
| GCTAACCAGTGGATAGAGCACTATGTGACGAC | 71 | BK049466.1 | / | Phage |
| GCTACTGCATCCACGGCGTACATGCTCAGTGT | 71 | BK049466.1 | / | Phage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurić, I.; Jelić, M.; Markanović, M.; Kanižaj, L.; Bošnjak, Z.; Budimir, A.; Kuliš, T.; Tambić-Andrašević, A.; Ivančić-Baće, I.; Mareković, I. CRISPR-Cas Dynamics in Carbapenem-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Clinical Isolates from a Croatian Tertiary Hospital. Pathogens 2025, 14, 604. https://doi.org/10.3390/pathogens14060604
Jurić I, Jelić M, Markanović M, Kanižaj L, Bošnjak Z, Budimir A, Kuliš T, Tambić-Andrašević A, Ivančić-Baće I, Mareković I. CRISPR-Cas Dynamics in Carbapenem-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Clinical Isolates from a Croatian Tertiary Hospital. Pathogens. 2025; 14(6):604. https://doi.org/10.3390/pathogens14060604
Chicago/Turabian StyleJurić, Ivana, Marko Jelić, Manda Markanović, Lucija Kanižaj, Zrinka Bošnjak, Ana Budimir, Tomislav Kuliš, Arjana Tambić-Andrašević, Ivana Ivančić-Baće, and Ivana Mareković. 2025. "CRISPR-Cas Dynamics in Carbapenem-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Clinical Isolates from a Croatian Tertiary Hospital" Pathogens 14, no. 6: 604. https://doi.org/10.3390/pathogens14060604
APA StyleJurić, I., Jelić, M., Markanović, M., Kanižaj, L., Bošnjak, Z., Budimir, A., Kuliš, T., Tambić-Andrašević, A., Ivančić-Baće, I., & Mareković, I. (2025). CRISPR-Cas Dynamics in Carbapenem-Resistant and Carbapenem-Susceptible Klebsiella pneumoniae Clinical Isolates from a Croatian Tertiary Hospital. Pathogens, 14(6), 604. https://doi.org/10.3390/pathogens14060604







